Antibody data
- Antibody Data
- Antigen structure
- References [4]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [4]
- Other assay [2]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-21049 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- MFSD2A Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- A suggested positive control is rat lung tissue lysate. PA5-21049 can be used with blocking peptide PEP-1163.
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- 4°C
Submitted references Selective sphingosine-1-phosphate receptor 1 modulator attenuates blood-brain barrier disruption following traumatic brain injury by inhibiting vesicular transcytosis.
Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain.
MiR-155 deletion reduces ischemia-induced paralysis in an aortic aneurysm repair mouse model: Utility of immunohistochemistry and histopathology in understanding etiology of spinal cord paralysis.
Mfsd2a and Glut1 Brain Nutrient Transporters Expression Increase with 32-Week Low and High Lard Compared with Fish-Oil Dietary Treatment in C57Bl/6 Mice.
Zhang Y, Wang L, Pan Q, Yang X, Cao Y, Yan J, Wang Y, Tao Y, Fan R, Sun X, Li L
Fluids and barriers of the CNS 2022 Jul 11;19(1):57
Fluids and barriers of the CNS 2022 Jul 11;19(1):57
Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain.
Zhao L, Li Z, Vong JSL, Chen X, Lai HM, Yan LYC, Huang J, Sy SKH, Tian X, Huang Y, Chan HYE, So HC, Ng WL, Tang Y, Lin WJ, Mok VCT, Ko H
Nature communications 2020 Sep 4;11(1):4413
Nature communications 2020 Sep 4;11(1):4413
MiR-155 deletion reduces ischemia-induced paralysis in an aortic aneurysm repair mouse model: Utility of immunohistochemistry and histopathology in understanding etiology of spinal cord paralysis.
Awad H, Bratasz A, Nuovo G, Burry R, Meng X, Kelani H, Brown M, Ramadan ME, Williams J, Bouhliqah L, Popovich PG, Guan Z, Mcallister C, Corcoran SE, Kaspar B, Michele Basso D, Otero JJ, Kirsch C, Davis IC, Croce CM, Michaille JJ, Tili E
Annals of diagnostic pathology 2018 Oct;36:12-20
Annals of diagnostic pathology 2018 Oct;36:12-20
Mfsd2a and Glut1 Brain Nutrient Transporters Expression Increase with 32-Week Low and High Lard Compared with Fish-Oil Dietary Treatment in C57Bl/6 Mice.
Sandoval KE, Wooten JS, Harris MP, Schaller ML, Umbaugh DS, Witt KA
Current developments in nutrition 2018 Oct;2(10):nzy065
Current developments in nutrition 2018 Oct;2(10):nzy065
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
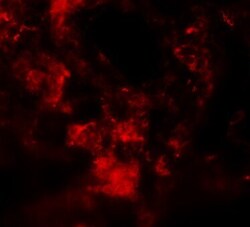
- Experimental details
- Immunofluorescence of MFSD2A in Rat Lung cells with MFSD2A Polyclonal Antibody (Product # PA5-21049) at 20 µg/mL.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
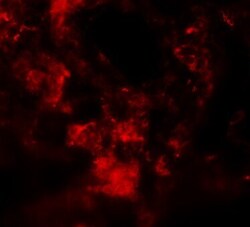
- Experimental details
- Immunofluorescence of MFSD2A in Rat Lung cells with MFSD2A Polyclonal Antibody (Product # PA5-21049) at 20 µg/mL.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
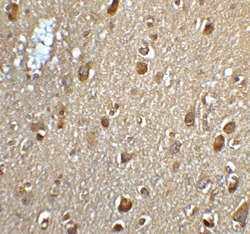
- Experimental details
- Immunohistochemistry of MFSD2A in human brain tissue with MFSD2A Polyclonal Antibody (Product # PA5-21049) at 5 µg/mL.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
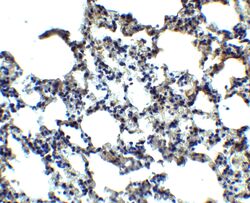
- Experimental details
- Immunohistochemistry of MFSD2A in mouse lung tissue with MFSD2A Polyclonal Antibody (Product # PA5-21049) at 2.5 µg/mL.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
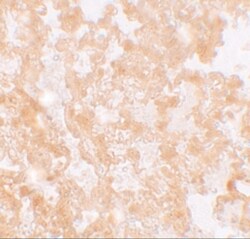
- Experimental details
- Immunohistochemistry of MFSD2A in rat lung tissue with MFSD2A Polyclonal Antibody (Product # PA5-21049) at 5 µg/mL.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
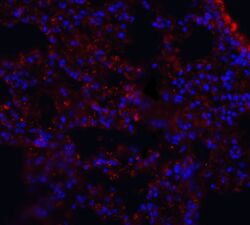
- Experimental details
- Immunofluorescence of MFSD2A in mouse lung tissue with MFSD2A Polyclonal Antibody (Product # PA5-21049) at 20 µg/mL. Red: MFSD2A Blue: DAPI staining
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
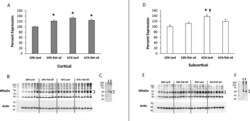
- Experimental details
- FIGURE 2 Western blot analysis bar charts show differences in Mfsd2a (~50 kDa) protein expression in cortical (A) and subcortical (D) brain tissue at the end of the 32-wk diet treatment. Representative blots of cortical (B) and subcortical (E) brain tissue with actin reprobes are shown. Deglycosylation evaluation blots of cortical (C) and subcortical (F) brain tissue show nondeglycosylated control (1) and deglycosylated (2) samples (41% lard). Values are means +- SEMs, n = 11-12/group. Two-factor ANOVA with Tukey-Kramer post hoc test was conducted. *Different from 10% lard, P < 0.05; + Different from 10% fish oil, P < 0.05. Mfsd2a, major facilitator super family domain containing 2a.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
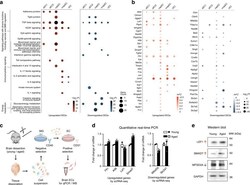
- Experimental details
- Fig. 2 Pathway analysis and differential expression validation. a Dot plots of important signaling pathways whose functions include vascular and BBB regulation, immune/cytokine signaling, respiratory electron transport chain, and glucose/energy metabolism pathways with significant enrichment in the aged brain for upregulated (left panel) and downregulated (right panel) EC DEGs. b Dot plots showing the differential expression profile of selected upregulated (left panel) and downregulated (right panel) genes in aged brain ECs associated with enriched pathways shown in a in the different EC subtypes. c Schematics of validation experiments by quantitative PCR and western blot for selected DEGs found by scRNA-seq (including Flt1 , Klf6 , Lef1 , Smad7 , Mfsd2a , and Slc2a1 ), in whole-brain ECs isolated by immunopanning. d Expression changes of selected genes in immunopanned ECs by quantitative PCR (for each gene, quantified as fold change relative to young adult group mean, error bars represent S.E.M. ; Flt1 : P = 0.036; Klf6 : P = 0.011; Lef1 : P = 0.28; Smad7 : P = 0.012; Mfsd2a : P = 0.022; Slc2a1 : P = 0.73; asterisks in the plot indicates P < 0.05 for easy visualization, two-sided unpaired t -test without adjustment for multiple comparisons; for each group, samples from n = 3 mice were pooled and measured in duplicate, resulting in two measurement values for each gene). e Western blot assays of the encoded proteins of a subset of aged brain EC-upregulated (LEF1, SMAD7) and do
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry