MA1-26528
antibody from Invitrogen Antibodies
Targeting: ABCB1
ABC20, CD243, CLCS, GP170, MDR1, P-gp, PGY1
Antibody data
- Antibody Data
- Antigen structure
- References [26]
- Comments [0]
- Validations
- Other assay [10]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA1-26528 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- P-Glycoprotein Monoclonal Antibody (C219)
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Recommended positive controls: Cell lines or frozen tissue, such as liver or colon. MA1-26528 is expected to cross react with a wide range of mammals due to sequence homology. Store product as a concentrated solution. Centrifuge briefly prior to opening the vial. This clone recognizes an internal, highly conserved amino acid sequence (VQEALD and VQAALD) found in both protein isoforms, MDR1 P-glycoprotein (~170kDa) and MDR3 P-glycoprotein (~140kDa). In Western blot, boiling samples may reduce visibility of the protein. For flow cytometry, cells should be fixed in 3.7% formaldehyde for 10 min at room temperature to permeabilize the cell membrane. Incubate cells with 5-10 µg antibody for 30-60 min at 4ºC.
- Reactivity
- Human, Mouse, Rat, Bovine, Canine, Zebrafish
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- C219
- Vial size
- 250 μL
- Concentration
- Conc. Not Determined
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Is P-Glycoprotein Functionally Expressed in the Limiting Membrane of Endolysosomes? A Biochemical and Ultrastructural Study in the Rat Liver.
Essential Oils, Pituranthos chloranthus and Teucrium ramosissimum, Chemosensitize Resistant Human Uterine Sarcoma MES-SA/Dx5 Cells to Doxorubicin by Inducing Apoptosis and Targeting P-Glycoprotein.
Protecting P-glycoprotein at the blood-brain barrier from degradation in an Alzheimer's disease mouse model.
P-glycoprotein Expression Is Upregulated in a Pre-Clinical Model of Traumatic Brain Injury.
Vascularized human cortical organoids (vOrganoids) model cortical development in vivo.
The potential role of human multidrug resistance protein 1 (MDR1) and multidrug resistance-associated protein 2 (MRP2) in the transport of Huperzine A in vitro.
APOE genotype-dependent pharmacogenetic responses to rapamycin for preventing Alzheimer's disease.
An Orally Available Tubulin Inhibitor, VERU-111, Suppresses Triple-Negative Breast Cancer Tumor Growth and Metastasis and Bypasses Taxane Resistance.
ABC transporters in gills of rainbow trout (Oncorhynchus mykiss).
Porphyrin-lipid assemblies and nanovesicles overcome ABC transporter-mediated photodynamic therapy resistance in cancer cells.
Preventing P-gp Ubiquitination Lowers Aβ Brain Levels in an Alzheimer's Disease Mouse Model.
Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice.
Age Drives Distortion of Brain Metabolic, Vascular and Cognitive Functions, and the Gut Microbiome.
Establishment and characterization of arsenic trioxide resistant KB/ATO cells.
Molecular Imaging of P-glycoprotein in Chemoresistant Tumors Using a Dual-Modality PET/Fluorescence Probe.
A multifunctional lipid nanoparticle for co-delivery of paclitaxel and curcumin for targeted delivery and enhanced cytotoxicity in multidrug resistant breast cancer cells.
In Vitro Modeling of Blood-Brain Barrier with Human iPSC-Derived Endothelial Cells, Pericytes, Neurons, and Astrocytes via Notch Signaling.
Structures of the Multidrug Transporter P-glycoprotein Reveal Asymmetric ATP Binding and the Mechanism of Polyspecificity.
Selective induction of P-glycoprotein at the CNS barriers during symptomatic stage of an ALS animal model.
Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions.
Aβ40 Reduces P-Glycoprotein at the Blood-Brain Barrier through the Ubiquitin-Proteasome Pathway.
Inhibition of P-glycoprotein, multidrug resistance-associated protein 2 and cytochrome P450 3A4 improves the oral absorption of octreotide in rats with portal hypertension.
Instruction of circulating endothelial progenitors in vitro towards specialized blood-brain barrier and arterial phenotypes.
Reversal of multidrug resistance phenotype in human breast cancer cells using doxorubicin-liposome-microbubble complexes assisted by ultrasound.
P-gp efflux pump inhibition potential of common environmental contaminants determined in vitro.
Protein phosphatase complex PP5/PPP2R3C dephosphorylates P-glycoprotein/ABCB1 and down-regulates the expression and function.
Gericke B, Wienböker I, Brandes G, Löscher W
Cells 2022 May 5;11(9)
Cells 2022 May 5;11(9)
Essential Oils, Pituranthos chloranthus and Teucrium ramosissimum, Chemosensitize Resistant Human Uterine Sarcoma MES-SA/Dx5 Cells to Doxorubicin by Inducing Apoptosis and Targeting P-Glycoprotein.
Lahmar A, Mathey A, Aires V, Elgueder D, Vejux A, Khlifi R, Sioud F, Chekir-Ghedira L, Delmas D
Nutrients 2021 May 19;13(5)
Nutrients 2021 May 19;13(5)
Protecting P-glycoprotein at the blood-brain barrier from degradation in an Alzheimer's disease mouse model.
Ding Y, Zhong Y, Baldeshwiler A, Abner EL, Bauer B, Hartz AMS
Fluids and barriers of the CNS 2021 Mar 6;18(1):10
Fluids and barriers of the CNS 2021 Mar 6;18(1):10
P-glycoprotein Expression Is Upregulated in a Pre-Clinical Model of Traumatic Brain Injury.
Vita SM, Redell JB, Maynard ME, Zhao J, Grill RJ, Dash PK, Grayson BE
Neurotrauma reports 2020;1(1):207-217
Neurotrauma reports 2020;1(1):207-217
Vascularized human cortical organoids (vOrganoids) model cortical development in vivo.
Shi Y, Sun L, Wang M, Liu J, Zhong S, Li R, Li P, Guo L, Fang A, Chen R, Ge WP, Wu Q, Wang X
PLoS biology 2020 May;18(5):e3000705
PLoS biology 2020 May;18(5):e3000705
The potential role of human multidrug resistance protein 1 (MDR1) and multidrug resistance-associated protein 2 (MRP2) in the transport of Huperzine A in vitro.
Fei Z, Hu M, Baum L, Kwan P, Hong T, Zhang C
Xenobiotica; the fate of foreign compounds in biological systems 2020 Mar;50(3):354-362
Xenobiotica; the fate of foreign compounds in biological systems 2020 Mar;50(3):354-362
APOE genotype-dependent pharmacogenetic responses to rapamycin for preventing Alzheimer's disease.
Lin AL, Parikh I, Yanckello LM, White RS, Hartz AMS, Taylor CE, McCulloch SD, Thalman SW, Xia M, McCarty K, Ubele M, Head E, Hyder F, Sanganahalli BG
Neurobiology of disease 2020 Jun;139:104834
Neurobiology of disease 2020 Jun;139:104834
An Orally Available Tubulin Inhibitor, VERU-111, Suppresses Triple-Negative Breast Cancer Tumor Growth and Metastasis and Bypasses Taxane Resistance.
Deng S, Krutilina RI, Wang Q, Lin Z, Parke DN, Playa HC, Chen H, Miller DD, Seagroves TN, Li W
Molecular cancer therapeutics 2020 Feb;19(2):348-363
Molecular cancer therapeutics 2020 Feb;19(2):348-363
ABC transporters in gills of rainbow trout (Oncorhynchus mykiss).
Kropf C, Fent K, Fischer S, Casanova A, Segner H
The Journal of experimental biology 2020 Aug 11;223(Pt 15)
The Journal of experimental biology 2020 Aug 11;223(Pt 15)
Porphyrin-lipid assemblies and nanovesicles overcome ABC transporter-mediated photodynamic therapy resistance in cancer cells.
Baglo Y, Liang BJ, Robey RW, Ambudkar SV, Gottesman MM, Huang HC
Cancer letters 2019 Aug 10;457:110-118
Cancer letters 2019 Aug 10;457:110-118
Preventing P-gp Ubiquitination Lowers Aβ Brain Levels in an Alzheimer's Disease Mouse Model.
Hartz AMS, Zhong Y, Shen AN, Abner EL, Bauer B
Frontiers in aging neuroscience 2018;10:186
Frontiers in aging neuroscience 2018;10:186
Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice.
Ma D, Wang AC, Parikh I, Green SJ, Hoffman JD, Chlipala G, Murphy MP, Sokola BS, Bauer B, Hartz AMS, Lin AL
Scientific reports 2018 Apr 27;8(1):6670
Scientific reports 2018 Apr 27;8(1):6670
Age Drives Distortion of Brain Metabolic, Vascular and Cognitive Functions, and the Gut Microbiome.
Hoffman JD, Parikh I, Green SJ, Chlipala G, Mohney RP, Keaton M, Bauer B, Hartz AMS, Lin AL
Frontiers in aging neuroscience 2017;9:298
Frontiers in aging neuroscience 2017;9:298
Establishment and characterization of arsenic trioxide resistant KB/ATO cells.
Zhang YK, Dai C, Yuan CG, Wu HC, Xiao Z, Lei ZN, Yang DH, Le XC, Fu L, Chen ZS
Acta pharmaceutica Sinica. B 2017 Sep;7(5):564-570
Acta pharmaceutica Sinica. B 2017 Sep;7(5):564-570
Molecular Imaging of P-glycoprotein in Chemoresistant Tumors Using a Dual-Modality PET/Fluorescence Probe.
Wang M, Mao C, Wang H, Ling X, Wu Z, Li Z, Ming X
Molecular pharmaceutics 2017 Oct 2;14(10):3391-3398
Molecular pharmaceutics 2017 Oct 2;14(10):3391-3398
A multifunctional lipid nanoparticle for co-delivery of paclitaxel and curcumin for targeted delivery and enhanced cytotoxicity in multidrug resistant breast cancer cells.
Baek JS, Cho CW
Oncotarget 2017 May 2;8(18):30369-30382
Oncotarget 2017 May 2;8(18):30369-30382
In Vitro Modeling of Blood-Brain Barrier with Human iPSC-Derived Endothelial Cells, Pericytes, Neurons, and Astrocytes via Notch Signaling.
Yamamizu K, Iwasaki M, Takakubo H, Sakamoto T, Ikuno T, Miyoshi M, Kondo T, Nakao Y, Nakagawa M, Inoue H, Yamashita JK
Stem cell reports 2017 Mar 14;8(3):634-647
Stem cell reports 2017 Mar 14;8(3):634-647
Structures of the Multidrug Transporter P-glycoprotein Reveal Asymmetric ATP Binding and the Mechanism of Polyspecificity.
Esser L, Zhou F, Pluchino KM, Shiloach J, Ma J, Tang WK, Gutierrez C, Zhang A, Shukla S, Madigan JP, Zhou T, Kwong PD, Ambudkar SV, Gottesman MM, Xia D
The Journal of biological chemistry 2017 Jan 13;292(2):446-461
The Journal of biological chemistry 2017 Jan 13;292(2):446-461
Selective induction of P-glycoprotein at the CNS barriers during symptomatic stage of an ALS animal model.
Chan GN, Evans RA, Banks DB, Mesev EV, Miller DS, Cannon RE
Neuroscience letters 2017 Feb 3;639:103-113
Neuroscience letters 2017 Feb 3;639:103-113
Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions.
Parikh I, Guo J, Chuang KH, Zhong Y, Rempe RG, Hoffman JD, Armstrong R, Bauer B, Hartz AM, Lin AL
Aging 2016 Nov 8;8(11):2814-2826
Aging 2016 Nov 8;8(11):2814-2826
Aβ40 Reduces P-Glycoprotein at the Blood-Brain Barrier through the Ubiquitin-Proteasome Pathway.
Hartz AM, Zhong Y, Wolf A, LeVine H 3rd, Miller DS, Bauer B
The Journal of neuroscience : the official journal of the Society for Neuroscience 2016 Feb 10;36(6):1930-41
The Journal of neuroscience : the official journal of the Society for Neuroscience 2016 Feb 10;36(6):1930-41
Inhibition of P-glycoprotein, multidrug resistance-associated protein 2 and cytochrome P450 3A4 improves the oral absorption of octreotide in rats with portal hypertension.
Sun XY, Duan ZJ, Liu Z, Tang SX, Li Y, He SC, Wang QM, Chang QY
Experimental and therapeutic medicine 2016 Dec;12(6):3716-3722
Experimental and therapeutic medicine 2016 Dec;12(6):3716-3722
Instruction of circulating endothelial progenitors in vitro towards specialized blood-brain barrier and arterial phenotypes.
Boyer-Di Ponio J, El-Ayoubi F, Glacial F, Ganeshamoorthy K, Driancourt C, Godet M, Perrière N, Guillevic O, Couraud PO, Uzan G
PloS one 2014;9(1):e84179
PloS one 2014;9(1):e84179
Reversal of multidrug resistance phenotype in human breast cancer cells using doxorubicin-liposome-microbubble complexes assisted by ultrasound.
Deng Z, Yan F, Jin Q, Li F, Wu J, Liu X, Zheng H
Journal of controlled release : official journal of the Controlled Release Society 2014 Jan 28;174:109-16
Journal of controlled release : official journal of the Controlled Release Society 2014 Jan 28;174:109-16
P-gp efflux pump inhibition potential of common environmental contaminants determined in vitro.
Georgantzopoulou A, Skoczyńska E, Van den Berg JH, Brand W, Legay S, Klein SG, Rietjens IM, Murk AJ
Environmental toxicology and chemistry 2014 Apr;33(4):804-13
Environmental toxicology and chemistry 2014 Apr;33(4):804-13
Protein phosphatase complex PP5/PPP2R3C dephosphorylates P-glycoprotein/ABCB1 and down-regulates the expression and function.
Katayama K, Yamaguchi M, Noguchi K, Sugimoto Y
Cancer letters 2014 Apr 1;345(1):124-31
Cancer letters 2014 Apr 1;345(1):124-31
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
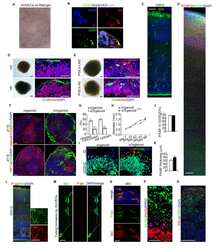
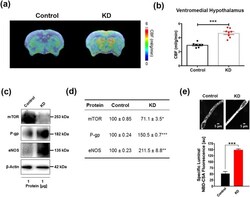
- Figure 1 Ketogenic diet enhances neurovascular functions. ( a ) Representative cerebral blood flow (CBF) maps superimposed on structural images; color code indicates level of CBF in a linear scale. KD mice exhibited significantly higher CBF in the ( b ) ventromedial hypothalamus. Data are presented as mean +- SEM, *** p < 0.001. ( c ) Western blot (WB) images for mTOR, P-gp, and eNOS from the cortical vasculature, beta-Actin was used as loading control. ( d ) The corresponding values of the levels of protein expression. WB data from KD mice were normalized to beta-Actin and compared to the control mice (100%), * p < 0.05, ** p < 0.01, *** p < 0.001. ( e ) Representative confocal images showing increased luminal accumulation of NBD-CSA fluorescence in brain capillaries isolated from KD mice compared to control mice, indicating higher P-gp transport activity. Corresponding quantitative fluorescence data; images are shown in arbitrary fluorescence units (scale 0-255). Data are mean +- SEM for 10 capillaries from one preparation of 10 mice per group, *** p < 0.001. mTOR: mechanistic target of Rapamycin; P-gp: P-glycoprotein; eNOS; endothelial nitric oxide synthase.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
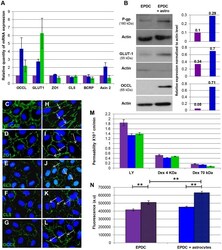
- Figure 3 Specialized EPDC's BBB characteristics. ( A ) Quantitative RT-PCR expression analysis of specific BBB genes at day 14 of culture, in EPDCs alone (purple), with astrocytes (blue) and hCMEC-D3 (green) (qRT-PCR were performed on EPDCs RNA isolated from 6 BBB differentiation independent experiences, each individual experience was performed in triplicate). ( B ) Western blot of P-gp, GLUT-1 and OCCL in EPDCs alone (purple) or with astrocytes (blue) at day 14. Densiometric quantification was performed for each immunoblot using ImageJ software. ( C - L ) VE-CAD, ZO1, CL3, CL5 and OCCL immunofluorescence staining in EPDCs alone ( C - G ) or with astrocytes ( H - L ). Arrows show continuous junctions. ( M ) Permeability for LY (0.457 kDa) and Dextran-FITC (4 and 70 kDa) in EPDCs alone (purple) or with astrocytes (blue) and in hCMEC/D3 (green) (EPDC permeability to dextran-FITC molecules was performed once in triplicate). ( N ) Accumulation of Calcein into EPDCs alone or with astrocytes, in the presence (hatched area) or absence of Verapamil (**p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
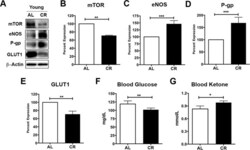
- Figure 2 Caloric restriction enriches vascular signaling markers and shifts metabolism in young mice ( A ) Western blotting (WB) of mTOR, eNOS, P-gp and GLUT1 from the cortical vasculature, beta-Actin was used as loading control; corresponding values of ( B ) mTOR, ( C ) eNOS, ( D ) P-gp, and ( E ) GLUT1 between the young AL and CR mice. All the WB data were normalized to beta-Actin and compared to young AL (100%). ( F ) Blood glucose and ( G ) Blood Ketone levels of the mice. * p < 0.05; ** p < 0.01; *** p < 0.001; AL: ad libitum; CR: caloric restriction.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
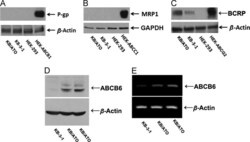
- Figure 4 Protein expression profile in KB/ATO cells and parental KB-3-1 cells. Expression level of (A) P-gp, (B) MRP1, (C) BCRP and (D) ABCB6 were shown in western blot results. (E) RT-PCR was performed to confirm the mRNA levels of ABCB6. Cell lysate from HEK/ABCB1, HEK/ABCC1 and HEK/ABCG2 was used as positive control for P-gp, MRP1 and BCRP respectively. Fig. 4
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
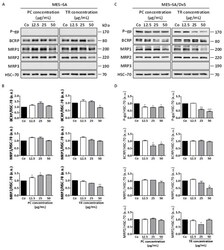
- Figure 10 Differential expression of ABC transporters in MES-SA and MES-SA/Dx5 to Pituranthos chloranthus (PC) and Teucrium ramosissimum Desf. (TR) extracts. ( A ) Immunoblot analysis of P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance proteins (MRP1, MRP2, and MRP3) after a 72 h treatment with vehicle (dimethyl sulfoxide, DMSO; Control, Co) or indicated concentrations of PC and TR in MES-SA cells. Heat shock cognate 70 (HSC-70) was used as the loading control. A representative blot from three independent experiments is shown. ( B ) Densitometric quantification of Western blotting obtained in (A). Data are expressed as mean fold induction +- Standard Error of the Mean (SEM) of three independent experiments; p -values were determined by a one-way ANOVA followed by Tukey's multiple comparison test. * p < 0.05, ** p < 0.01, and *** p < 0.001 compared to control (Co) condition. F values of ANOVA tests (significant results): MRP2 expression, F = 15.53 and F = 28.46 for PC and TR, respectively; BCRP expression, F = 10.18 for TR. ( C ) Immunoblot analysis of P-gp, BCRP, MRP1, MRP2, and MRP3 after a 72 h treatment with vehicle (dimethyl sulfoxide, DMSO; Control, Co) or indicated concentrations of PC and TR in MES-SA/Dx5 cells. HSC-70 was used as the loading control. A representative blot from three independent experiments is shown. ( D ) Densitometric quantification of Western blotting obtained in ( C ). Data are expressed as mean fold inductio
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
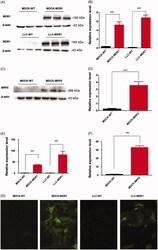
- Figure 1. P-gp and MRP2 expression in different cell lines. (A) Protein expression of P-gp was detected by western blot. (B) Quantification of P-gp by scanning of the protein bands in A. (C) Protein expression of MRP2 was detected by western blot. (D) Quantification of MRP2 by scanning of the protein bands in C. (E) mRNA expression of P-gp in MDCK, MDCK-MDR1, LLC and LLC-MDR1 cells. (F) mRNA expression of MRP2 in MDCK-WT and MDCK-MRP2 cells. Relative mRNA and protein levels of P-gp or MRP2 were scaled to the mean relative expression level of b-actin. The relative levels of P-gp or MRP2 in wild-type cells were regarded as 1. (G) Immunostaining of P-gp in wild type and MDR1 -transfected cells. Experiments were performed in triplicate, and values are shown as mean +- SEM ( n = 3, * p < 0.05, ** p < 0.01, *** p < 0.001).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
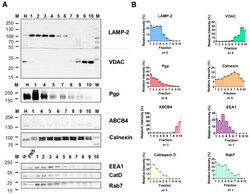
- Biochemical characterization of rat liver subcellular fractions and Pgp localization in endolysosomal-enriched fractions. Rat liver subcellular fractionation was performed by low speed centrifugation and subsequent density gradient ultracentrifugation. Recovery and purity of the cell organelles from collected gradient fractions 1-10 (top to bottom) were analyzed by separation of equal protein amounts (5 ug) of each fraction and whole-cell homogenate (H) and post-nuclear supernatant (PNS) by SDS-PAGE and immunoblotting. Homogenate and PNS data were similar; thus, PNS is shown here for only some proteins. Endolysosomes were detected using the LAMP-2 and Rab7 marker proteins and the luminal lysosomal protease cathepsin D (CatD). EEA1 was used as a marker for early endosomes. The purity of the endolysosomal fractions was assessed by organelle markers for mitochondria (VDAC), canalicular plasma membrane (ABCB4), and ER (calnexin). ( A ) Representative Western blots showing the distribution of the different organelle protein markers and Pgp in the gradient fractions. Some fractions (mainly 2 and 3) contained low protein amounts and thus could not be characterized for the presence of all marker proteins for each individual gradient. Therefore, marker quantification in the gradient fractions was repeatedly performed for different fractionations. ( B ) Quantification of the protein band intensities for the marker proteins in the different gradient fractions. Immunoblotting revealed re
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
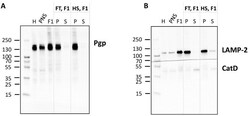
- Detection of Pgp in the membrane fraction of F1. The endolysosome-enriched fraction was separated into soluble (luminal) and membrane subcompartments and analyzed by Western blotting for Pgp localization ( A ). The organelle membranes were ruptured applying two different methods in comparison, either repeated freeze-thaw (FT) cycles or a hypoosmotic shock (HS). The pellet (P) enriched in membrane proteins and the supernatant (S) containing soluble luminal proteins were analyzed by Western blotting (5 ug total protein/lane of P and corresponding amount of S). Liver homogenate (H), post-nuclear supernatant (PNS), and fraction 1 (F1) (5 ug total protein each) were analyzed for comparison. ( B ) LAMP-2 was used as a marker protein for the membrane fraction, whereas cathepsin D (CatD) served as a control protein for the soluble endolysosomal protein fraction.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
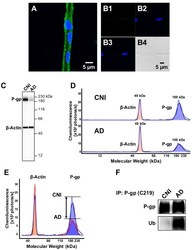
- Figure 1 P-glycoprotein (P-gp) protein expression levels are decreased and P-gp ubiquitination levels are increased in brain capillaries from Alzheimer's disease (AD) patients. (A) Representative image of a P-gp-immunostained (green) brain capillary isolated from brain tissue of a cognitive normal individual (CNI); nuclei were counterstained with DAPI (blue). (B) Negative control (no primary antibody): (B1 ; green channel); (B2 ; blue channel); (B3 ; overlay of green and blue channel) and (B4 ; transmitted light channel). (C) Representative WES(tm) image and (D) electropherogram showing reduced P-gp (blue shaded area) protein expression levels in brain capillaries isolated from human brain tissue (frontal cortex) of AD patients ( n = 3) vs. CNIs ( n = 3). (E) Overlay of electropherograms displayed in (D) show a reduction in the area under the curve (AUC) that represents P-gp protein expression levels (blue shaded area) in brain capillaries from AD patients (red line) relative to CNI (blue line). In contrast, beta-actin levels (orange shaded area) in brain capillaries from AD patients (red line) and CNI (blue line) were the same. (F) Western blot showing that ubiquitin levels in P-gp-immunoprecipitates are increased in capillaries from AD patients compared to those from CNI.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Other assay
Other assay