Antibody data
- Antibody Data
- Antigen structure
- References [1]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [7]
- Flow cytometry [3]
- Other assay [2]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-78806 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Aquaporin 1 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- Reconstitute with 0.2 mL of distilled water to yield a concentration of 500 µg/mL. Positive Control - WB: rat heart tissue, rat kidney tissue, rat lung tissue, mouse heart tissue, mouse kidney tissue, mouse lung tissue. IHC: human kidney tissue, mouse kidney tissue, rat kidney tissue. Flow: U2OS cell.
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 500 μg/mL
- Storage
- -20°C
Submitted references Vascular Inflammation Is Associated with Loss of Aquaporin 1 Expression on Endothelial Cells and Increased Fluid Leakage in SARS-CoV-2 Infected Golden Syrian Hamsters.
Allnoch L, Beythien G, Leitzen E, Becker K, Kaup FJ, Stanelle-Bertram S, Schaumburg B, Mounogou Kouassi N, Beck S, Zickler M, Herder V, Gabriel G, Baumgärtner W
Viruses 2021 Apr 8;13(4)
Viruses 2021 Apr 8;13(4)
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
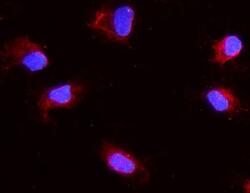
- Experimental details
- Immunocytochemistry analysis of Aquaporin 1 using anti-Aquaporin 1 antibody (Product # PA5-78806). Aquaporin 1 was detected in a section of NRK cells. Enzyme antigen retrieval was performed using IHC enzyme antigen retrieval reagent for 15 mins. The cells were blocked with 10% goat serum and then incubated with 2μg/mL rabbit anti-Aquaporin 1 antibody (Product # PA5-78806)overnight at 4°C. DyLight®550 Conjugated Goat Anti-Rabbit IgG was used as secondary antibody at 1:100 dilution and incubated for 30 minutes at 37°C. The section was counterstained with DAPI. Visualize using a fluorescence microscope and filter sets appropriate for the label used.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
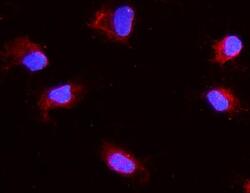
- Experimental details
- Immunocytochemistry analysis of Aquaporin 1 using anti-Aquaporin 1 antibody (Product # PA5-78806). Aquaporin 1 was detected in a section of NRK cells. Enzyme antigen retrieval was performed using IHC enzyme antigen retrieval reagent for 15 mins. The cells were blocked with 10% goat serum and then incubated with 2μg/mL rabbit anti-Aquaporin 1 antibody (Product # PA5-78806)overnight at 4°C. DyLight®550 Conjugated Goat Anti-Rabbit IgG was used as secondary antibody at 1:100 dilution and incubated for 30 minutes at 37°C. The section was counterstained with DAPI. Visualize using a fluorescence microscope and filter sets appropriate for the label used.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
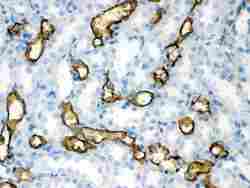
- Experimental details
- Immunohistochemistry analysis of Aquaporin 1 on paraffin-embedded rat kidney tissue. Sample was incubated with Aquaporin 1 polyclonal antibody (Product# PA5-78806).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
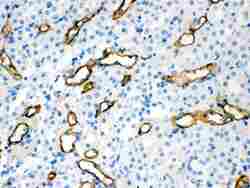
- Experimental details
- Immunohistochemistry analysis of Aquaporin 1 on paraffin-embedded mouse kidney tissue. Sample was incubated with Aquaporin 1 polyclonal antibody (Product# PA5-78806).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
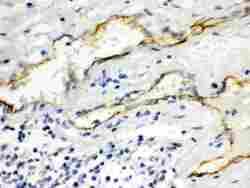
- Experimental details
- Immunohistochemistry analysis of Aquaporin 1 on paraffin-embedded human intestinal cancer tissue. Sample was incubated with Aquaporin 1 polyclonal antibody (Product# PA5-78806).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
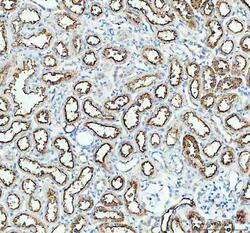
- Experimental details
- Immunohistochemistry (Paraffin) analysis of Aquaporin 1 in paraffin-embedded section of rat kidney tissue using Aquaporin 1 Polyclonal Antibody (Product # PA5-78806). Heat mediated antigen retrieval was performed in EDTA buffer (pH 8.0, epitope retrieval solution). The tissue section was blocked with 10% goat serum. The tissue section was then incubated with the primary antibody at a 2 µg/mL dilution overnight at 4°C. Peroxidase conjugated goat anti-rabbit IgG was used as secondary antibody and incubated for 30 minutes at 37°C. The tissue section was developed using HRP Conjugated Rabbit IgG Super Vision Assay Kit with DAB as the chromogen.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
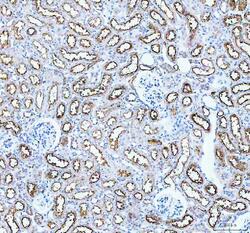
- Experimental details
- Immunohistochemistry (Paraffin) analysis of Aquaporin 1 in paraffin-embedded section of mouse kidney tissue using Aquaporin 1 Polyclonal Antibody (Product # PA5-78806). Heat mediated antigen retrieval was performed in EDTA buffer (pH 8.0, epitope retrieval solution). The tissue section was blocked with 10% goat serum. The tissue section was then incubated with the primary antibody at a 2 µg/mL dilution overnight at 4°C. Peroxidase conjugated goat anti-rabbit IgG was used as secondary antibody and incubated for 30 minutes at 37°C. The tissue section was developed using HRP Conjugated Rabbit IgG Super Vision Assay Kit with DAB as the chromogen.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunohistochemistry (Paraffin) analysis of Aquaporin 1 in paraffin-embedded section of human kidney tissue using Aquaporin 1 Polyclonal Antibody (Product # PA5-78806). Heat mediated antigen retrieval was performed in EDTA buffer (pH 8.0, epitope retrieval solution). The tissue section was blocked with 10% goat serum. The tissue section was then incubated with the primary antibody at a 2 µg/mL dilution overnight at 4°C. Peroxidase conjugated goat anti-rabbit IgG was used as secondary antibody and incubated for 30 minutes at 37°C. The tissue section was developed using HRP Conjugated Rabbit IgG Super Vision Assay Kit with DAB as the chromogen.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
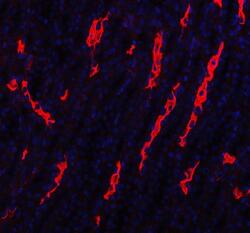
- Experimental details
- Immunohistochemistry (Paraffin) analysis of Aquaporin 1 in paraffin-embedded section of rat kidney tissue using Aquaporin 1 Polyclonal Antibody (Product # PA5-78806). Heat mediated antigen retrieval was performed in EDTA buffer (pH 8.0, epitope retrieval solution). The tissue section was blocked with 10% goat serum. The tissue section was then incubated with the primary antibody at a 5 µg/mL dilution overnight at 4°C. Cy3 conjugated goat anti-rabbit IgG was used as secondary antibody at 1:500 dilution and incubated for 30 minutes at 37°C. The section was counterstained with DAPI. Visualize using a fluorescence microscope and filter sets appropriate for the label used.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
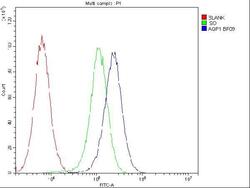
- Experimental details
- Flow Cytometry of Aquaporin 1 in U2OS cells (blue line), isotype control rabbit IgG (green line) and unlabeled (red line). Samples were blocked with 10% goat serum, incubated with Aquaporin 1 Polyclonal Antibody (Product # PA5-78806) at a dilution of 1 μg (per 1x10^6 cells), followed by DyLight®488 conjugated goat anti-rabbit IgG (for 30 minutes at 20°C) using 5-10 μg (per 1x10^6 cells) dilution.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
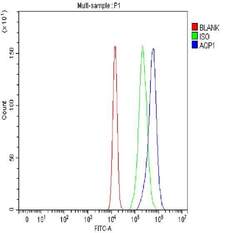
- Experimental details
- Flow cytometry analysis of Aquaporin 1 in U2OS cells using Aquaporin 1 Polyclonal Antibody (Product # PA5-78806), shown in overlay histogram (blue line). The cells were fixed with 4% paraformaldehyde and blocked with 10% normal goat serum, and incubated with the primary antibody (1 μg/1x10^6 cells) for 30 min at 20°C. DyLight 488 conjugated goat anti-rabbit IgG (5-10 µg/1x10^6 cells) was used as secondary antibody for 30 minutes at 20°C. Isotype control antibody (Green line) was rabbit IgG (1 µg/1x10^6) used under the same conditions. Unlabelled sample without incubation with primary antibody and secondary antibody (Red line) was used as a blank control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
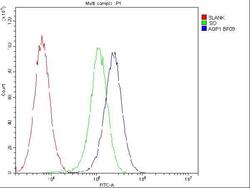
- Experimental details
- Flow Cytometry of Aquaporin 1 in U2OS cells (blue line), isotype control rabbit IgG (green line) and unlabeled (red line). Samples were blocked with 10% goat serum, incubated with Aquaporin 1 Polyclonal Antibody (Product # PA5-78806) at a dilution of 1 μg (per 1x10^6 cells), followed by DyLight®488 conjugated goat anti-rabbit IgG (for 30 minutes at 20°C) using 5-10 μg (per 1x10^6 cells) dilution.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 6 Aquaporin 1 expression during perivascular edema in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infected hamster lungs. Immunohistochemistry targeting aquaporin 1 (AQP1) water channels, as well as a subsequent alcian blue staining, were applied to evaluate the effects of the inflammatory vascular alterations upon vascular integrity. In SARS-CoV-2 infected animals perivascular edema could be detected in both small and medium-sized vessels at 1, 3, 6, and 14 days post-infection (dpi) compared to controls ( A - C ). From 1 to 6 dpi, edema affecting medium-sized vessels increased ( C ). Small vessels were also affected but to a lower degree ( B ). The stacked bar charts represent the circumferential expression of the water channel AQP1 in small ( D ) and medium-sized ( E ) vessels depending on the presence (stacked bars with dotted pattern) or absence of edema (stacked bars with striped pattern). Interestingly, vessels exhibiting edema more often reveal a decreased expression of AQP1 compared to vessels without edema. Furthermore, numbers of vessels lacking AQP1 expression increased over time with partial to complete loss of the water channel at 3, 6, and 14 dpi. Triangles indicate a strong positive relationship between AQP1 = 0% and edema formation (r s = 0.903; p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 7 Evaluation of the basement membrane and endothelial integrity during severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induced perivascular edema. To evaluate the integrity of the basement membrane aquaporin 1 (AQP1) immunostaining ( A , D ) was compared with immunohistochemistry targeting laminin ( B , E ). The vascular endothelium was visualized using immunohistochemistry detecting CD31 ( C , F ). To investigate the primary antibodies with the perivascular edema in a comparative manner, an alcian blue counterstaining was applied additionally. While vascular leakage ( A , asterisk) was associated with a segmental ( A , arrows) or even complete circumferential loss ( D ) of the water channel AQP1, the same vessels showed continuous labeling for laminin ( B , E ) and CD31 ( C , F ). Occasionally the circumferential expression of CD31 appeared segmentally disrupted ( C , insert). Representative images of vessels were taken at 100x magnification, bars represent 100 um in overviews and 20 um in the insert.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry