Antibody data
- Antibody Data
- Antigen structure
- References [29]
- Comments [0]
- Validations
- Flow cytometry [1]
- Other assay [17]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 13-9800 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CaMKII beta Monoclonal Antibody (CB-beta-1)
- Antibody type
- Monoclonal
- Antigen
- Purifed from natural sources
- Reactivity
- Human, Mouse, Rat
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- CB-beta-1
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- -20°C
Submitted references Herpes Simplex Virus Type 1 Neuronal Infection Triggers the Disassembly of Key Structural Components of Dendritic Spines.
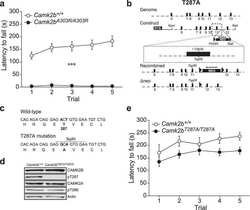
Gene-environment interactions mediate stress susceptibility and resilience through the CaMKIIβ/TARPγ-8/AMPAR pathway.
A simple DMSO-based method for cryopreservation of primary hippocampal and cortical neurons.
CAMK2-Dependent Signaling in Neurons Is Essential for Survival.
A novel role for CAMKIIβ in the regulation of cortical neuron migration: implications for neurodevelopmental disorders.
Calmodulin shuttling mediates cytonuclear signaling to trigger experience-dependent transcription and memory.
CaMKIIβ is localized in dendritic spines as both drebrin-dependent and drebrin-independent pools.
Differential Involvement of Kinase Activity of Ca(2+)/Calmodulin-Dependent Protein Kinase IIα in Hippocampus- and Amygdala-Dependent Memory Revealed by Kinase-Dead Knock-In Mouse.
Cyclophilin D regulates neuronal activity-induced filopodiagenesis by fine-tuning dendritic mitochondrial calcium dynamics.
Reciprocal control of excitatory synapse numbers by Wnt and Wnt inhibitor PRR7 secreted on exosomes.
Dynamic Arc SUMOylation and Selective Interaction with F-Actin-Binding Protein Drebrin A in LTP Consolidation In Vivo.
Regulation of spinogenesis in mature Purkinje cells via mGluR/PKC-mediated phosphorylation of CaMKIIβ.
A Novel Human CAMK2A Mutation Disrupts Dendritic Morphology and Synaptic Transmission, and Causes ASD-Related Behaviors.
The molecular, temporal and region-specific requirements of the beta isoform of Calcium/Calmodulin-dependent protein kinase type 2 (CAMK2B) in mouse locomotion.
Targeting the CaMKII/ERK Interaction in the Heart Prevents Cardiac Hypertrophy.
Electron tomographic structure and protein composition of isolated rat cerebellar, hippocampal and cortical postsynaptic densities.
Evolutionary and functional perspectives on signaling from neuronal surface to nucleus.
CaMKII regulates diacylglycerol lipase-α and striatal endocannabinoid signaling.
Electron cryotomography of postsynaptic densities during development reveals a mechanism of assembly.
GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis.
Structure and composition of the postsynaptic density during development.
Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning.
Identification and validation of novel spinophilin-associated proteins in rodent striatum using an enhanced ex vivo shotgun proteomics approach.
Interactions of the NPXY microdomains of the low density lipoprotein receptor-related protein 1.
Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning.
Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning.
Inositol 1,4,5-trisphosphate 3-kinase A associates with F-actin and dendritic spines via its N terminus.
PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain.
PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain.
Acuña-Hinrichsen F, Covarrubias-Pinto A, Ishizuka Y, Stolzenbach MF, Martin C, Salazar P, Castro MA, Bramham CR, Otth C
Frontiers in cellular neuroscience 2021;15:580717
Frontiers in cellular neuroscience 2021;15:580717
Gene-environment interactions mediate stress susceptibility and resilience through the CaMKIIβ/TARPγ-8/AMPAR pathway.
Sakai Y, Li H, Inaba H, Funayama Y, Ishimori E, Kawatake-Kuno A, Yamagata H, Seki T, Hobara T, Nakagawa S, Watanabe Y, Tomita S, Murai T, Uchida S
iScience 2021 May 21;24(5):102504
iScience 2021 May 21;24(5):102504
A simple DMSO-based method for cryopreservation of primary hippocampal and cortical neurons.
Ishizuka Y, Bramham CR
Journal of neuroscience methods 2020 Mar 1;333:108578
Journal of neuroscience methods 2020 Mar 1;333:108578
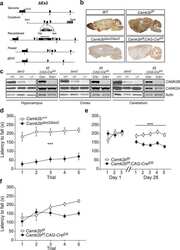
CAMK2-Dependent Signaling in Neurons Is Essential for Survival.
Kool MJ, Proietti Onori M, Borgesius NZ, van de Bree JE, Elgersma-Hooisma M, Nio E, Bezstarosti K, Buitendijk GHS, Aghadavoud Jolfaei M, Demmers JAA, Elgersma Y, van Woerden GM
The Journal of neuroscience : the official journal of the Society for Neuroscience 2019 Jul 10;39(28):5424-5439
The Journal of neuroscience : the official journal of the Society for Neuroscience 2019 Jul 10;39(28):5424-5439
A novel role for CAMKIIβ in the regulation of cortical neuron migration: implications for neurodevelopmental disorders.
Nicole O, Bell DM, Leste-Lasserre T, Doat H, Guillemot F, Pacary E
Molecular psychiatry 2018 Nov;23(11):2209-2226
Molecular psychiatry 2018 Nov;23(11):2209-2226
Calmodulin shuttling mediates cytonuclear signaling to trigger experience-dependent transcription and memory.
Cohen SM, Suutari B, He X, Wang Y, Sanchez S, Tirko NN, Mandelberg NJ, Mullins C, Zhou G, Wang S, Kats I, Salah A, Tsien RW, Ma H
Nature communications 2018 Jun 22;9(1):2451
Nature communications 2018 Jun 22;9(1):2451
CaMKIIβ is localized in dendritic spines as both drebrin-dependent and drebrin-independent pools.
Yamazaki H, Sasagawa Y, Yamamoto H, Bito H, Shirao T
Journal of neurochemistry 2018 Jul;146(2):145-159
Journal of neurochemistry 2018 Jul;146(2):145-159
Differential Involvement of Kinase Activity of Ca(2+)/Calmodulin-Dependent Protein Kinase IIα in Hippocampus- and Amygdala-Dependent Memory Revealed by Kinase-Dead Knock-In Mouse.
Yamagata Y, Yanagawa Y, Imoto K
eNeuro 2018 Jul-Aug;5(4)
eNeuro 2018 Jul-Aug;5(4)
Cyclophilin D regulates neuronal activity-induced filopodiagenesis by fine-tuning dendritic mitochondrial calcium dynamics.
Sui S, Tian J, Gauba E, Wang Q, Guo L, Du H
Journal of neurochemistry 2018 Aug;146(4):403-415
Journal of neurochemistry 2018 Aug;146(4):403-415
Reciprocal control of excitatory synapse numbers by Wnt and Wnt inhibitor PRR7 secreted on exosomes.
Lee SH, Shin SM, Zhong P, Kim HT, Kim DI, Kim JM, Heo WD, Kim DW, Yeo CY, Kim CH, Liu QS
Nature communications 2018 Aug 24;9(1):3434
Nature communications 2018 Aug 24;9(1):3434
Dynamic Arc SUMOylation and Selective Interaction with F-Actin-Binding Protein Drebrin A in LTP Consolidation In Vivo.
Nair RR, Patil S, Tiron A, Kanhema T, Panja D, Schiro L, Parobczak K, Wilczynski G, Bramham CR
Frontiers in synaptic neuroscience 2017;9:8
Frontiers in synaptic neuroscience 2017;9:8
Regulation of spinogenesis in mature Purkinje cells via mGluR/PKC-mediated phosphorylation of CaMKIIβ.
Sugawara T, Hisatsune C, Miyamoto H, Ogawa N, Mikoshiba K
Proceedings of the National Academy of Sciences of the United States of America 2017 Jun 27;114(26):E5256-E5265
Proceedings of the National Academy of Sciences of the United States of America 2017 Jun 27;114(26):E5256-E5265
A Novel Human CAMK2A Mutation Disrupts Dendritic Morphology and Synaptic Transmission, and Causes ASD-Related Behaviors.
Stephenson JR, Wang X, Perfitt TL, Parrish WP, Shonesy BC, Marks CR, Mortlock DP, Nakagawa T, Sutcliffe JS, Colbran RJ
The Journal of neuroscience : the official journal of the Society for Neuroscience 2017 Feb 22;37(8):2216-2233
The Journal of neuroscience : the official journal of the Society for Neuroscience 2017 Feb 22;37(8):2216-2233
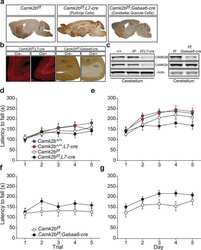
The molecular, temporal and region-specific requirements of the beta isoform of Calcium/Calmodulin-dependent protein kinase type 2 (CAMK2B) in mouse locomotion.
Kool MJ, van de Bree JE, Bodde HE, Elgersma Y, van Woerden GM
Scientific reports 2016 May 31;6:26989
Scientific reports 2016 May 31;6:26989
Targeting the CaMKII/ERK Interaction in the Heart Prevents Cardiac Hypertrophy.
Cipolletta E, Rusciano MR, Maione AS, Santulli G, Sorriento D, Del Giudice C, Ciccarelli M, Franco A, Crola C, Campiglia P, Sala M, Gomez-Monterrey I, De Luca N, Trimarco B, Iaccarino G, Illario M
PloS one 2015;10(6):e0130477
PloS one 2015;10(6):e0130477
Electron tomographic structure and protein composition of isolated rat cerebellar, hippocampal and cortical postsynaptic densities.
Farley MM, Swulius MT, Waxham MN
Neuroscience 2015 Sep 24;304:286-301
Neuroscience 2015 Sep 24;304:286-301
Evolutionary and functional perspectives on signaling from neuronal surface to nucleus.
Cohen SM, Li B, Tsien RW, Ma H
Biochemical and biophysical research communications 2015 Apr 24;460(1):88-99
Biochemical and biophysical research communications 2015 Apr 24;460(1):88-99
CaMKII regulates diacylglycerol lipase-α and striatal endocannabinoid signaling.
Shonesy BC, Wang X, Rose KL, Ramikie TS, Cavener VS, Rentz T, Baucum AJ 2nd, Jalan-Sakrikar N, Mackie K, Winder DG, Patel S, Colbran RJ
Nature neuroscience 2013 Apr;16(4):456-63
Nature neuroscience 2013 Apr;16(4):456-63
Electron cryotomography of postsynaptic densities during development reveals a mechanism of assembly.
Swulius MT, Farley MM, Bryant MA, Waxham MN
Neuroscience 2012 Jun 14;212:19-29
Neuroscience 2012 Jun 14;212:19-29
GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis.
Wills TA, Klug JR, Silberman Y, Baucum AJ, Weitlauf C, Colbran RJ, Delpire E, Winder DG
Proceedings of the National Academy of Sciences of the United States of America 2012 Jan 31;109(5):E278-87
Proceedings of the National Academy of Sciences of the United States of America 2012 Jan 31;109(5):E278-87
Structure and composition of the postsynaptic density during development.
Swulius MT, Kubota Y, Forest A, Waxham MN
The Journal of comparative neurology 2010 Oct 15;518(20):4243-60
The Journal of comparative neurology 2010 Oct 15;518(20):4243-60
Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning.
Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A
The Journal of neuroscience : the official journal of the Society for Neuroscience 2010 Mar 31;30(13):4590-600
The Journal of neuroscience : the official journal of the Society for Neuroscience 2010 Mar 31;30(13):4590-600
Identification and validation of novel spinophilin-associated proteins in rodent striatum using an enhanced ex vivo shotgun proteomics approach.
Baucum AJ 2nd, Jalan-Sakrikar N, Jiao Y, Gustin RM, Carmody LC, Tabb DL, Ham AJ, Colbran RJ
Molecular & cellular proteomics : MCP 2010 Jun;9(6):1243-59
Molecular & cellular proteomics : MCP 2010 Jun;9(6):1243-59
Interactions of the NPXY microdomains of the low density lipoprotein receptor-related protein 1.
Guttman M, Betts GN, Barnes H, Ghassemian M, van der Geer P, Komives EA
Proteomics 2009 Nov;9(22):5016-28
Proteomics 2009 Nov;9(22):5016-28
Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning.
Elgersma Y, Fedorov NB, Ikonen S, Choi ES, Elgersma M, Carvalho OM, Giese KP, Silva AJ
Neuron 2002 Oct 24;36(3):493-505
Neuron 2002 Oct 24;36(3):493-505
Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning.
Elgersma Y, Fedorov NB, Ikonen S, Choi ES, Elgersma M, Carvalho OM, Giese KP, Silva AJ
Neuron 2002 Oct 24;36(3):493-505
Neuron 2002 Oct 24;36(3):493-505
Inositol 1,4,5-trisphosphate 3-kinase A associates with F-actin and dendritic spines via its N terminus.
Schell MJ, Erneux C, Irvine RF
The Journal of biological chemistry 2001 Oct 5;276(40):37537-46
The Journal of biological chemistry 2001 Oct 5;276(40):37537-46
PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain.
Christopherson KS, Hillier BJ, Lim WA, Bredt DS
The Journal of biological chemistry 1999 Sep 24;274(39):27467-73
The Journal of biological chemistry 1999 Sep 24;274(39):27467-73
PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain.
Christopherson KS, Hillier BJ, Lim WA, Bredt DS
The Journal of biological chemistry 1999 Sep 24;274(39):27467-73
The Journal of biological chemistry 1999 Sep 24;274(39):27467-73
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
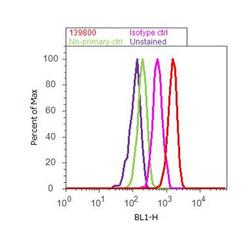
- Experimental details
- Flow cytometry analysis of CaM Kinase II beta Antibody (CB-beta-1) was done on U-87 MG cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with CaM Kinase II beta Antibody Mouse Monoclonal Antibody (139800, red histogram) or with mouse isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Rabbit Anti-Mouse Secondary Antibody (A11059) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
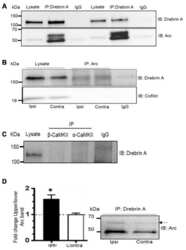
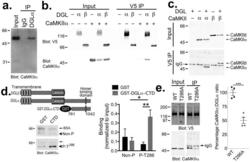
- Figure 1 DGLalpha interacts with CaMKII (a) Western blot confirms that CaMKIIalpha specifically associates with DGLalpha immune complexes, but not control IgG complexes, isolated from mouse striatal extracts. (b) CaMKIIalpha was detected in DGLalpha-V5 but not DGLbeta-V5 immune complexes isolated from co-transfected HEK293T cells. Control cells were not transfected or expressed either CaMKIIalpha or DGLalpha/beta-V5 alone. (c) Both CaMKII-alpha and -beta associated with DGLalpha-V5 immune complexes isolated from co-transfected HEK293T cells. The band between CaMKII-alpha and -beta in all of the IP samples is the IgG heavy chain used for the immunoprecipitation, as indicated. Panels a-c are representative of at least two experiments. (d) Alignment of DGLalpha and DGLbeta highlights a unique C-terminal domain in DGLalpha. A GST fusion protein containing the C-terminal domain (CTD; residues 761-1042) of DGLalpha directly interacts with purified Thr 286 -autophosphorylated, but not non-phosphorylated, CaMKIIalpha in vitro . The amount of CaMKII detected in each sample was normalized to the amount detected in an aliquot of the input for each incubation (Non-P (n=5 independent experiments): 0.15+-0.09 and 0.11+-0.03 for GST and GST-DGLalpha-CTD, respectively. P-T286 (n=6 independent experiments): 0.07+-0.03 and 0.37+-0.07 for GST and GST-DGLalpha-CTD, respectively. 2-way ANOVA: Interaction, F(1,18)=7.739, P=0.0123. Autophosphorylation, F 1,18 =4.661, P=0.0446. Protein, F 1,18 =2.22
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
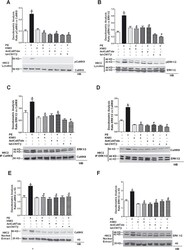
- Fig 2 Activation of CaMKII pathway in H9C2 cardiomyoblasts. To investigate the role of the cross talk between CaMKII and ERK on cardiac hypertrophy, we assessed the effect of CaMKII inhibition on PE-induced ERK activation. H9C2s were pretreated with KN93 (5 mumol/L) or AntCaNtide (10 mumol/L) and tat-CN17beta (10 mumol/L) for 30 min and then stimulated with PE (100 nmol/L for 15 min.). A: H9C2 total cell lysates were analyzed by Western blotting (WB) for phosphothreonine 286 CaMKII (pCaMKII) and total CaMKII (CaMKII), with specific antibodies. Data from immunoblots were quantified by densitometric analysis (DA). pCaMKII levels were corrected by total CaMKII densitometry. * = p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig 4 PKA inhibition does not modify PE-induced CaMKII/ERK activation in H9C2 cardiomyoblasts. A: H9C2 cardiomyoblasts were transfected with PKA-I and then stimulated with 100 nm phenylephrine (PE) for 15 min. Total cell extracts were analyzed by western blot for phosphothreonine 286 CaMKII (pCaMKII) and CaMKII with specific antibodies. pCaMKII levels were corrected by CaMKII densitometry. *, P < 0.05 vs . Ctrl. B: H9C2s were stimulated with PE after transfection with PKA-I. Total lysates were analysed by WB for pERK with specific antibody. pERK1/2 levels were corrected by ERK1/2 densitometry. * = p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
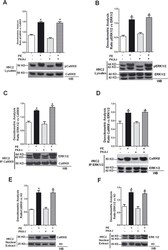
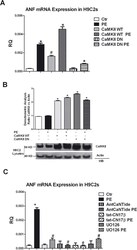
- Fig 5 CaMKII/ERK-dependent regulation of the hypertrophy marker ANF. A: H9C2 cells at 70% confluence were incubated 1 h at 37degC with 5 mL DMEM containing purified adenovirus at a multiplicity of infection (moi) of 100:1, encoding either the kinase-dead (CaMKII-DN, rCaMKIIdelta, K42M), or the wild type (CaMKII-WT, rCaMKIIdelta) variant of CaMKII or the empty virus as a negative control (Ctr). 48 h after the infection, the cells were stimulated with PE 100 nM for 24 h. Total RNA was isolated from H9C2s using TRIzol reagent, and cDNA was synthesized by means of a Thermo-Script RT-PCR System, following the manufacturer's instruction. Then ANP gene expression was evaluated by real-time PCR. Results are expressed as mean+-SEM from 3 independent experiments. The ratio of fold change was calculated using the Pfaffl method[ 36 ]. * = p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
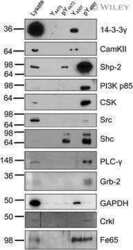
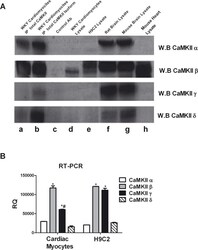
- Fig 1 CaMKII isoforms. A: To evaluate the expression of CaMKII isoforms in ventricular adult cardiomyocytes from WKY rats, the total cell lysates were immunoprecipitated using anti-CaMKII antibody (lane a) or anti-CaMKII alpha, beta gamma or delta specific antibodies (lane b) and together to total samples from WKY cardiomyocytes (lane d) and H9C2 cardiomyoblasts (lane e) were analyzed by western blot with anti-CaMKII alpha, beta gamma or delta antibodies as indicated. Total extracts from rat brain (lane f), mouse brain (lane g) and mouse heart (lane h) were used as controls. Total lysates from WKY cardiomyocytes with specific antibody without A/G agarose beads were used as negative control (lane c). B: Total RNA from H9C2 cell line and isolated ventricular cardiomyocytes was extracted with standard methods. RT-PCR for CaMKII alpha, beta, gamma, and delta was performed as indicated in methods. The representative graph indicates the relative amounts of transcripts for CaMKII isoforms in H9C2s and ventricular adult myocytes from WKY rats. Cycle threshold (Ct) values from 3 independent experiments were normalized to the internal beta-actin control. The ratio of fold change was calculated using the Pfaffl method. * = p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
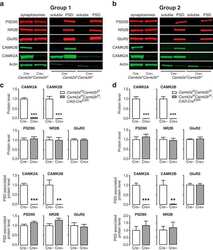
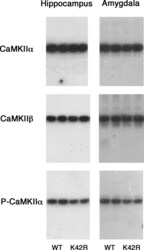
- Figure 2. Representative immunoblots showing the CaMKIIalpha, CaMKIIbeta and phospho-T286-CaMKIIalpha levels in the hippocampus and amygdala of the kinase-dead CaMKIIalpha (K42R)-KI mouse as compared to the wild-type mouse. The amounts of protein used were 2, 4, and 8 mug for the detection of CaMKIIalpha, CaMKIIbeta, and phospho-T286-CaMKIIalpha (P-CaMKIIalpha), respectively, from hippocampal or amygdala homogenates. Autoradiography of duplicated samples from a pair of wild-type (WT) and kinase-dead CaMKIIalpha (K42R)-KI mice in the same experimental group used for quantitative immunoblot analyses are shown. See also Table 1.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
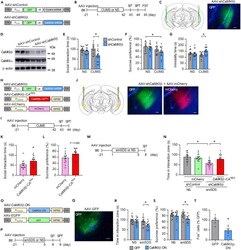
- Experimental details
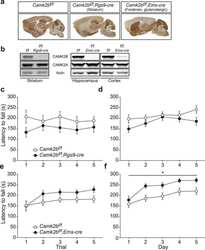
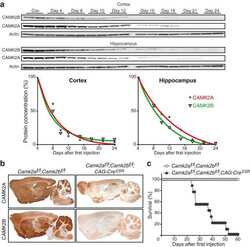
- Figure 3 CaMKIIbeta inhibition in the vCA1 increases stress susceptibility (A) AAV vectors used for the control construct (AAV-shControl) and CaMKIIbeta knockdown (AAV-shCaMKIIbeta). ITR, inverted terminal repeats; P CMV , cytomegalovirus promoter; P Camk2a , Camk2a promoter; U6, U6 promoter; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element. (B) Experimental paradigm for behavioral testing. CUMS, chronic ultra-mild stress; SIT, social interaction test; SPT, sucrose preference test; FST, forced swim test. (C) AAV microinjection into the vCA1. Region-specific expression of GFP in vCA1 is shown. Scale bar, 100 mum. (D) Specific knockdown of CaMKIIbeta but not CaMKIIalpha by the AAV-shCaMKIIbeta. (E-G) CaMKIIbeta knockdown in the vCA1 of B6 mice leads to significantly decreased social interaction time in the SIT (E), decreased sucrose preference in SPT (F), and increased immobility time in FST (G) following CUMS exposure. n = 12-15 mice per group. (H) AAV vectors engineered to overexpress a constitutively active form of CaMKIIbeta (CaMKIIbeta-CA) with resistance against shCaMKIIbeta (AAV-CaMKIIbeta-CA Res ) or a control construct (AAV-mCherry). (I) Experimental paradigm for behavioral testing. (J) AAV microinjection into the vCA1. Region-specific expression of GFP and mCherry in the vCA1 is shown. Scale bar, 100 mum. (K and L) Overexpression of CaMKIIbeta-CA Res together with shCaMKIIbeta increases time in the interaction zone in the SIT (K) and tends to inc
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Flow cytometry
Flow cytometry