Antibody data
- Antibody Data
- Antigen structure
- References [20]
- Comments [0]
- Validations
- Western blot [3]
- Immunocytochemistry [2]
- Immunoprecipitation [1]
- Immunohistochemistry [2]
- Flow cytometry [1]
- Other assay [20]
Submit
Validation data
Reference
Comment
Report error
- Product number
- AHO0802 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- EIF2S1 Monoclonal Antibody (EIF2-alpha)
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Description
- This antibody recognizes the alpha subunit of eukaryotic translation initiation factor 2 (EIF2-alpha). Recommended positive control are human Jurkat, CEM, and HeLa cells; mouse 3T3L1 cells; and rat PC-12 cells.
- Reactivity
- Human, Mouse, Rat
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- EIF2-alpha
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- -20°C
Submitted references Salubrinal Enhances Cancer Cell Death during Glucose Deprivation through the Upregulation of xCT and Mitochondrial Oxidative Stress.
Mitochondrial stress is relayed to the cytosol by an OMA1-DELE1-HRI pathway.
Irp2 regulates insulin production through iron-mediated Cdkal1-catalyzed tRNA modification.
Nucleocytoplasmic Proteomic Analysis Uncovers eRF1 and Nonsense-Mediated Decay as Modifiers of ALS/FTD C9orf72 Toxicity.
Fission Yeast Asc1 Stabilizes the Interaction between Eukaryotic Initiation Factor 3a and Rps0A/uS2 for Protein Synthesis.
Inactivation of Ppp1r15a minimises weight gain and insulin resistance during caloric excess in female mice.
A Rapid Extraction Method for mammalian cell cultures, suitable for quantitative immunoblotting analysis of proteins, including phosphorylated GCN2 and eIF2α.
Extracellular and ER-stored Ca(2+) contribute to BIRD-2-induced cell death in diffuse large B-cell lymphoma cells.
Infantile Cirrhosis, Growth Impairment, and Neurodevelopmental Anomalies Associated with Deficiency of PPP1R15B.
Perturbations in actin dynamics reconfigure protein complexes that modulate GCN2 activity and promote an eIF2 response.
Ebola Virus Does Not Induce Stress Granule Formation during Infection and Sequesters Stress Granule Proteins within Viral Inclusions.
Actin dynamics tune the integrated stress response by regulating eukaryotic initiation factor 2α dephosphorylation.
Generation and characterization of an analog-sensitive PERK allele.
Endoplasmic reticulum stress induced by aqueous extracts of cigarette smoke in 3T3 cells activates the unfolded-protein-response-dependent PERK pathway of cell survival.
Enhanced antiglioma activity of chimeric HCMV/HSV-1 oncolytic viruses.
VSV replication in neurons is inhibited by type I IFN at multiple stages of infection.
Deficiency of ATP2C1, a Golgi ion pump, induces secretory pathway defects in endoplasmic reticulum (ER)-associated degradation and sensitivity to ER stress.
Human cytomegalovirus TRS1 and IRS1 gene products block the double-stranded-RNA-activated host protein shutoff response induced by herpes simplex virus type 1 infection.
The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas.
The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas.
Chen MC, Hsu LL, Wang SF, Pan YL, Lo JF, Yeh TS, Tseng LM, Lee HC
Biomedicines 2021 Aug 28;9(9)
Biomedicines 2021 Aug 28;9(9)
Mitochondrial stress is relayed to the cytosol by an OMA1-DELE1-HRI pathway.
Guo X, Aviles G, Liu Y, Tian R, Unger BA, Lin YT, Wiita AP, Xu K, Correia MA, Kampmann M
Nature 2020 Mar;579(7799):427-432
Nature 2020 Mar;579(7799):427-432
Irp2 regulates insulin production through iron-mediated Cdkal1-catalyzed tRNA modification.
Santos MCFD, Anderson CP, Neschen S, Zumbrennen-Bullough KB, Romney SJ, Kahle-Stephan M, Rathkolb B, Gailus-Durner V, Fuchs H, Wolf E, Rozman J, de Angelis MH, Cai WM, Rajan M, Hu J, Dedon PC, Leibold EA
Nature communications 2020 Jan 15;11(1):296
Nature communications 2020 Jan 15;11(1):296
Nucleocytoplasmic Proteomic Analysis Uncovers eRF1 and Nonsense-Mediated Decay as Modifiers of ALS/FTD C9orf72 Toxicity.
Ortega JA, Daley EL, Kour S, Samani M, Tellez L, Smith HS, Hall EA, Esengul YT, Tsai YH, Gendron TF, Donnelly CJ, Siddique T, Savas JN, Pandey UB, Kiskinis E
Neuron 2020 Apr 8;106(1):90-107.e13
Neuron 2020 Apr 8;106(1):90-107.e13
Fission Yeast Asc1 Stabilizes the Interaction between Eukaryotic Initiation Factor 3a and Rps0A/uS2 for Protein Synthesis.
Wang YT, Chien YC, Hsiao WY, Wang CC, Wang SW
Molecular and cellular biology 2019 Oct 1;39(19)
Molecular and cellular biology 2019 Oct 1;39(19)
Inactivation of Ppp1r15a minimises weight gain and insulin resistance during caloric excess in female mice.
Patel V, Bidault G, Chambers JE, Carobbio S, Everden AJT, Garcés C, Dalton LE, Gribble FM, Vidal-Puig A, Marciniak SJ
Scientific reports 2019 Feb 27;9(1):2903
Scientific reports 2019 Feb 27;9(1):2903
A Rapid Extraction Method for mammalian cell cultures, suitable for quantitative immunoblotting analysis of proteins, including phosphorylated GCN2 and eIF2α.
Silva RC, Castilho BA, Sattlegger E
MethodsX 2018;5:75-82
MethodsX 2018;5:75-82
Extracellular and ER-stored Ca(2+) contribute to BIRD-2-induced cell death in diffuse large B-cell lymphoma cells.
Bittremieux M, La Rovere RM, Schuermans M, Luyten T, Mikoshiba K, Vangheluwe P, Parys JB, Bultynck G
Cell death discovery 2018;4:101
Cell death discovery 2018;4:101
Infantile Cirrhosis, Growth Impairment, and Neurodevelopmental Anomalies Associated with Deficiency of PPP1R15B.
Mohammad S, Wolfe LA, Stöbe P, Biskup S, Wainwright MS, Melin-Aldana H, Malladi P, Muenke M, Gahl WA, Whitington PF
The Journal of pediatrics 2016 Dec;179:144-149.e2
The Journal of pediatrics 2016 Dec;179:144-149.e2
Perturbations in actin dynamics reconfigure protein complexes that modulate GCN2 activity and promote an eIF2 response.
Silva RC, Sattlegger E, Castilho BA
Journal of cell science 2016 Dec 15;129(24):4521-4533
Journal of cell science 2016 Dec 15;129(24):4521-4533
Ebola Virus Does Not Induce Stress Granule Formation during Infection and Sequesters Stress Granule Proteins within Viral Inclusions.
Nelson EV, Schmidt KM, Deflubé LR, Doğanay S, Banadyga L, Olejnik J, Hume AJ, Ryabchikova E, Ebihara H, Kedersha N, Ha T, Mühlberger E
Journal of virology 2016 Aug 15;90(16):7268-7284
Journal of virology 2016 Aug 15;90(16):7268-7284
Actin dynamics tune the integrated stress response by regulating eukaryotic initiation factor 2α dephosphorylation.
Chambers JE, Dalton LE, Clarke HJ, Malzer E, Dominicus CS, Patel V, Moorhead G, Ron D, Marciniak SJ
eLife 2015 Mar 16;4
eLife 2015 Mar 16;4
Generation and characterization of an analog-sensitive PERK allele.
Maas NL, Singh N, Diehl JA
Cancer biology & therapy 2014 Aug;15(8):1106-11
Cancer biology & therapy 2014 Aug;15(8):1106-11
Endoplasmic reticulum stress induced by aqueous extracts of cigarette smoke in 3T3 cells activates the unfolded-protein-response-dependent PERK pathway of cell survival.
Hengstermann A, Müller T
Free radical biology & medicine 2008 Mar 15;44(6):1097-107
Free radical biology & medicine 2008 Mar 15;44(6):1097-107
Enhanced antiglioma activity of chimeric HCMV/HSV-1 oncolytic viruses.
Shah AC, Parker JN, Gillespie GY, Lakeman FD, Meleth S, Markert JM, Cassady KA
Gene therapy 2007 Jul;14(13):1045-54
Gene therapy 2007 Jul;14(13):1045-54
VSV replication in neurons is inhibited by type I IFN at multiple stages of infection.
Trottier MD Jr, Palian BM, Reiss CS
Virology 2005 Mar 15;333(2):215-25
Virology 2005 Mar 15;333(2):215-25
Deficiency of ATP2C1, a Golgi ion pump, induces secretory pathway defects in endoplasmic reticulum (ER)-associated degradation and sensitivity to ER stress.
Ramos-Castañeda J, Park YN, Liu M, Hauser K, Rudolph H, Shull GE, Jonkman MF, Mori K, Ikeda S, Ogawa H, Arvan P
The Journal of biological chemistry 2005 Mar 11;280(10):9467-73
The Journal of biological chemistry 2005 Mar 11;280(10):9467-73
Human cytomegalovirus TRS1 and IRS1 gene products block the double-stranded-RNA-activated host protein shutoff response induced by herpes simplex virus type 1 infection.
Cassady KA
Journal of virology 2005 Jul;79(14):8707-15
Journal of virology 2005 Jul;79(14):8707-15
The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas.
Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR
Molecular and cellular biology 2002 Jun;22(11):3864-74
Molecular and cellular biology 2002 Jun;22(11):3864-74
The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas.
Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR
Molecular and cellular biology 2002 Jun;22(11):3864-74
Molecular and cellular biology 2002 Jun;22(11):3864-74
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
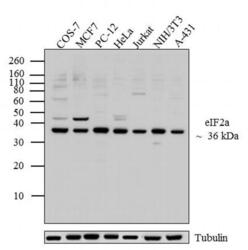
- Experimental details
- Western blot analysis was performed on whole cell extracts (20 µg lysate) of COS-7 (Lane 1), MCF7 (Lane 2), PC-12 (Lane 3), HeLa (lane 4), Jurkat (lane 5), NIH/3T3 (lane 6) and A-431 (lane 7). The blots were probed with Anti-eIF2a Mouse Monoclonal Antibody (Product # AHO0802, 1:500-1:1000 dilution) and detected by chemiluminescence using Goat anti-Mouse IgG (H+L) Secondary Antibody, HRP conjugate (Product # 62-6520, 1:4000 dilution). A 36 kDa band corresponding to eIF2a was observed across cell lines tested. Known quantity of protein samples were electrophoresed using Novex® NuPAGE® 12 % Bis-Tris gel (Product # NP0342BOX), XCell SureLock™ Electrophoresis System (Product # EI0002) and Novex® Sharp Pre-Stained Protein Standard (Product # LC5800). Resolved proteins were then transferred onto a nitrocellulose membrane with iBlot® 2 Dry Blotting System (Product # IB21001). The membrane was probed with the relevant primary and secondary Antibody following blocking with 5 % skimmed milk. Chemiluminescent detection was performed using Pierce™ ECL Western Blotting Substrate (Product # 32106).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
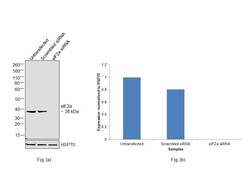
- Experimental details
- Knockdown of Eukaryotic translation initiation factor 2 subunit 1 was achieved by transfecting HeLa with Eukaryotic translation initiation factor 2 subunit 1 specific siRNAs (Silencer® select Product # S4556, S4555). Western Blot analysis (Fig. a) was performed using whole cell extracts from the Eukaryotic translation initiation factor 2 subunit 1 knockdown cells (lane 3), non-targeting scrambled siRNA transfected cells (lane 2) and untransfected cells (lane 1). The Blot was probed with eIF2a Monoclonal Antibody (EIF2-alpha) (Product # AHO0802, 1:1000 dilution) and Goat anti-Mouse IgG (H+L) Superclonal™ Recombinant Secondary Antibody, HRP (Product # A28177, 1:4000 dilution). Densitometric analysis of this western Blot is shown in histogram (Fig. b). Decrease in signal upon siRNA mediated knock down confirms that antibody is specific to Eukaryotic translation initiation factor 2 subunit 1.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
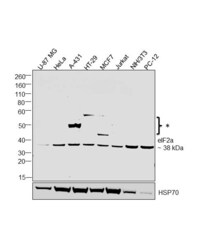
- Experimental details
- Western Blot was performed using Anti-eIF2a Monoclonal Antibody (EIF2-alpha) (Product # AHO0802) and a 38 kDa band corresponding to Eukaryotic translation initiation factor 2 subunit 1 was observed across tested cell lines along with uncharacterised bands (*) at ~50 and 60 kDa. Whole cell extracts (40 µg lysate) of U-87 MG (Lane 1), HeLa (Lane 2), A-431 (Lane 3), HT-29 (Lane 4), MCF7 (Lane 5), Jurkat (Lane 6), NIH/3T3 (Lane 7), PC-12 (Lane 8) were electrophoresed using NuPAGE™ 4-12% Bis-Tris Protein Gel (Product # NP0321BOX). Resolved proteins were then transferred onto a Nitrocellulose membrane (Product # LC2001) by iBlot® 2 Dry Blotting System (Product # IB21001). The Blot was probed with the primary antibody (1:1000 dilution) and detected by chemiluminescence with Goat anti-Mouse IgG (H+L) Superclonal™ Recombinant Secondary Antibody, HRP (Product # A28177, 1:4000 dilution) using the iBright FL 1000 (Product # A32752). Chemiluminescent detection was performed using SuperSignal™ West Dura Extended Duration Substrate (Product # 34076).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
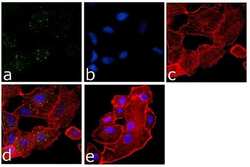
- Experimental details
- Immunofluorescence analysis of eIF2a was done on 70% confluent log phase A549 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with eIF2a Mouse Monoclonal Antibody at 1:250 dilution in1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing Cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
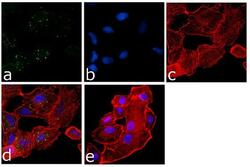
- Experimental details
- Immunofluorescence analysis of eIF2a was done on 70% confluent log phase A549 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with eIF2a Mouse Monoclonal Antibody at 1:250 dilution in1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing Cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
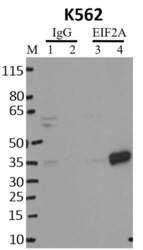
- Experimental details
- Immunoprecipitation of EIF2A was performed on K562 cells. Antigen-antibody complexes were formed by incubating approximately 500 µg whole cell lysate with 5 µg of monoclonal EIF2A antibody (Product # AHO0802( rotating 60 min at RT. The immune complexes were captured on 625 µg of anti-mouse coated Dynabeads (Product # 11202D), washed extensively, and eluted with NuPAGE™ LDS Sample Buffer (Product # NP0007). Samples were resolved onto NuPAGE™ 4-12% Bis-Tris gel (Product # NP0335BOX). Lanes 1 and 3 are input and lanes 2 and 4 are IP. Proteins were transferred to PVDF membrane (Product # IB23001). Membrane was blocked in 5% milk. Target was detected using a EIF2A monoclonal antibody (Product # AHO0802) at a dilution of 1:2000, followed by a 1:4000 dilution of secondary antibody. Chemiluminescent detection was performed using ECL Western Blotting Substrate (Product # 32106). Data courtesy of the Yeo lab as part of the ENCODE project (www.encodeproject.org).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
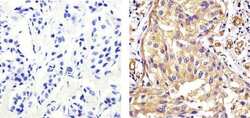
- Experimental details
- Immunohistochemistry analysis of EIF 2ALPHA (EIF2A) showing staining in the cytoplasm of paraffin-embedded human breast carcinoma tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a EIF 2ALPHA (EIF2A) Mouse Monoclonal Antibody (Product # AHO0802) diluted in 3% BSA-PBS at a dilution of 1:20 overnight at 4ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
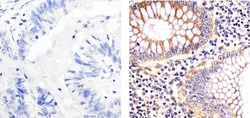
- Experimental details
- Immunohistochemistry analysis of EIF 2ALPHA (EIF2A) showing staining in the cytoplasm of paraffin-embedded human colon carcinoma (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a EIF 2ALPHA (EIF2A) Mouse Monoclonal Antibody (Product # AHO0802) diluted in 3% BSA-PBS at a dilution of 1:20 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
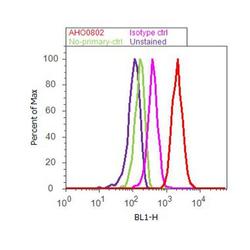
- Experimental details
- Flow cytometry analysis of EIF2-alpha was done on HeLa cells treated with Thapsigargin (50ng/mL, 20 minutes). Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with EIF2-alpha Mouse Monoclonal Antibody (AHO0802, red histogram) or with mouse isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Rabbit Anti-Mouse Secondary Antibody (A11059) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
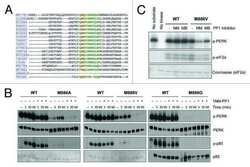
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
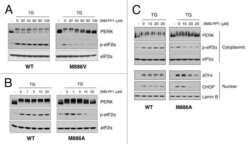
- Figure 5. Association of G-actin with PPP1R15 regulates eIF2alpha phosphatase activity. ( A ) Immunoblot for phosphorylated eIF2alpha (P-eIF2alpha), total eIF2alpha, and actin. Wild-type (WT) mouse embryonic fibroblasts (MEF) were treated with jasplakinolide 1 uM for the indicated times. Lysates were subjected to sedimentation assay and immunoblot for G-actin in the supernatant (G) or F-actin in the pellet (F). ( B ) Immunoblot for phosphorylated eIF2alpha (P-eIF2alpha), total eIF2alpha, and actin. WT MEFs were treated with the indicated concentrations of jasplakinolide for 1 hr. Lysates were analysed as in ' A '. ( C ) Immunoblot for phosphorylated eIF2alpha (P-eIF2alpha), total eIF2alpha, and PPP1R15A. WT MEFs were treated with thapsigargin 400 nM for the indicated times, without or with jasplakinolide 1 uM. ( D ) Immunoblot for P-eIF2alpha and PPP1R15A (hR15A). GFP-hPPP1R15A Tet-On HeLa cells were treated with doxycycline to induce transgene expression and then with thapsigargin 400 nM for the indicated times. Cells were co-treated with jasplakinolide or latrunculin B 1 uM or vehicle as indicated. Accompanying graphs show means +-SEM of n = 3 independent repeats. DOI: http://dx.doi.org/ Figure 5--figure supplement 1. Immunoblot for phosphorylated eIF2alpha (P-eIF2alpha), total eIF2alpha and PPP1R15A. WT MEFs were treated with thapsigargin 400 nM for the indicated times, without or with latrunculin B 1 uM. DOI: http://dx.doi.org/
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
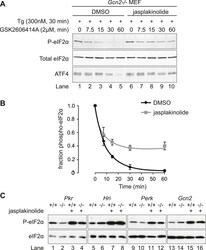
- Figure 6. Jasplakinolide diminishes eIF2alpha phosphatase activity in vivo. ( A ) Immunoblot for phosphorylated eIF2alpha (P-eIF2alpha), total eIF2alpha, and ATF4. Gcn2 -/- MEFs were pre-treated with thapsigargin 300 nM for 30 min to induce eIF2alpha phosphorylation and ATF4 protein levels. GSK2606414A at 2 uM was then added for the indicated times. Protein lysates were analysed by SDS-PAGE and subjected to immunoblot. ( B ) Quantification of ' A ' using ImageJ software. Mean +- SEM of n = 3 independent repeats. ( C ) Immunoblot for phosphorylated eIF2alpha (P-eIF2alpha) and total eIF2alpha. MEFs of the indicated genotypes were treated with or without jasplakinolide 1 uM for 1 hr. Protein lysates were analysed by SDS-PAGE and subjected to immunoblot. DOI: http://dx.doi.org/ Figure 6--figure supplement 1. Immunoblot for P-eIF2alpha, total eIF2alpha, and ATF4 (specific band marked with an asterisk) in lysates of wild type (WT) or eIF2alpha AA MEFs following treatment with thapsigargin 300 nM for 4 hr and/or jasplakinolide 1 uM for 4 hr. DOI: http://dx.doi.org/
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
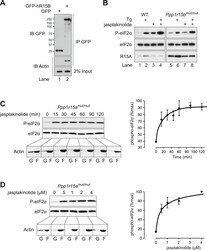
- Figure 7. Actin associates with PPP1R15B to alter the level of eIF2alpha phosphorylation. ( A ) Immunoblot for GFP and actin of HEK293T cell lysates expressing either GFP or GFP-PPP1R15B. Upper two panels indicate proteins immunoprecipitated by anti-GFP beads. Lower panel shows 2% of input lysate. ( B ) Immunoblot for P-eIF2alpha, total eIF2alpha, and PPP1R15A of lysates from WT or Ppp1r15b tm1Dron/tm1Dron MEFs treated for 1 hr with thapsigargin 400 nM, jasplakinolide 1 uM or both. ( C ) Immunoblot for phosphorylated eIF2alpha (P-eIF2alpha), total eIF2alpha and actin. Ppp1r15a tm1Dron/tm1Dron MEFs were treated with jasplakinolide 1 uM for the indicated times. Lysates were subjected to sedimentation assay and immunoblot for G-actin in the supernatant (G) or F-actin in the pellet (F). ( D ) Immunoblot for phosphorylated eIF2alpha (P-eIF2alpha), total eIF2alpha, and actin. Ppp1r15a tm1Dron/tm1Dron MEFs were treated with the indicated concentrations of jasplakinolide for 1 hr. Lysates were analysed as in ' C '. Accompanying graphs show mean +- SEM of n = 3 independent repeats. DOI: http://dx.doi.org/
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
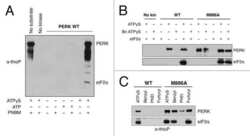
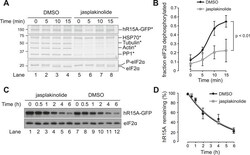
- Figure 8. Association of actin with PPP1R15A promotes eIF2alpha phosphatase activity in vitro. ( A ) Silver-stained SDS-PAGE or GFP-affinity purified PPP1R15A-GFP (hR15-GFP) and associated proteins. Asterisks signify identity confirmed by mass spectrometry. Purified complex incubated with phosphorylated recombinant eIF2alpha N-terminal lobe (eIF2alpha) for incubated time. Note size shift corresponds to dephosphorylation. ( B ) Quantification of ' A ' using ImageJ software. Mean +- SEM. p value calculated by two-way ANOVA, n = 3. ( C ) Immunoblot for PPP1R15A and eIF2alpha of HEK293T cells expressing hPPP1R15A-GFP (hR15A-GFP). Treated with cycloheximide 50 uM for indicated times. ( D ) Quantification of ' C '. Mean +- SEM, n = 3. DOI: http://dx.doi.org/
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
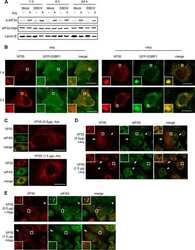
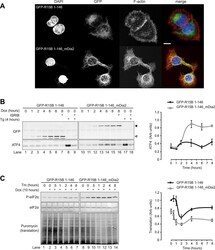
- Figure 9. Localised changes in the polymeric status of actin modulate the sensitivity of the ISR. ( A ) Fluorescence microscopy image of Flp-In T-REx HEK293 cells treated with 1 ug/ml doxycycline for 12 hr to express either ER membrane-localised GFP (GFP-R15B 1-146) or ER membrane-localised GFP-mDia2 fusion (GFP-R15B 1-146_mDia2) then fixed and stained with Alexa-Fluor 568 phalloidin and imaged by confocal microscopy. Bar = 5 um. ( B ) Immunoblot for GFP and ATF4 in lysates of GFP-R15B 1-146 or GFP-R15B 1-146_mDia2 Flp-In T-REx HEK293 cells following treatment with doxycycline (Dox) 0.1 ug/ml for indicated times or with ISRIB 100 nM and or thapsigargin 300 nM for 4 hr. Immunoreactivity to ATF4 was quantified using ImageJ software (ATF4 Intensity). Proteins of the expected sizes are marked with a solid triangle GFP-R15B 1-146_mDia2 or an open triangle GFP-R15B 1-146. ( C ) Immunoblot for P-eIF2alpha, total eIF2alpha, and puromycin in lysates of GFP-R15B 1-146 or GFP-R15B 1-146_mDia2 Flp-In T-REx HEK293 cells following pre-treatment--if indicated with doxycycline (Dox) 0.1 ug/ml for 10 hr followed by treatment with tunicamycin 2.5 ug/ml for indicated times. 10 min prior to harvesting, puromycin was added to the culture medium at a final concentration of 10 ug/ml. Immunoreactivity to puromycin within lysates served as a marker of protein translation and was quantified using ImageJ software (Puromycin intensity). Accompanying graphs of mean +- SEM of n = 3 independent repeats. DO
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
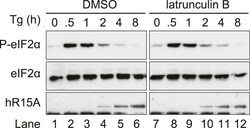
- Experimental details
- Figure 5--figure supplement 1. Immunoblot for phosphorylated eIF2alpha (P-eIF2alpha), total eIF2alpha and PPP1R15A. WT MEFs were treated with thapsigargin 400 nM for the indicated times, without or with latrunculin B 1 uM. DOI: http://dx.doi.org/
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
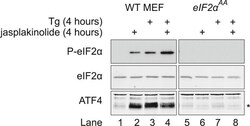
- Experimental details
- Figure 6--figure supplement 1. Immunoblot for P-eIF2alpha, total eIF2alpha, and ATF4 (specific band marked with an asterisk) in lysates of wild type (WT) or eIF2alpha AA MEFs following treatment with thapsigargin 300 nM for 4 hr and/or jasplakinolide 1 uM for 4 hr. DOI: http://dx.doi.org/
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
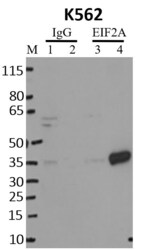
- Experimental details
- RNA immunoprecipitation (RIP) of EIF2A was performed on K562 cells. Antigen-antibody complexes were formed by incubating approximately 500 µg whole cell lysate with 5 µg of monoclonal EIF2A antibody (Product # AHO0802( rotating 60 min at RT. The immune complexes were captured on 625 µg of anti-mouse coated Dynabeads (Product # 11202D), washed extensively, and eluted with NuPAGE™ LDS Sample Buffer (Product # NP0007). Samples were resolved onto NuPAGE™ 4-12% Bis-Tris gel (Product # NP0335BOX). Lanes 1 and 3 are input and lanes 2 and 4 are IP. Proteins were transferred to PVDF membrane (Product # IB23001). Membrane was blocked in 5% milk. Target was detected using a EIF2A monoclonal antibody (Product # AHO0802) at a dilution of 1:2000, followed by a 1:4000 dilution of secondary antibody. Chemiluminescent detection was performed using ECL Western Blotting Substrate (Product # 32106). Data courtesy of the Yeo lab as part of the ENCODE project (www.encodeproject.org).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
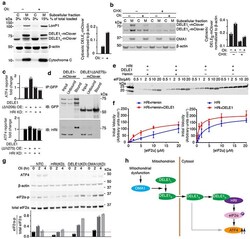
- Experimental details
- Fig. 4 Cytosolic DELE1 physically interacts with and activates HRI. (a) Immunoblot of biochemical fractionations of cells stably expressing DELE1-mClover treated with 1.25 ng/mL oligomycin (Oli) for 16 h where indicated. Left , representative immunoblot of the cytosolic (C) and mitochondrial (M) fractions. LonP1 and cytochrome c were probed as mitochondrial markers. Right , quantification of the cytosolic DELE1 S -mClover from n = 2 blots. (b) Accumulation of DELE1 S is protein-synthesis independent. Biochemical fractionation of cells stably expressing DELE1-mClover treated with 1.25 ng/mL oligomycin (Oli) and 20 mug/mL cycloheximide (CHX) for 4 h where indicated. Left , representative immunoblot of the cytosolic (C) and mitochondrial (M) fractions. OMA1 as probed as mitochondrial marker. Right , quantification of the cytosolic DELE1 S -mClover from n = 2 blots. (c) Transient overexpression (OE) of the cytosolically localized DELE1(DeltaN206), but not the DELE1(DeltaN275) construct induces the ATF4 reporter. Knockdown (KD) of HRI blocks reporter activation (mean +- s.d., n = 3 culture wells). (d) Co-immunoprecipitation of HRI with transiently expressed full-length DELE1-mClover but not with DELE1(DeltaN275)-mClover. Similar results in n = 2 independent experiments. (e,f) HRI enzyme kinetics in the presence or absence of DELE1 with or without 5 muM hemin using different concentrations of the substrate eIF2alpha. (e) Representative immunoblot. (f) Quantification and fitting to
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
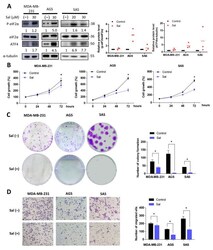
- Experimental details
- Figure 1 Salubrinal inhibited cell growth and migration in cancer cells. Three cancer cell lines (MDA-MB-231, AGS, and SAS) were treated with or without 30 muM of salubrinal in normal culture medium. ( A ) The protein levels of eIF2alpha, p-eIF2alpha, and ATF4 were detected using Western blotting after salubrinal treatment for 24 h; The value of the control group was set as 1. ( B ) The cell growth rate at 0, 24, 48, and 72 h after salubrinal treatment was detected by a sulforhodamine B (SRB) assay. ( C ) The colony formation ability was checked after treatment with or without salubrinal for 10 days in MDA-MB-231 cells, for 7 days in AGS cells, and for 9 days in SAS cells. ( D ) The ability of cell migration was detected using a transwell migration assay. After salubrinal pre-treatment for 24 h, the migration time was found to be 6 h for MDA-MB-231 cells, 8 h for AGS cells, and 24 h for SAS cells. The photograph was taken by a microscope (100x magnification). The data are presented as the means +-SEMs of the results from three independent experiments. * p < 0.05. Sal: salubrinal.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
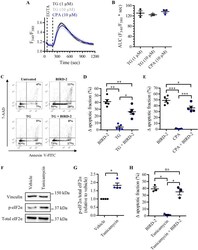
- Experimental details
- Fig. 7 BIRD-2-triggered apoptosis is reduced by depleting the ER Ca 2+ store in SU-DHL-4 cells. a Analysis of the cytosolic Ca 2+ response in SU-DHL-4 cells using Fura-2 AM. After the addition of 3 mM EGTA, 1 or 10 uM TG or 10 uM CPA were added to deplete the ER Ca 2+ store. The curves represent the mean +- SEM of three independent experiments. The TG/CPA-releasable Ca 2+ is quantified by measuring the AUC ( F 340 / F 380 x s), which is shown in b . c Representative scatter plots from flow cytometry analysis detecting apoptosis in SU-DHL-4 cells stained with Annexin V-FITC and 7-AAD. Cells were pre-treated with or without 1 uM TG 30 min prior to application of 10 uM BIRD-2. After 2 h of BIRD-2 treatment, apoptotic cell death was detected by measuring the Annexin V-FITC-positive fraction. d Quantitative analysis of five independent experiments detecting apoptosis in SU-DHL-4 cells treated for 2 h with 10 uM BIRD-2, with or without a pre-treatment of 30 min with 1 uM TG. e Quantitative analysis of five independent experiments detecting apoptosis in SU-DHL-4 cells treated for 2 h with 10 uM BIRD-2, with or without a pre-treatment of 30 min with 10 uM CPA. f A representative western blot of four independent experiments showing the expression levels of total eIF2alpha and p-eIF2alpha in SU-DHL-4 cells treated for 4 h with 5ug/ml tunicamycin. The expression level of vinculin was used as a loading control. g Quantification of the p-eIF2alpha/total eIF2alpha-protein
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
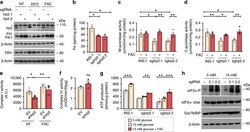
- Experimental details
- Fig. 7 Irp2 deficiency impairs Fe-S cluster protein function and causes ER stress. a CRISPR/Cas9 depletion of Irp2 in INS-1 832/13 cells (sgIrp2.1, sgIrp2.2) and control parental cells grown in the presence of FAC (10 µg/mL), DFO (50 µM), or no treatment (NT) for 18 h. Irp2, Fth1, Ftl1 and TfR1 were assessed by western blot analysis to show efficacy of Irp2 knockdown and appropriate iron regulation. beta-Actin is a loading control. Asterisk, nonspecific band. b The total iron content measured by ICP-MS and normalized to total cellular protein ( n = 3 independent biological experiments). c , d Mitochondrial ( c ) and cytosolic ( d ) aconitase activity in control, sgIrp2.1, and sgIrp2.2 INS-1 cells grown in medium with or without supplemental FAC and normalized to total cellular protein. e , f Complex I activity ( e ) and complex IV activity ( f ) in lysates from control EV and shIrp2 cells grown in medium with or without supplemental FAC and normalized to total cellular protein ( n >= 3 independent biological experiments). g ATP production in control INS-1, sgIrp2.1, and sgIrp2.2 cells grown in medium with or without supplemental FAC and assayed under basal (5 mM) glucose and after stimulation with 15 mM glucose for 1 h. ATP production was normalized to total cellular protein ( n = 5 independent biological experiments). h Western blot analysis of eIF2alpha-P, eIF2alpha-total, and Grp78/BiP levels in sgIrp2.1 and sgIrp2.2 cells under basal glucose (5
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
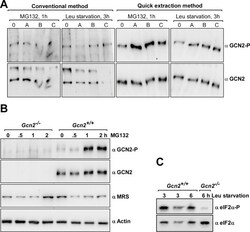
- Experimental details
- Fig. 2 Western blot results using samples generated via the Rapid Protein Extraction (RPE) Method. A) MEFs were grown in cell culture flasks to
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
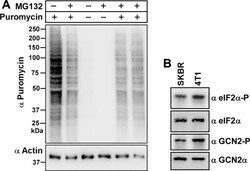
- Experimental details
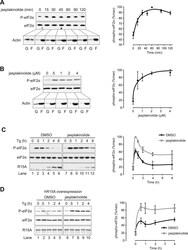
- Fig. 3 Further testing of the Rapid Protein Extraction (RPE) Method. A) Gcn2 +/+ MEF cells were grown in medium with or without 2 muM MG132 for 1 h, and then incubated in medium containing puromycin for 10 min prior to harvesting. 10 mul of samples were subjected to immunoblotting with anti-puromycin antibodies (1:5000, Millipore-MABE343, clone 12D10) [ 7 ] and, after stripping [ 10 ], with anti-actin antibodies, as described in [ 5 ]. Molecular markers are depicted in kDa on the left side of the image. B) 5 x 10 5 human (SKBR3) or mouse (4T1) breast cancer cell lines were each grown overnight in 6 cm plastic dishes containing 4 ml RPMI 1640 medium (Gibco Thermo Fisher Scientific, # 72400-047), supplemented with FBS, sodium pyruvate and antibiotics as above. After subjecting cells to the RPE method, 20 mul and 5 mul of the cell extract were resolved via SDS-PAGE in 6% (for detecting GCN2 and Gcn2-P, 20 mul equate to ~50 mug of total protein loaded) and 10% (eIF2alpha and eIF2alpha-P, 5 mul equate to ~12.5 mug of total protein loaded) denaturing Tris acrylamide gels, followed by immunoblotting to detect the indicated proteins. Working solutions containing primary antibodies were diluted in standard Tris buffered saline containing 5% (w/v) skim milk powder, or 3% (w/v) BSA (for eIF2alpha-P antibodies, BSA fraction-V IgG-free, Gibco Thermo Fisher Scientific, # 30063721). Primary antibodies were detected with horseradish peroxidase (HRP) conjugated to goat anti-rabbit antibodie
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot