14-0688-37
antibody from Invitrogen Antibodies
Targeting: CD68
DKFZp686M18236, GP110, LAMP4, macrosialin, SCARD1
 Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [23]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [4]
- Other assay [19]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-0688-37 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD68 Monoclonal Antibody (KP1), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The KP1 antibody reacts with human CD68 which belongs to the sialomucin family and is closely related to the family of lysosomal-associated membrane proteins (lamps) and scavenger receptor. CD68 is predominantly an intracellular protein, found mainly in the late endosomal compartment but can be detected in smaller amounts on the surface of mainly myeloid-derived cells; monocytes and macrophages, dendritic cells (and in some langerhan cells), neutrophils, basophils, mast cells, myeloid progenitor cells, and a subset of CD34+ hematopoietic bone marrow progenitor cells. The function has not been fully elucidated but based on homology and structure, CD68 may play a role in antigen processing or presentation and protection of the lysosomal membrane from hydrolytic enzymes. Reports have also shown expression in activated T cells, and about 40% of peripheral blood B-lymphocytes and 50% of all B-ALL. The KP1 antibody has also been reported to stain fibroblasts which may be due to a conserved epitope rather than fibroblasts expressing CD68. Applications Reported: This KP1 antibody has been reported for use in flow cytometric analysis, immunoprecipitation, western blotting, immunohistochemical staining of frozen tissue sections, and immunohistochemical staining of formalin-fixed paraffin embedded tissue sections. Applications Tested: This KP1 antibody has been tested by immunohistochemistry with both low and high pH antigen retrieval on FFPE tissue. Either antigen retrieval method can be used. This antibody can be used at less than or equal to 1 µg/mL. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- KP1
- Vial size
- 2 mg
- Concentration
- 0.5 mg/mL
- Storage
- 4°C
Submitted references Subtype-Specific Surface Proteins on Adipose Tissue Macrophages and Their Association to Obesity-Induced Insulin Resistance.
Multiple benign fibrous histiocytomas of the mandible: A case report and review of the literature.
Pericyte mediates the infiltration, migration, and polarization of macrophages by CD163/MCAM axis in glioblastoma.
Stem cell architecture drives myelodysplastic syndrome progression and predicts response to venetoclax-based therapy.
Evidence for the presence of synovial sheaths surrounding the extensor tendons at the metacarpophalangeal joints: a microscopy study.
Macrophage-derived IL-6 trans-signalling as a novel target in the pathogenesis of bronchopulmonary dysplasia.
SeqStain is an efficient method for multiplexed, spatialomic profiling of human and murine tissues.
Sialic acid-binding immunoglobulin-like lectin-15 expression on peritumoral macrophages is a favorable prognostic factor for primary central nervous system lymphoma patients.
DUOX2, a New Biomarker for Disseminated Gastric Cancer's Response to Low Dose Radiation in Mice.
CNS delivery of anti-CD52 antibodies modestly reduces disease severity in an animal model for multiple sclerosis.
Modeling COVID-19 with Human Pluripotent Stem Cell-Derived Cells Reveals Synergistic Effects of Anti-inflammatory Macrophages with ACE2 Inhibition Against SARS-CoV-2.
Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain.
Interplay between Liver X Receptor and Hypoxia Inducible Factor 1α Potentiates Interleukin-1β Production in Human Macrophages.
Tumor-Derived Retinoic Acid Regulates Intratumoral Monocyte Differentiation to Promote Immune Suppression.
CD36-mediated uptake of myelin debris by macrophages and microglia reduces neuroinflammation.
Exosomal miRNA-19a and miRNA-614 Induced by Air Pollutants Promote Proinflammatory M1 Macrophage Polarization via Regulation of RORα Expression in Human Respiratory Mucosal Microenvironment.
Evaluation of lipid coverage and high spatial resolution MALDI-imaging capabilities of oversampling combined with laser post-ionisation.
Protective Aptitude of Annexin A1 in Arterial Neointima Formation in Atherosclerosis-Prone Mice-Brief Report.
LRP1 expression in microglia is protective during CNS autoimmunity.
Tumor-Infiltrating Immune Cells Are Associated With Prognosis of Gastric Cancer.
Rhinovirus colocalizes with CD68- and CD11b-positive macrophages following experimental infection in humans.
Lenalidomide inhibits lymphangiogenesis in preclinical models of mantle cell lymphoma.
Macrophage specificity of three anti-CD68 monoclonal antibodies (KP1, EBM11, and PGM1) widely used for immunohistochemistry and flow cytometry.
Strand K, Stiglund N, Haugstøyl ME, Kamyab Z, Langhelle V, Lawrence-Archer L, Busch C, Cornillet M, Hjellestad ID, Nielsen HJ, Njølstad PR, Mellgren G, Björkström NK, Fernø J
Frontiers in endocrinology 2022;13:856530
Frontiers in endocrinology 2022;13:856530
Multiple benign fibrous histiocytomas of the mandible: A case report and review of the literature.
Wang Y, Huang Y, Cai WX, Tao Q
Experimental and therapeutic medicine 2022 Sep;24(3):593
Experimental and therapeutic medicine 2022 Sep;24(3):593
Pericyte mediates the infiltration, migration, and polarization of macrophages by CD163/MCAM axis in glioblastoma.
Zhang H, Zhang N, Wu W, Wang Z, Dai Z, Liang X, Zhang L, Peng Y, Luo P, Zhang J, Liu Z, Cheng Q, Liu Z
iScience 2022 Sep 16;25(9):104918
iScience 2022 Sep 16;25(9):104918
Stem cell architecture drives myelodysplastic syndrome progression and predicts response to venetoclax-based therapy.
Ganan-Gomez I, Yang H, Ma F, Montalban-Bravo G, Thongon N, Marchica V, Richard-Carpentier G, Chien K, Manyam G, Wang F, Alfonso A, Chen S, Class C, Kanagal-Shamanna R, Ingram JP, Ogoti Y, Rose A, Loghavi S, Lockyer P, Cambo B, Muftuoglu M, Schneider S, Adema V, McLellan M, Garza J, Marchesini M, Giuliani N, Pellegrini M, Wang J, Walker J, Li Z, Takahashi K, Leverson JD, Bueso-Ramos C, Andreeff M, Clise-Dwyer K, Garcia-Manero G, Colla S
Nature medicine 2022 Mar;28(3):557-567
Nature medicine 2022 Mar;28(3):557-567
Evidence for the presence of synovial sheaths surrounding the extensor tendons at the metacarpophalangeal joints: a microscopy study.
Dakkak YJ, van Dijk BT, Jansen FP, Wisse LJ, Reijnierse M, van der Helm-van Mil AHM, DeRuiter MC
Arthritis research & therapy 2022 Jun 24;24(1):154
Arthritis research & therapy 2022 Jun 24;24(1):154
Macrophage-derived IL-6 trans-signalling as a novel target in the pathogenesis of bronchopulmonary dysplasia.
Hirani D, Alvira CM, Danopoulos S, Milla C, Donato M, Tian L, Mohr J, Dinger K, Vohlen C, Selle J, V Koningsbruggen-Rietschel S, Barbarino V, Pallasch C, Rose-John S, Odenthal M, Pryhuber GS, Mansouri S, Savai R, Seeger W, Khatri P, Al Alam D, Dötsch J, Alejandre Alcazar MA
The European respiratory journal 2022 Feb;59(2)
The European respiratory journal 2022 Feb;59(2)
SeqStain is an efficient method for multiplexed, spatialomic profiling of human and murine tissues.
Rajagopalan A, Venkatesh I, Aslam R, Kirchenbuechler D, Khanna S, Cimbaluk D, Kordower JH, Gupta V
Cell reports methods 2021 Jun 21;1(2)
Cell reports methods 2021 Jun 21;1(2)
Sialic acid-binding immunoglobulin-like lectin-15 expression on peritumoral macrophages is a favorable prognostic factor for primary central nervous system lymphoma patients.
Fudaba H, Momii Y, Hirakawa T, Onishi K, Asou D, Matsushita W, Kawasaki Y, Sugita K, Fujiki M
Scientific reports 2021 Jan 13;11(1):1206
Scientific reports 2021 Jan 13;11(1):1206
DUOX2, a New Biomarker for Disseminated Gastric Cancer's Response to Low Dose Radiation in Mice.
Parekh PR, Solano-Gonzalez E, Martins MB, Ma X, Tighe K, Casildo A, Zodda A, Johnstone C, Poirier Y, Mahmood J, Bhalla K, Li S, Lapidus RG, Carrier F
Cancers 2021 Aug 20;13(16)
Cancers 2021 Aug 20;13(16)
CNS delivery of anti-CD52 antibodies modestly reduces disease severity in an animal model for multiple sclerosis.
Bogie JF, Grajchen E, Wouters E, Broux B, Stinissen P, Van Wijmeersch B, Hendriks JJ
Therapeutic advances in chronic disease 2020;11:2040622320947378
Therapeutic advances in chronic disease 2020;11:2040622320947378
Modeling COVID-19 with Human Pluripotent Stem Cell-Derived Cells Reveals Synergistic Effects of Anti-inflammatory Macrophages with ACE2 Inhibition Against SARS-CoV-2.
Duan F, Guo L, Yang L, Han Y, Thakur A, Nilsson-Payant BE, Wang P, Zhang Z, Yan Ma C, Zhou X, Han T, Zhang T, Wang X, Xu D, Duan X, Xiang J, Tse HF, Liao C, Luo W, Huang FP, Chen YW, Evans T, Schwartz RE, tenOever B, Ho DD, Chen S, Na J, Lian Q, Chen HJ
Research square 2020 Sep 15;
Research square 2020 Sep 15;
Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain.
Bogie JFJ, Grajchen E, Wouters E, Corrales AG, Dierckx T, Vanherle S, Mailleux J, Gervois P, Wolfs E, Dehairs J, Van Broeckhoven J, Bowman AP, Lambrichts I, Gustafsson JÅ, Remaley AT, Mulder M, Swinnen JV, Haidar M, Ellis SR, Ntambi JM, Zelcer N, Hendriks JJA
The Journal of experimental medicine 2020 May 4;217(5)
The Journal of experimental medicine 2020 May 4;217(5)
Interplay between Liver X Receptor and Hypoxia Inducible Factor 1α Potentiates Interleukin-1β Production in Human Macrophages.
Ménégaut L, Thomas C, Jalil A, Julla JB, Magnani C, Ceroi A, Basmaciyan L, Dumont A, Le Goff W, Mathew MJ, Rébé C, Dérangère V, Laubriet A, Crespy V, Pais de Barros JP, Steinmetz E, Venteclef N, Saas P, Lagrost L, Masson D
Cell reports 2020 May 19;31(7):107665
Cell reports 2020 May 19;31(7):107665
Tumor-Derived Retinoic Acid Regulates Intratumoral Monocyte Differentiation to Promote Immune Suppression.
Devalaraja S, To TKJ, Folkert IW, Natesan R, Alam MZ, Li M, Tada Y, Budagyan K, Dang MT, Zhai L, Lobel GP, Ciotti GE, Eisinger-Mathason TSK, Asangani IA, Weber K, Simon MC, Haldar M
Cell 2020 Mar 19;180(6):1098-1114.e16
Cell 2020 Mar 19;180(6):1098-1114.e16
CD36-mediated uptake of myelin debris by macrophages and microglia reduces neuroinflammation.
Grajchen E, Wouters E, van de Haterd B, Haidar M, Hardonnière K, Dierckx T, Van Broeckhoven J, Erens C, Hendrix S, Kerdine-Römer S, Hendriks JJA, Bogie JFJ
Journal of neuroinflammation 2020 Jul 27;17(1):224
Journal of neuroinflammation 2020 Jul 27;17(1):224
Exosomal miRNA-19a and miRNA-614 Induced by Air Pollutants Promote Proinflammatory M1 Macrophage Polarization via Regulation of RORα Expression in Human Respiratory Mucosal Microenvironment.
Shin CH, Byun J, Lee K, Kim B, Noh YK, Tran NL, Park K, Kim SH, Kim TH, Oh SJ
Journal of immunology (Baltimore, Md. : 1950) 2020 Dec 1;205(11):3179-3190
Journal of immunology (Baltimore, Md. : 1950) 2020 Dec 1;205(11):3179-3190
Evaluation of lipid coverage and high spatial resolution MALDI-imaging capabilities of oversampling combined with laser post-ionisation.
Bowman AP, Bogie JFJ, Hendriks JJA, Haidar M, Belov M, Heeren RMA, Ellis SR
Analytical and bioanalytical chemistry 2020 Apr;412(10):2277-2289
Analytical and bioanalytical chemistry 2020 Apr;412(10):2277-2289
Protective Aptitude of Annexin A1 in Arterial Neointima Formation in Atherosclerosis-Prone Mice-Brief Report.
de Jong RJ, Paulin N, Lemnitzer P, Viola JR, Winter C, Ferraro B, Grommes J, Weber C, Reutelingsperger C, Drechsler M, Soehnlein O
Arteriosclerosis, thrombosis, and vascular biology 2017 Feb;37(2):312-315
Arteriosclerosis, thrombosis, and vascular biology 2017 Feb;37(2):312-315
LRP1 expression in microglia is protective during CNS autoimmunity.
Chuang TY, Guo Y, Seki SM, Rosen AM, Johanson DM, Mandell JW, Lucchinetti CF, Gaultier A
Acta neuropathologica communications 2016 Jul 11;4(1):68
Acta neuropathologica communications 2016 Jul 11;4(1):68
Tumor-Infiltrating Immune Cells Are Associated With Prognosis of Gastric Cancer.
Liu K, Yang K, Wu B, Chen H, Chen X, Chen X, Jiang L, Ye F, He D, Lu Z, Xue L, Zhang W, Li Q, Zhou Z, Mo X, Hu J
Medicine 2015 Sep;94(39):e1631
Medicine 2015 Sep;94(39):e1631
Rhinovirus colocalizes with CD68- and CD11b-positive macrophages following experimental infection in humans.
Bentley JK, Sajjan US, Dzaman MB, Jarjour NN, Lee WM, Gern JE, Hershenson MB
The Journal of allergy and clinical immunology 2013 Sep;132(3):758-761.e3
The Journal of allergy and clinical immunology 2013 Sep;132(3):758-761.e3
Lenalidomide inhibits lymphangiogenesis in preclinical models of mantle cell lymphoma.
Song K, Herzog BH, Sheng M, Fu J, McDaniel JM, Chen H, Ruan J, Xia L
Cancer research 2013 Dec 15;73(24):7254-64
Cancer research 2013 Dec 15;73(24):7254-64
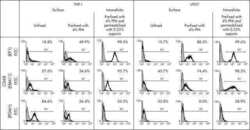
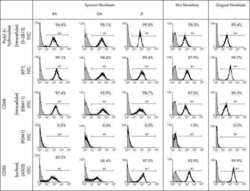
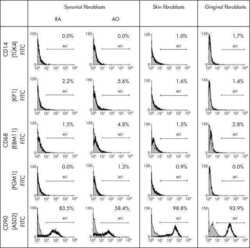
Macrophage specificity of three anti-CD68 monoclonal antibodies (KP1, EBM11, and PGM1) widely used for immunohistochemistry and flow cytometry.
Kunisch E, Fuhrmann R, Roth A, Winter R, Lungershausen W, Kinne RW
Annals of the rheumatic diseases 2004 Jul;63(7):774-84
Annals of the rheumatic diseases 2004 Jul;63(7):774-84
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
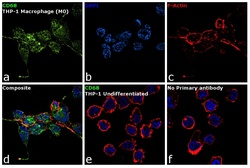
- Experimental details
- Immunofluorescence analysis of CD68 was performed using 70% confluent log phase THP-1 cells differentiated into Macrophage (M0 phase). The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with CD68 Mouse Monoclonal Antibody (KP1) (Product # 14-0688-80) at 5 µg/mL in 0.1% BSA, incubated at 4 degree Celsius overnight and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing membrane localization. Panel e shows untreated cells with no signal. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
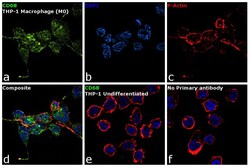
- Experimental details
- Immunofluorescence analysis of CD68 was performed using 70% confluent log phase THP-1 cells differentiated into Macrophage (M0 phase). The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with CD68 Mouse Monoclonal Antibody (KP1) (Product # 14-0688-80) at 5 µg/mL in 0.1% BSA, incubated at 4 degree Celsius overnight and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing membrane localization. Panel e shows untreated cells with no signal. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunohistochemistry on formalin-fixed paraffin embedded human cerebellar white matter, using 1 µg/mL of Mouse IgG1 Isotype Control (left) or 1 µg/mL of Anti-Human CD68 Purified (right) followed by biotinylated Anti-Mouse IgG, and DAB visualization. Nuclei are counterstained with hematoxylin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
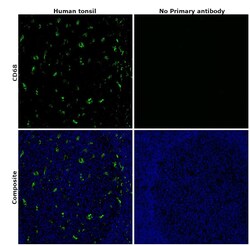
- Experimental details
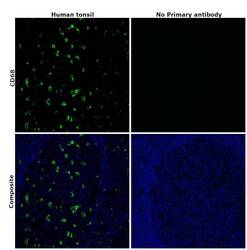
- Immunohistochemical analysis of CD68 was performed using formalin-fixed paraffin-embedded human tonsil tissue sections. To expose the target protein, heat-induced epitope retrieval was performed on de-paraffinized sections using eBioscience™ IHC Antigen Retrieval Solution - High pH (10X) (Product # 00-4956-58) diluted to 1X solution in water in a microwave oven at 100 degree Celsius for 15 minutes. Following antigen retrieval, the sections were blocked with 2% normal goat serum in 1X PBS for 45 minutes at room temperature and then probed with or without CD68 Monoclonal Antibody (KP1), eBioscience™ (Product #14-0688-82) at 5 µg/mL in 0.1% normal goat serum overnight at 4 degree Celsius in a humidified chamber. Detection was performed using Goat anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32723) at a dilution of 1:2000 in 0.1% normal goat serum for 45 minutes at room temperature. ReadyProbes™ Tissue Autofluorescence Quenching Kit (Product # R37630) was used to quench autofluorescence from the tissues. Nuclei were stained with DAPI (Product # D1306) and the sections were mounted using ProLong™ Glass Antifade Mountant (Product # P36984). The images were captured on EVOS™ M7000 Imaging System (Product # AMF7000) at 20X magnification and externally deconvoluted.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
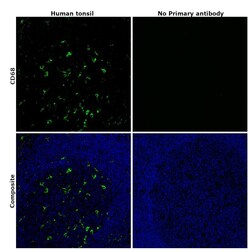
- Experimental details
- Immunohistochemical analysis of CD68 was performed using formalin-fixed paraffin-embedded human tonsil tissue sections. To expose the target protein, heat-induced epitope retrieval was performed on de-paraffinized sections using eBioscience™ IHC Antigen Retrieval Solution - High pH (10X) (Product # 00-4956-58) diluted to 1X solution in water in a microwave oven at 100 degree Celsius for 15 minutes. Following antigen retrieval, the sections were blocked with 2% normal goat serum in 1X PBS for 45 minutes at room temperature and then probed with or without CD68 Monoclonal Antibody (KP1), eBioscience™ (Product #14-0688-82) at 5 µg/mL in 0.1% normal goat serum overnight at 4 degree Celsius in a humidified chamber. Detection was performed using Goat anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32723) at a dilution of 1:2000 in 0.1% normal goat serum for 45 minutes at room temperature. ReadyProbes™ Tissue Autofluorescence Quenching Kit (Product # R37630) was used to quench autofluorescence from the tissues. Nuclei were stained with DAPI (Product # D1306) and the sections were mounted using ProLong™ Glass Antifade Mountant (Product # P36984). The images were captured on EVOS™ M7000 Imaging System (Product # AMF7000) at 20X magnification and externally deconvoluted.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunohistochemical analysis of CD68 was performed using formalin-fixed paraffin-embedded human tonsil tissue sections. To expose the target protein, heat-induced epitope retrieval was performed on de-paraffinized sections using eBioscience™ IHC Antigen Retrieval Solution - High pH (10X) (Product # 00-4956-58) diluted to 1X solution in water in a microwave oven at 100 degree Celsius for 15 minutes. Following antigen retrieval, the sections were blocked with 2% normal goat serum in 1X PBS for 45 minutes at room temperature and then probed with or without CD68 Monoclonal Antibody (KP1), eBioscience™ (Product #14-0688-82) at 5 µg/mL in 0.1% normal goat serum overnight at 4 degree Celsius in a humidified chamber. Detection was performed using Goat anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32723) at a dilution of 1:2000 in 0.1% normal goat serum for 45 minutes at room temperature. ReadyProbes™ Tissue Autofluorescence Quenching Kit (Product # R37630) was used to quench autofluorescence from the tissues. Nuclei were stained with DAPI (Product # D1306) and the sections were mounted using ProLong™ Glass Antifade Mountant (Product # P36984). The images were captured on EVOS™ M7000 Imaging System (Product # AMF7000) at 20X magnification and externally deconvoluted.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
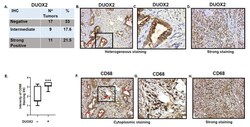
- Figure 6 Immuno-Histo-Chemistry (IHC) in 37 human adenocarcinoma samples. ( A ) Staining scored blinded by a pathologist at Pantomics, Inc. Staining > 2.8 was considered strong DUOX2 positive, score of ""0"" was considered DUOX2 negative. Percentage of strong staining and negative DUOX2 staining are indicated. ( B ) Example of heterogeneous (positive and negative) DUOX2 staining in well differentiated/low grade gastric adenocarcinoma (Malignant 1). Red arrows indicate cells expressing DUOX2. ( C ) Expanded view of inset from ( B ). ( D ) Example of strong DUOX2 staining in poorly differentiated/high grade stomach adenocarcinoma. ( E ) Intensity of CD68 staining in DUOX2 negative and positive samples. Student's t -test, *** p = 0.0005. ( F ) CD68, red arrows indicate macrophages tumor and stroma infiltration in same tissue as ( B ) expressing DUOX2. ( G ) Expanded view of inset from ( F ). ( H ) IHC in poorly differentiated/high grade stomach adenocarcinoma showing strong CD68 staining. Images dimensions are 450 x 383 pixels (W x H).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
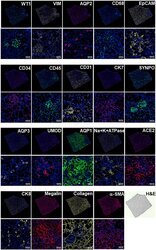
- Experimental details
- Figure 6. SeqStain-based multiplex imaging of whole human kidney tissue provides a 20-plex image Immunofluorescent images of whole kidney tissue sections after each round of staining with unique SeqStain antibodies (as indicated) and DAPI (as indicated in the panel). Zoomed-in sections of images are presented below each panel. A serial section stained with H&E is also presented. Scale bars, 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
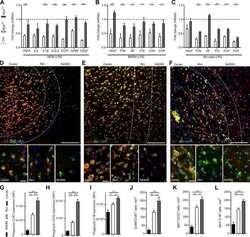
- Figure 7. SeqStain multiplex imaged panels identify major substructures in the human kidney (A) Image showing a composite overlay of aligned immunofluorescence image stack of 20 unique markers from the whole kidney tissue section. Scale bar, 100 mum. (B) Image of a zoomed-in region from the whole kidney tissue section in (A) (white square) showing various components of the kidney tissue, including the glomerular endothelial cells (GECs), proximal tubule (PT), collecting duct (CD), distal convoluted tubules (DCT), and podocytes. Scale bar, 100 mum. (C) Boxed dot plot showing computed co-expression of EpCAM and the indicated markers on a per-cell basis (mean +- SD). (D) Image of a zoomed-in region from the whole kidney tissue section in (A) (red square) showing one glomerulus (two panels), false colored for the indicated markers. Scale bar, 100 mum. (E) Representative images showing zoomed-in regions of composite overlay of aligned immunofluorescence image stacks from (A) for the identification of various immunophenotypes of cells and tissue sections based on co-localization of various markers (as labeled). Scale bar, 50 mum. (F) Boxed dot plot of cellular neighborhoods showing the computed distances of the indicated cells from glomerular basement membrane of a selected glomerulus in the kidney (mean +- SD). Glom refers to cells residing inside the glomerulus, whereas RI refers to cells residing outside the glomerulus, in the renal interstitium.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
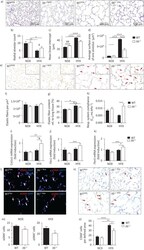
- Experimental details
- FIGURE 3 Loss of interleukin 6 ( Il6 ) preserves alveolar as well as elastic fibre formation, and reduces macrophage invasion in neonatal lungs after hyperoxia. a) Representative images of haematoxylin- and eosin-stained tissue sections showing alveolarisation in mice at postnatal day 28 (P28). Wildtype (WT) and Il6 knockout mice ( Il6 -/- ) were exposed to hyperoxia (HYX; 85% O 2 ) or normoxia (NOX; 21% O 2 ) from postnatal day 1 (P1) to P28. b-d) Summary data of quantitative histomorphometric analyses: radial alveolar count (b), mean linear intercept (c), average surface area of one alveolus (d). e) Representative images of Hart's-stained tissue sections showing distribution and localisation of elastic fibres in mice at P28. Arrows indicate elastic fibres at the secondary tips after hyperoxia. f-g) Summary data of quantitative analyses of elastic fibres per defined area (um 3 ) (f) and elastic fibre content relative (rel.) to lung tissue (%) (g). h) Measurement of respiratory (Resp.) system compliance using whole-body plethysmography in Il6 -/- and WT mice at P28. i-k) Assessment of the expression of genes encoding components of lung matrix, including elastic fibres. i) Collagen 1alpha1 ( Col1a1 ). j) Fibrillin 1 ( Fbn1 ). k) Fibulin5 ( Fbln5 ). l-m) Representative immunofluorescent staining of alpha-smooth muscle actin (alphaSMA) as a marker of myofibroblasts. White arrows depict positive stained cells (l); quantification data of alphaSMA + cells relative to all 4',6-diami
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
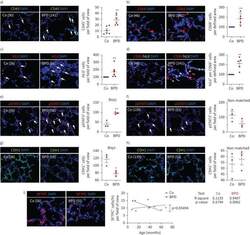
- Experimental details
- FIGURE 9 Inflammation and interleukin 6 (IL-6)/signal transducer and activator of transcription 3 (STAT3) signalling in lungs of infants with bronchopulmonary dysplasia (BPD) and non-BPD. a) Representative immunofluorescent staining for immune cells using CD45 as a marker (green) in age-matched BPD and control lungs (Co); the lung identification numbers of the infants are indicated in brackets. Immune cells (CD45 + cells) were counted in 4-12 fields of view per lung. Summary data of the quantification of immune cells (CD45 + cells) per field of view for all infants (n=6 per group). DAPI: 4',6-diamidino-2-phenylindole. b) Representative co-immunofluorescent localisation of CD68 (marker of macrophages, red) in age-matched BPD and Co lungs; white arrows are indicating CD68 + cells. The analysis of CD68 + cells per 6-12 fields of view is shown next to the images; n=6 per group. c) Representative localisation of human IL6 (h IL6 , red) in lungs with BPD and Co; white arrows are indicating h IL6 + in situ hybridisation. The analysis of h IL6 + cells per 6-12 fields of view is shown next to the images; n=6 per group. d) Representative co-immunofluorescent localisation of CD68 (marker of macrophages, red) with h IL6 (white; in situ hybridisation) in age-matched BPD and Co lungs; white arrows are indicating CD68 + and h Il6 + . The analysis of h IL6 mRNA expression per CD68 + cells per 6-12 fields of view is shown next to the images; n=6 per group. e, f) Representative immunofluoresce
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Insulin resistance, dyslipidemia, and inflammatory markers in SAT and VAT. (A) Pearson correlations between monocytes, M1-, and M2-like macrophages and M1/M2 ratio in SAT and VAT and biochemical parameters, corrected for BMI (n=80). Size and color intensities of circles indicate correlation coefficients. Red squares indicate significant (p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
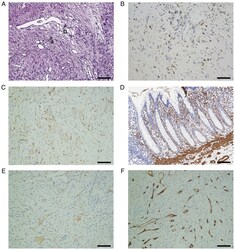
- Experimental details
- Pathological examination of the tumors. (A) Histology indicated that the tumors consisted of spindle-shaped cells and collagen fibers, and the former were arranged in a storiform pattern and surrounded by the latter. The arrows a and b indicated the collagen fiber and cell, respectively. (H&E staining; magnification, x200). Immunohistochemistry suggested that the tumor cells were strongly positive for (B) CD68 and (C) XIIIa factor, while they were weakly positive for (D) alpha-SMA and (E) desmin. (F) The tumor cells were negative for CD34 (magnification, x200; scale bars, 100 um).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
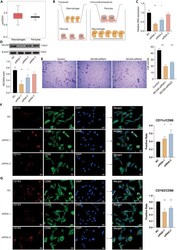
- Pericytes mediate the migration and polarization of macrophages (A) The relative expression of macrophages and pericytes in the TME of GBM based on the ssGSEA algorithm. (B) The flow diagram of the co-culture system between macrophages and pericytes. (C) The relative RNA expression of MCAM in the NC and three siRNA target groups. (D) The protein expression of MCAM in the NC group and three siRNA target groups. (E) Transwell assay for the co-cultured pericytes. Statistical analysis of the migrated pericytes in different siRNA groups. (F)Multiplex immunofluorescence staining of M1 macrophage markers CD68, CD11c in the cocultured system between macrophages and pericytes. Statistical analysis of the positive M1 macrophages in different siRNA groups (scale bar: 25um). (G)Multiplex immunofluorescence staining of M2 macrophage markers CD68, CD163 in the cocultured system between macrophages and pericytes (scale bar: 25um). Statistical analysis of the positive M2 macrophages in different siRNA groups. Data are represented as mean +-SD.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
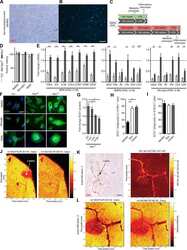
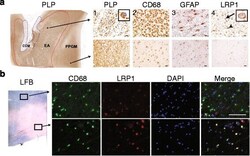
- Fig. 1 LRP1 expression is increased in MS lesions. a Immunohistochemistry on consecutive sections from an early active (EA) MS lesion (upper row) shows: (1) myelin (PLP) laden macrophages consistent with ongoing demyelinating activity, (2) abundant macrophage infiltration (CD68), (3) hypertrophic reactive astrocytes indicating gliosis (GFAP), and (4) LRP1 immunoreactivity present on both astrocytes (arrowhead) and macrophages (arrow and inset). In contrast, the periplaque gray matter (PPGM, lower row) shows: (1) normal appearing myelin (PLP), (2) limited microglial reactivity (CD68), (3) astrocytes with regular size and morphology (GFAP), and (4) limited LRP1 immunoreactivity. (Scale bars = 20 mum). b Luxol Fast Blue histology (LFB) and immunofluorescence for CD68 and LRP1 shows that myeloid cells express LRP1 within the lesion (Scale bar = 100 mum)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
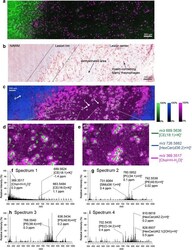
- Fig. 6 a CD68 (macrophages, purple) and Bodipy (myelin/neutral lipids, green) immunostaining and b Oil Red O staining of human brain tissue slices acquired from the same patient used to collect the MSI data shown in c - e with several tissue regions indicated. c Ion distributions images of m / z 689.5636 ([CE(18:1)+K] + , green), m / z 726.5882 ([HexCer(d36:2)+H] + , blue) and m / z 369.3517 ([Chol+H-H 2 O] + , pink) acquired using MALDI-2 and a pixel size of 6 mum from human multiple sclerosis brain tissue. d , e Selected enlarged regions of the MSI data shown in c . f - i Single pixel spectra acquired from the regions indicated by the white arrows in c . All MSI data is visualised using the total ion current normalisation and hotspot removal (99% quantile). NAWM = normal appearing white matter
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
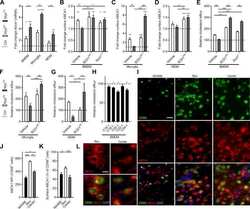
- Figure 1 Macrophages were highly involved at the severe stage of COVID-19(A) H+E (Hematoxylin and Eosin) staining on the bronchial or alveolar region in healthy or severe COVID-19 case. Pulmonary hemorrhagic infarct (denoted by arrowheads) (B) Immunohistochemistry (IHC) using antibody against CD68 revealed macrophage with aggregated phenotype and enlarged nuclei in COVID-19 lung, compared to the ones in the healthy lung. (C) Immunofluorescence (IF) staining on healthy or COVID-19 distal lung tissues using antibodies against CD68 (pan-macrophage marker), and CD80 (M1 macrophage marker) (D) Quantification on CD68+ or CD80+ macrophages in healthy or COVID-19 distal lung tissues. (E) IF staining on healthy or COVID distal lung tissues using antibodies against CD68 and CD163 (M2 macrophage marker) (F) Quantification on CD68+ or CD163+ macrophages in healthy or COVID-19 distal lung tissues. (G) IF staining on healthy or COVID-19 distal lung tissues using antibodies against CD68 and IL-6. Scale bar = 100 mum in all images in Figure 1. Data were presented as mean +- STDEV. P values were calculated by unpaired two-tailed Student's t-test. **P < 0.01, ***P < 0.001, and ****P < 0.0001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
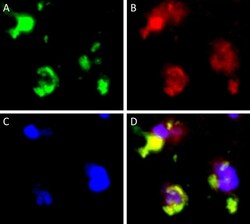
- Figure 4 Double-immunofluorescent staining of CD68 and Siglec-15. CD68 staining ( A green). Siglec-15 staining ( B red). DAPI staining ( C blue). Merged images ( D ). Representative images of PCNSL peritumoral tissue with the co-expression of CD68 and Siglec-15. (magnification x 1000).
 Explore
Explore Validate
Validate Learn
Learn