Antibody data
- Antibody Data
- Antigen structure
- References [1]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [1]
- Other assay [4]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-29696 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- AKT3 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Recombinant full-length protein
- Description
- Recommended positive controls: Raji, mouse brain, AKT3-transfected 293T cells. Predicted reactivity: Mouse (100%), Rat (99%), Pig (100%), Rhesus Monkey (100%), Chimpanzee (100%), Bovine (100%). Store product as a concentrated solution. Centrifuge briefly prior to opening the vial.
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- 2.15 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references AKT3 deficiency in M2 macrophages impairs cutaneous wound healing by disrupting tissue remodeling.
Gu S, Dai H, Zhao X, Gui C, Gui J
Aging 2020 Apr 14;12(8):6928-6946
Aging 2020 Apr 14;12(8):6928-6946
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
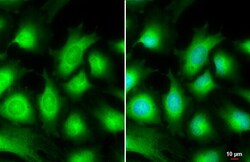
- Experimental details
- Immunocytochemistry-Immunofluorescence analysis of AKT3 was performed in HeLa cells fixed in 4% paraformaldehyde at RT for 15 min. Green: AKT3 Polyclonal Antibody (Product # PA5-29696) diluted at 1:500. Blue: Hoechst 33342 staining. Scale bar = 10 µm.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
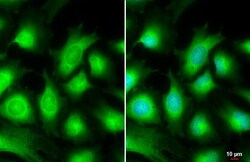
- Experimental details
- Immunocytochemistry-Immunofluorescence analysis of AKT3 was performed in HeLa cells fixed in 4% paraformaldehyde at RT for 15 min. Green: AKT3 Polyclonal Antibody (Product # PA5-29696) diluted at 1:500. Blue: Hoechst 33342 staining. Scale bar = 10 µm.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
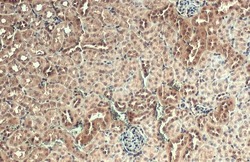
- Experimental details
- AKT3 Polyclonal Antibody detects AKT3 protein at nucleus by immunohistochemical analysis. Sample: Paraffin-embedded mouse kidney. AKT3 stained by AKT3 Polyclonal Antibody (Product # PA5-29696) diluted at 1:500. Antigen Retrieval: Citrate buffer, pH 6.0, 15 min.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
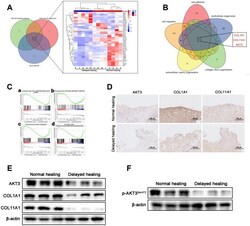
- Experimental details
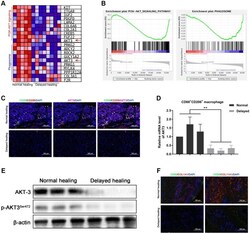
- Figure 3 Downregulation of AKT3, COL1A1, and COL11A1 in delayed cutaneous wound tissue. ( A ) Venn diagram of the KEGG pathway. ( a ) Venn analysis identified 35 genes that were enriched in PI3K-AKT signaling, ECM-receptor interactions, and focal adhesion. ( b ) The heatmap expression profile of the 35 changed genes. ( B ) Venn diagram of GO analysis for the tissue remodeling-associated biological functions. AKT3, COL1A1, and COL11A1 were enriched. ( C a - d ) Gene set enrichment analysis (GSEA) of cutaneous wound tissue. The genes associated with ( a ) cell adhesion molecules, ( b ) collagen metabolic processes, ( c ) focal adhesion, and ( d ) extracellular structural organization were negatively enriched in the delayed cutaneous wound tissue. ( D ) IHC staining of AKT3, COL1A1, and COL11A1 in cutaneous wound tissue (200 x). The levels of all three proteins were reduced in the delayed wound tissue. ( E ) Decreased AKT3, COL1A1, and COL11A1 protein levels in delayed cutaneous wound tissue. ( F ) Total AKT3 and phosphorylated-Ser472 AKT3 levels were decreased in delayed cutaneous wound tissue. All the experiments were repeated at least three times.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 4 Loss of AKT3 in M2 macrophages inhibited extracellular COL1A1 and COL11A1 expression. ( A ) GSEA showed that negatively enriched genes were associated with PI3K-AKT signaling and phagosomes in delayed cutaneous wound tissue. ( B ) Heatmap of the top 10 genes related to PI3K-AKT signaling and phagosomes; AKT3 was downregulated in both functional enrichment sets in the delayed cutaneous wound tissue. ( C ) Immunofluorescence of cutaneous wound tissue (n = 6). CD68- (green) and CD206-(red) positive M2 macrophages were reduced in the delayed cutaneous wound tissue. AKT3 (pink) was decreased in the M2 macrophages. ( D ) qRT-PCR showed decreased AKT3 mRNA expression in the delayed cutaneous wound tissue-derived M2 macrophages. ( E ) Western blotting verified the reduction and loss of AKT3 in M2 macrophages from delayed cutaneous wound tissue. ( F ) Immunofluorescence of COL1A1 and COL11A1 in CD68-positive macrophages in cutaneous wound tissue. ( a ) Decreased CD68-positive macrophage infiltration and COL1A1 protein expression were observed in delayed cutaneous wound tissue. ( b ) Decreased COL11A1 protein expression also accompanied the reduced CD68-positive macrophage infiltration. All the experiments were repeated at least three times.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
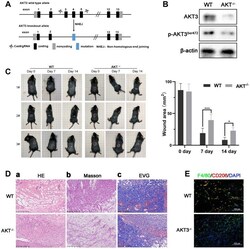
- Figure 6 Loss of AKT3 delayed cutaneous wound healing in mice. ( A ) Schematic of AKT3 knockout in mice. ( B ) Western blotting for AKT3 levels in AKT3 +/+ and AKT3 -/- mice (n = 6). ( C ) AKT3 knockout delayed cutaneous wound healing in mice by days 7 and 14 post-injury. ( D a - c ) Histological staining of mouse cutaneous wound tissue. ( a ) H&E staining showed more inflammatory cells in the wound tissue of AKT3 -/- mice and incomplete tissue integrity (n = 6). ( b ) Masson staining showed the numbers of collagenous and muscular fibers were reduced in the wound tissue of AKT3 -/- mice (n = 6). ( c ) EVG staining showed that number of elastin fibers were decreased in the wound tissue of AKT3 -/- mice (n = 6). ( E ) IF staining showed the F40/80 and CD206 expression in mouse cutaneous wound tissue. All the experiments were repeated at least three times.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
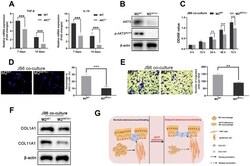
- Figure 7 M2 macrophages from AKT3 -/- mice failed to promote cell proliferation and migration ex vivo. ( A ) TGF-beta and IL-10 mRNA levels were decreased in delayed cutaneous wound tissue 7 th and 14 th day post-injury in mice (n = 3). ( B ) Western blotting demonstrated the loss of AKT3 in M2 macrophages from AKT3 -/- mice. ( C , D ) CCK-8 and EdU assays demonstrated that M2 macrophages from AKT3 -/- mice were incapable of promoting JB6 cell proliferation ( C ) or DNA replication ( D ), respectively. ( E ) Transwell migration assay showed M2 macrophages from AKT3 -/- mice could not promote JB6 cell migration. ( F ) COL1A1 and COL11A1 protein levels in JB6 were not increased by co-culture with M2 macrophages from AKT3 -/- mice. ( G ) The schematic illustration of the role of M2 macrophage AKT3 deficiency in delayed cutaneous wound healing. All the experiments were repeated at least three times.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry