Antibody data
- Antibody Data
- Antigen structure
- References [5]
- Comments [0]
- Validations
- Immunocytochemistry [3]
- Immunohistochemistry [1]
- Flow cytometry [2]
- Other assay [4]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-9865-37 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Phospho-Histone H2A.X (Ser139) Monoclonal Antibody (CR55T33), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The CR55T33 monoclonal antibody recognizes phosphorylated serine 139 of human and mouse H2AX. H2AX is a member of the H2A histone family that complex with DNA and other histones to form the repeating nucleosome units characteristic of eukaryotic chromatin. Nucleosomes consist of approximately 147 base pairs of DNA wrapped around an octamer of histones composed of two each of the four histone proteins: H2A, H2B, H3 and H4. After induction of DNA damage such as double-strand breaks by irradiation, genotoxic stresses, replication errors or gene recombination, PI3K-like kinases (e.g., ataxia telangiectasia mutated (ATM), ataxia telangiectasia Rad-3-related (ATR), and DNA-dependent protein kinase (DNA-PK) are activated to phosphorylate serine 139 in H2AX. This early phosphorylation event plays a critical role in recruiting proteins involved in DNA repair. The monoclonal antibody CR55T33 recognizes a single band of approximately 15 kDa on reduced cell lysates from Jurkat cells stimulated with etoposide. Applications Reported: This CR55T33 antibody has been reported for use in intracellular staining followed by flow cytometric analysis, western blotting, immunohistochemical staining of formalin-fixed paraffin embedded tissue sections, and immunocytochemistry (fluorochrome-conjugated CR55T33 is recommended for use in intracellular flow cytometry). Applications Tested: The CR55T33 has been tested by immunocytochemistry of methanol-fixed cells and by immunohistochemistry of human tissue using either low or high pH antigen retrieval and can be used at 5 µg/mL. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. This CR55T33 antibody has also been tested by intracellular staining and flow cytometric analysis of stimulated Jurkat cells using the Foxp3/Transcription Factor Buffer Set (Product # 00-5523-00) and protocol. This can be used at 0.03 µg per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. Protocols: We recommend Protocol B: One-step protocol: intracellular (nuclear) proteins. Alternatively, Protocol C: Two-step protocol: Fixation/Methanol can also be used. Protocol A: Two-step protocol: intracellular (cytoplasmic) proteins cannot be used. All Protocols can be found in the "Staining intracellular Antigens for Flow Cytometry Protocol" located in the BestProtocols® Section under the Resources tab online. eFluor® 660 is a replacement for Alexa Fluor® 647. eFluor® 660 emits at 659 nm and is excited with the red laser (633 nm). Please make sure that your instrument is capable of detecting this fluorochrome. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human, Mouse
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- CR55T33
- Vial size
- 2 mg
- Concentration
- 0.5 mg/mL
- Storage
- 4°C
Submitted references The mitotic checkpoint is a targetable vulnerability of carboplatin-resistant triple negative breast cancers.
MAU2 and NIPBL Variants Impair the Heterodimerization of the Cohesin Loader Subunits and Cause Cornelia de Lange Syndrome.
Synergistic lethality between PARP-trapping and alantolactone-induced oxidative DNA damage in homologous recombination-proficient cancer cells.
Evaluation of cisplatin-induced injury in human kidney organoids.
ATR maintains chromosomal integrity during postnatal cerebellar neurogenesis and is required for medulloblastoma formation.
Moens S, Zhao P, Baietti MF, Marinelli O, Van Haver D, Impens F, Floris G, Marangoni E, Neven P, Annibali D, Sablina AA, Amant F
Scientific reports 2021 Feb 4;11(1):3176
Scientific reports 2021 Feb 4;11(1):3176
MAU2 and NIPBL Variants Impair the Heterodimerization of the Cohesin Loader Subunits and Cause Cornelia de Lange Syndrome.
Parenti I, Diab F, Gil SR, Mulugeta E, Casa V, Berutti R, Brouwer RWW, Dupé V, Eckhold J, Graf E, Puisac B, Ramos F, Schwarzmayr T, Gines MM, van Staveren T, van IJcken WFJ, Strom TM, Pié J, Watrin E, Kaiser FJ, Wendt KS
Cell reports 2020 May 19;31(7):107647
Cell reports 2020 May 19;31(7):107647
Synergistic lethality between PARP-trapping and alantolactone-induced oxidative DNA damage in homologous recombination-proficient cancer cells.
Wang H, Zhang S, Song L, Qu M, Zou Z
Oncogene 2020 Apr;39(14):2905-2920
Oncogene 2020 Apr;39(14):2905-2920
Evaluation of cisplatin-induced injury in human kidney organoids.
Digby JLM, Vanichapol T, Przepiorski A, Davidson AJ, Sander V
American journal of physiology. Renal physiology 2020 Apr 1;318(4):F971-F978
American journal of physiology. Renal physiology 2020 Apr 1;318(4):F971-F978
ATR maintains chromosomal integrity during postnatal cerebellar neurogenesis and is required for medulloblastoma formation.
Lang PY, Nanjangud GJ, Sokolsky-Papkov M, Shaw C, Hwang D, Parker JS, Kabanov AV, Gershon TR
Development (Cambridge, England) 2016 Nov 1;143(21):4038-4052
Development (Cambridge, England) 2016 Nov 1;143(21):4038-4052
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
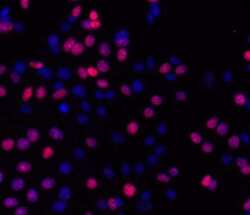
- Experimental details
- Immunocytochemistry of fixed and permeabilized MCF7 cells treated for 30 minutes with 10 uM camptothecin and stained with 5 µg/mL of Anti-Human/Mouse phospho-H2AX (S139) Purified followed by 5 µg/mL of F (ab')2 Anti-Mouse IgG eFluor® 570.Nuclei are stained with DAPI.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
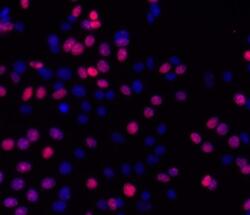
- Experimental details
- Immunocytochemistry of fixed and permeabilized MCF7 cells treated for 30 minutes with 10 uM camptothecin and stained with 5 µg/mL of Anti-Human/Mouse phospho-H2AX (S139) Purified followed by 5 µg/mL of F (ab')2 Anti-Mouse IgG eFluor® 570.Nuclei are stained with DAPI.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
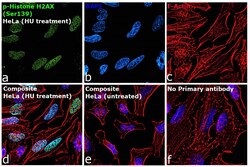
- Experimental details
- Immunofluorescence analysis of phospho-H2A.X was performed using 70% confluent log phase HeLa cells treated with hydroxyurea (3 mM for 6 hr). The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with Phospho-Histone H2A.X (Ser139) Monoclonal Antibody (CR55T33), eBioscience™ (Product # 14-9865-82, 5 µg/mL) in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Mouse IgG (H+L), Superclonal™ Recombinant Secondary Antibody, Alexa Fluor™ 488 (Product # A28175), (1:2500), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing nuclear localization. Panel e represents untreated HeLa cells. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
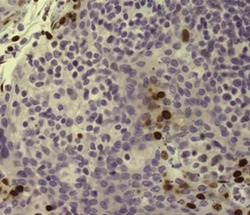
- Experimental details
- Immunohistochemistry of formalin-fixed paraffin embedded human tonsil stained with 5 µg/mL of Anti-Human/Mouse phospho-H2AX (S139) Purified followed by Anti-Mouse IgG Biotin, Streptavidin HRP, and DAB visualization.Nuclei are counterstained with hematoxylin.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
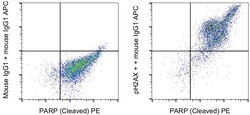
- Experimental details
- Jurkat cells were stimulated for 4 hours with staurosporine and then stained intracellularly, using the Foxp3/Transcription Factor Staining Buffer Set (Product # 00-5523-00) and protocol, with PARP1 (Cleaved) Monoclonal Antibody, PE (Product # 12-6668-42) and 0.015 µg Mouse IgG1 kappa Isotype Control (Product # 14-4714-85) (left) or 0.015 µg phospho-H2AX (S139) Monoclonal Antibody (right) followed by IgG1 Monoclonal Antibody, APC (Product # 17-4015-82). Total cells were used for analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
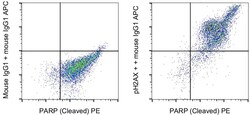
- Experimental details
- Jurkat cells were stimulated for 4 hours with staurosporine and then stained intracellularly, using the Foxp3/Transcription Factor Staining Buffer Set (Product # 00-5523-00) and protocol, with PARP1 (Cleaved) Monoclonal Antibody, PE (Product # 12-6668-42) and 0.015 µg Mouse IgG1 kappa Isotype Control (Product # 14-4714-85) (left) or 0.015 µg phospho-H2AX (S139) Monoclonal Antibody (right) followed by IgG1 Monoclonal Antibody, APC (Product # 17-4015-82). Total cells were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
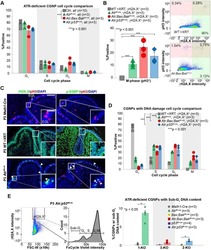
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
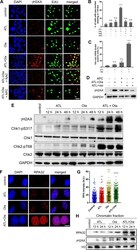
- Experimental details
- Fig. 4 ATL synergizes with olaparib to induce intense replication stress in cancer cells. a Immunofluorescent staining of gammaH2AX and EdU. PC-3 cells were treated by 10 muM ATL, 10 muM olaparib (Ola) or the combination of the two, with or without 10 mM NAC or 5 muM aphidicolin (APC), for 12 h. At the end of drug treatment, cells were pulse-labeled with 10 uM EdU for 20 min (scale bar: 20 mum). b , c gammaH2AX and EdU positive cells were measured using the ImageJ software and the data were processed by the Prism software. d Western blot detection of gammaH2AX. PC-3 cells were treated by the combination of 10 muM ATL and 10 muM Ola or 10 muM ATL and 10 muM veliparib (Vel), with or without 5 muM aphidicolin (APC), for 24 h. e Western blot analysis of the indicated proteins. PC-3 cells were treated by 10 muM ATL, 10 muM olaparib (Ola) or the combination of the two for the indicated times. f Immunofluorescent staining of RPA32 foci. PC-3 cells were treated by 10 muM ATL, 10 muM olaparib (Ola) or the combination of the two for 12 h (scale bar: 10 mum). g Nuclear RPA32 intensity was measured using the ImageJ software and the data were processed by the Prism software. h Western blot detection of chromatin bound RPA32 and gammaH2AX. PC-3 cells were treated by 10 muM ATL, 10 muM olaparib (Ola) or the combination of the two for the indicated times. n.s. not significant, ** p < 0.01, **** p < 0.0001 vs. vehicle control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
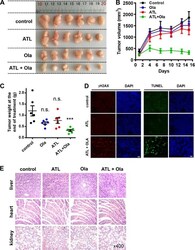
- Fig. 7 Coadministration of ATL and olaparib induces regression of tumor xenografts. PC-3 cells (2 x 10 6 ) in 1:1 matrigel were inoculated subcutaneously into the left flanks of male athymic BALB/c nude mice. When the tumor volume reached approximately 150 mm 3 (15 days after inoculation), mice were treated once daily with 50 mg/kg ATL oral gavage or 50 mg/kg olaparib intraperitoneal injection or both for 15 days. a Photograph of tumors dissected out from each mouse at the time of study termination. b Tumor volumes measured on the indicated days of treatment. Results were shown as mean +- SD. c Tumor weight measured at the end of the study. d Representative images of immunohistochemical staining of gammaH2AX and TUNEL in tumor tissues (scale bar: 20 mum). e Hematoxylin-eosin staining of liver, heart, and kidney tissue sections (magnification: x400). n.s. not significant, *** p < 0.001 vs. vehicle control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Proteome re-wiring of carboplatin-resistant 468-R cells protects from carboplatin-induced oxidative and genotoxic stress. ( A,B ) Hierarchical clustering of differentially expressed proteins (DE) ( A ) and Ingenuity Pathway Analysis ( B ) of the proteome alterations in MDA-MB-468 and 468-R cells treated with vehicle or 2 uM carboplatin for 5 days. Protein expression was analyzed by LC/MS-MS, and DE proteins were identified by ANOVA followed by Tukey's HSD test with an adjusted P value < 0.05. The top 10 significantly enriched pathways are shown. P value < 0.05 was used to determine significantly enriched pathways; the -log10 ( P value) was not shown if the pathway was not significantly enriched. N = 3. ( C,D ) ROS levels in MDA-MB-468 and 468-R cells treated with vehicle or 2 uM carboplatin for 5 days. Total ROS levels ( C ) were measured by the cellROX assay, and mitochondrial specific superoxide levels ( D ) were measured by the mitoSOX assay. Median flow of cytometric intensities were normalized to MDA-MB-468 vehicle condition. Data are shown as Mean +- SEM. P values were calculated by two-way ANOVA with Geisser-Greenhouse and Tukey's correction. N = 3. ( E ) NAD/NADH ratio as measured by NAD/NADH-Glo kit. Data are shown as Mean +- SEM. P values were calculated by two-way ANOVA with Geisser-Greenhouse and Tukey's correction. N = 3. ( F ) gamma-H2AX staining of MDA-MB-468 and 468-R cells treated with vehicle or 2 uM carboplatin for 5 days as analyzed by flow cytometry. P va
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry