Antibody data
- Antibody Data
- Antigen structure
- References [17]
- Comments [0]
- Validations
- Western blot [1]
- Immunocytochemistry [1]
- Immunohistochemistry [1]
- Other assay [9]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-16668 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Lysozyme Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Purifed from natural sources
- Description
- PA5-16668 targets Lysozyme in IHC (P) applications and shows reactivity with Canine, Feline, Human, Non-human primate, and Porcine samples. The PA5-16668 immunogen is human lysozyme purified from the urine of patients with monocytic leukemia.
- Reactivity
- Human, Mouse, Canine, Feline, Porcine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 500 µL
- Storage
- 4° C
Submitted references Single-Cell Analysis Reveals Unexpected Cellular Changes and Transposon Expression Signatures in the Colonic Epithelium of Treatment-Naïve Adult Crohn's Disease Patients.
Oxidized phospholipids cause changes in jejunum mucus that induce dysbiosis and systemic inflammation.
Wnt signaling is boosted during intestinal regeneration by a CD44-positive feedback loop.
Human AGR2 Deficiency Causes Mucus Barrier Dysfunction and Infantile Inflammatory Bowel Disease.
Aberrant Epithelial Differentiation Contributes to Pathogenesis in a Murine Model of Congenital Tufting Enteropathy.
Morphological and histochemical characterization of the secretory epithelium in the canine lacrimal gland.
ILC1 drive intestinal epithelial and matrix remodelling.
Protein Kinase D1 (PKD1) Signaling Induces Growth-Promoting Effects in Murine Enteroids.
Ubiquitination of RIPK1 regulates its activation mediated by TNFR1 and TLRs signaling in distinct manners.
NAD(+) supplementation rejuvenates aged gut adult stem cells.
Ex vivo gut culture for studying differentiation and migration of small intestinal epithelial cells.
Canine Salivary Glands: Analysis of Rab and SNARE Protein Expression and SNARE Complex Formation With Diverse Tissue Properties.
High-fat diet enhances stemness and tumorigenicity of intestinal progenitors.
Characterization of antigen-presenting cells from the porcine respiratory system.
The CSF-1 receptor fashions the intestinal stem cell niche.
mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake.
Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium.
Kanke M, Kennedy Ng MM, Connelly S, Singh M, Schaner M, Shanahan MT, Wolber EA, Beasley C, Lian G, Jain A, Long MD, Barnes EL, Herfarth HH, Isaacs KL, Hansen JJ, Kapadia M, Guillem JG, Feschotte C, Furey TS, Sheikh SZ, Sethupathy P
Cellular and molecular gastroenterology and hepatology 2022;13(6):1717-1740
Cellular and molecular gastroenterology and hepatology 2022;13(6):1717-1740
Oxidized phospholipids cause changes in jejunum mucus that induce dysbiosis and systemic inflammation.
Mukherjee P, Chattopadhyay A, Grijalva V, Dorreh N, Lagishetty V, Jacobs JP, Clifford BL, Vallim T, Mack JJ, Navab M, Reddy ST, Fogelman AM
Journal of lipid research 2022 Jan;63(1):100153
Journal of lipid research 2022 Jan;63(1):100153
Wnt signaling is boosted during intestinal regeneration by a CD44-positive feedback loop.
Walter RJ, Sonnentag SJ, Munoz-Sagredo L, Merkel M, Richert L, Bunert F, Heneka YM, Loustau T, Hodder M, Ridgway RA, Sansom OJ, Mely Y, Rothbauer U, Schmitt M, Orian-Rousseau V
Cell death & disease 2022 Feb 21;13(2):168
Cell death & disease 2022 Feb 21;13(2):168
Human AGR2 Deficiency Causes Mucus Barrier Dysfunction and Infantile Inflammatory Bowel Disease.
Al-Shaibi AA, Abdel-Motal UM, Hubrack SZ, Bullock AN, Al-Marri AA, Agrebi N, Al-Subaiey AA, Ibrahim NA, Charles AK, COLORS in IBD-Qatar Study Group, Elawad M, Uhlig HH, Lo B
Cellular and molecular gastroenterology and hepatology 2021;12(5):1809-1830
Cellular and molecular gastroenterology and hepatology 2021;12(5):1809-1830
Aberrant Epithelial Differentiation Contributes to Pathogenesis in a Murine Model of Congenital Tufting Enteropathy.
Das B, Okamoto K, Rabalais J, Young JA, Barrett KE, Sivagnanam M
Cellular and molecular gastroenterology and hepatology 2021;12(4):1353-1371
Cellular and molecular gastroenterology and hepatology 2021;12(4):1353-1371
Morphological and histochemical characterization of the secretory epithelium in the canine lacrimal gland.
Yasui T, Miyata K, Nakatsuka C, Tsukise A, Gomi H
European journal of histochemistry : EJH 2021 Nov 2;65(4)
European journal of histochemistry : EJH 2021 Nov 2;65(4)
ILC1 drive intestinal epithelial and matrix remodelling.
Jowett GM, Norman MDA, Yu TTL, Rosell Arévalo P, Hoogland D, Lust ST, Read E, Hamrud E, Walters NJ, Niazi U, Chung MWH, Marciano D, Omer OS, Zabinski T, Danovi D, Lord GM, Hilborn J, Evans ND, Dreiss CA, Bozec L, Oommen OP, Lorenz CD, da Silva RMP, Neves JF, Gentleman E
Nature materials 2021 Feb;20(2):250-259
Nature materials 2021 Feb;20(2):250-259
Protein Kinase D1 (PKD1) Signaling Induces Growth-Promoting Effects in Murine Enteroids.
Shimizu Y, Sinnett-Smith J, Tenggara M, Martin MG, Rozengurt E
Cellular and molecular gastroenterology and hepatology 2020;10(2):430-433.e9
Cellular and molecular gastroenterology and hepatology 2020;10(2):430-433.e9
Ubiquitination of RIPK1 regulates its activation mediated by TNFR1 and TLRs signaling in distinct manners.
Li X, Zhang M, Huang X, Liang W, Li G, Lu X, Li Y, Pan H, Shi L, Zhu H, Qian L, Shan B, Yuan J
Nature communications 2020 Dec 11;11(1):6364
Nature communications 2020 Dec 11;11(1):6364
NAD(+) supplementation rejuvenates aged gut adult stem cells.
Igarashi M, Miura M, Williams E, Jaksch F, Kadowaki T, Yamauchi T, Guarente L
Aging cell 2019 Jun;18(3):e12935
Aging cell 2019 Jun;18(3):e12935
Ex vivo gut culture for studying differentiation and migration of small intestinal epithelial cells.
Sun X, Fu X, Du M, Zhu MJ
Open biology 2018 Apr;8(4)
Open biology 2018 Apr;8(4)
Canine Salivary Glands: Analysis of Rab and SNARE Protein Expression and SNARE Complex Formation With Diverse Tissue Properties.
Gomi H, Osawa H, Uno R, Yasui T, Hosaka M, Torii S, Tsukise A
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2017 Nov;65(11):637-653
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2017 Nov;65(11):637-653
High-fat diet enhances stemness and tumorigenicity of intestinal progenitors.
Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, Pinello L, Katz Y, Shinagare S, Abu-Remaileh M, Mihaylova MM, Lamming DW, Dogum R, Guo G, Bell GW, Selig M, Nielsen GP, Gupta N, Ferrone CR, Deshpande V, Yuan GC, Orkin SH, Sabatini DM, Yilmaz ÖH
Nature 2016 Mar 3;531(7592):53-8
Nature 2016 Mar 3;531(7592):53-8
Characterization of antigen-presenting cells from the porcine respiratory system.
López-Robles G, Silva-Campa E, Burgara-Estrella A, Hernández J
Research in veterinary science 2015 Jun;100:80-7
Research in veterinary science 2015 Jun;100:80-7
The CSF-1 receptor fashions the intestinal stem cell niche.
Akcora D, Huynh D, Lightowler S, Germann M, Robine S, de May JR, Pollard JW, Stanley ER, Malaterre J, Ramsay RG
Stem cell research 2013 Mar;10(2):203-12
Stem cell research 2013 Mar;10(2):203-12
mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake.
Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, Nielsen GP, Mino-Kenudson M, Zukerberg LR, Bhan AK, Deshpande V, Sabatini DM
Nature 2012 Jun 28;486(7404):490-5
Nature 2012 Jun 28;486(7404):490-5
Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium.
Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, Jay P
The Journal of cell biology 2011 Mar 7;192(5):767-80
The Journal of cell biology 2011 Mar 7;192(5):767-80
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
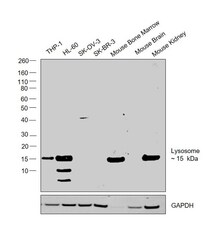
- Experimental details
- Western blot was performed using Anti-Lysozyme Polyclonal Antibody (Product # PA5-16668) and a 15kDa band corresponding to Lysozyme was observed across cell lines and tissues tested except SK-O-V3, SK-BR-3 and Mouse Brain. Whole cell extracts (30 µg lysate) of THP-1 (Lane 1), HL-60 (Lane 2), SK-O-V3 (Lane 3), SK-BR-3 (Lane 4), Mouse Bone Marrow (Lane 5), Mouse Brain (Lane 6) and Mouse Kidney (Lane 7) were electrophoresed using Novex® NuPAGE® 4-12 % Bis-Tris gel (Product # NP0322BOX). Resolved proteins were then transferred onto a nitrocellulose membrane (Product # IB23001) by iBlot® 2 Dry Blotting System (Product # IB21001). The blot was probed with the primary antibody (1:500 dilution) and detected by chemiluminescence with Goat anti-Rabbit IgG (H+L), Superclonal™ Recombinant Secondary Antibody, HRP (Product # A27036, 1:4000 dilution) using the iBright FL 1000 (Product # A32752). Chemiluminescent detection was performed using Novex® ECL Chemiluminescent Substrate Reagent Kit (Product # WP20005).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
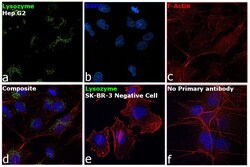
- Experimental details
- Immunofluorescence analysis of Lysozyme was performed using 70% confluent log phase Hep G2 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with Lysozyme Polyclonal Antibody (Product # PA5-16668) at 1:100 dilution in 0.1% BSA, incubated at 4 degree Celsius overnight and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Recombinant Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing localization to golgi network and cytoplasm. Panel e shows SK-BR-3 cells with no expression of Lysozyme. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
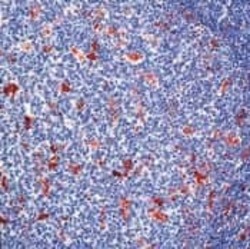
- Experimental details
- Formalin-fixed, paraffin-embedded human tonsil stained with Lysozyme antibody using peroxidase-conjugate and AEC chromogen. Note cytoplasmic staining of myeloid cells.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
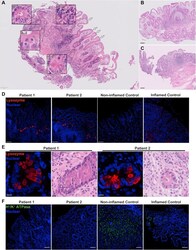
- Figure 1 Patients developed severe intestinal metaplasia in the gastric epithelium with the appearance of Paneth cells and loss of parietal cells. ( A-C ) Scans of H&E staining on formalin-fixed paraffin-embedded (FFPE) gastric sections were used to highlight metaplastic and inflammatory features of the patients. Insets : Arrowheads indicate the feature described in the legend pertaining to that particular inset. ( A ) Gastric section of patient 1 taken at 1 year of age showing severe intestinal metaplasia and patchy lymphocytic infiltration. Metaplasia was shown by the presence of villiforms ( arrows ), crypt-like structures ( A-1 ), granulated Paneth-like cells ( A-2 ), and a lack of recognizable parietal and foveolar cells. Inflammation is evident by the presence of patchy lymphocytic infiltrates ( A-3 ), and mitotic figures indicate a regenerative state ( A-4 ). Scans from gastric section of ( B ) patient 2 taken at 2 months of age alongside ( C ) patient 1 at 6 months of age showing the same extensive metaplasia and metaplastic features. Biopsies were performed at ( A and B ) Hamad General Hospital, and ( C ) Al Wakra Hospital. Each biopsy was performed by a different physician. ( A-C ) H&E slides were acquired with a slide scanner using a 40x objective. Insets were generated using digital zoom. ( D ) Lysozyme was detected by fluorescent immunohistochemistry (IHC) and confocal microscopy on FFPE sections. Nuclei were stained with Hoechst (blue). Lysozyme (red, polyclonal
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
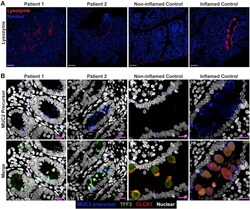
- Figure 3 Small bowel of the patients show unremarkable localization of lysozyme in Paneth cells and depletion of nonglycosylated MUC2 precursor compared with inflamed controls. ( A ) Confocal images of fluorescent immunohistochemical staining for lysozyme (red, polyclonal) shows normal localization of Paneth cells in the small intestines of both siblings. Confocal acquisition was performed using a 10x objective. Scale bars : 100 mum. Lysozyme staining was replicated in a minimum of 3 independent experiments including a total of 3 noninflamed controls and 4 inflamed controls. ( B ) Maximum intensity projection of confocal Z-stacks acquired from small-bowel sections stained for MUC2 precursor (blue, CCP58), TFF3 (green, B-1), and CLCA1 (red, EPR12254-88) with Hoechst nuclear counterstain (white). Staining shows little accumulation of MUC2 precursor in the patients compared with inflamed controls, but not with noninflamed controls, which appeared to have the lowest accumulation of the precursor. Patient sections also show reduced size of goblet cell thecae. Images were acquired using a 63x objective. Scale bar : 10 mum. Replicates included 2 noninflamed controls and 4 inflamed controls. Identical thresholding parameters for each marker were applied to all images in each respective panel.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
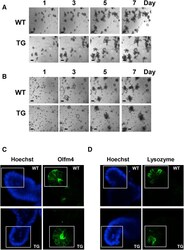
- Supplementary Figure 1 ( A and B ) PKD1 overexpression increases the area and complexity of enteroids. Representative low-magnification images of enteroids derived from the ileum of wild-type (WT) or PKD1 Tg ( A ) at passage 4 or ( B ) primary enteroids. These images complement the high-magnification images of enteroids shown in Figure 1 . ( C and D ) Impact of PKD1 overexpression on abundance of ISCs and Paneth cells. Additional representative ( C ) olfactomedin 4 (Olfm4) and ( D ) lysozyme staining from WT and PKD1-Tg mice-derived enteroids at passage 4. Hoechst staining is overexposed to show nuclei in the enteroids. Boxes represent the portion of enteroids used for quantification and shown in Figure 2 . Similar results were obtained in 4 independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
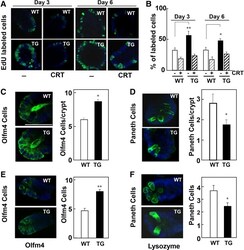
- Figure 2 Impact of PKD1 overexpression on cell proliferation and abundance of ISCs and Paneth cells. ( A ) 5-Ethynyl-2'-deoxyuridine (EdU) incorporation in enteroids derived from wild-type (WT, open bars ) and PKD1-Tg (TG, solid bars ) crypts in the absence or presence of CRT0066101 (1.0 mumol/L, added 24 h before EdU). ( B ) Values are means +- SEM; n = 10 buds. Similar results were obtained in 3 (day 3) and 4 (day 6) independent experiments. Supplementary Figure 4 shows a different assay of EdU incorporation. ( C-F ) Representative images of ( C and E ) olfactomedin 4 (Olfm4) and ( D and F ) lysozyme staining from ( C and D ) WT ( open bars ) and PKD1-Tg ( solid bars ) mice-derived enteroids at passage 4, or ( E and F ) freshly isolated crypts. Bars represent means +- SEM; n = 7-8 buds. Similar results were obtained in 4 independent experiments. * P < .05; ** P < .01; TG vs WT.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 5 ( See previous page ). Restoration of secretory epithelial lineages with Notch inhibition in mutant enteroids. ( A ) mRNA expression of EPCAM exons 3-4 (mutated region) over EPCAM exons 6-7 (unchanged region) in EDM from control (Con), control EDM treated with DAPT (Con w DAPT), mutant EDM (Mut), and mutant EDM treated with DAPT (Mut w DAPT) group. Decreased expression of EPCAM exons 3-4 in the Mut and Mut w DAPT groups confirms induction of mutation in the respective group. ( B ) mRNA expression for cell-type markers (lysozyme, mucin 2, CHGA) and transcription factors ( ATOH1 , HES1 , and NOTCH , neurogenin 3) in lysates from Control w DAPT or Con (n = 5 in all except neurogenin 3 where n = 3). The mRNA expression of lysozyme, mucin 2, CHGA, and neurogenin 3 were increased upon treatment with DAPT, while expression of HES1 and NOTCH was decreased. ( C ) mRNA expression for cell type markers (lysozyme, mucin 2, CHGA) and transcription factors ( ATOH1 , HES1 , and NOTCH , neurogenin 3) in lysates from Mut w DAPT or Mut (n = 4). The mRNA expression of lysozyme (n = 5), mucin 2 (n = 6), CHGA (n = 6), Atoh1 (n = 5), and neurogenin 3 (n = 3) were increased upon treatment with DAPT, while expression of HES1 and NOTCH (n = 6 in both cases) was not significantly changed. ( D ) Representative immunofluorescent images of CHGA for enteroendocrine cells (n = 4), lysozyme for Paneth Cells (n = 3), and bright field of PAS staining for goblet cells (n = 5) with quantification of ce
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
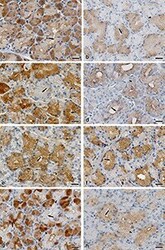
- Figure 7. Immunohistochemical staining for the detection of antimicrobial, Rab and SNARE proteins in the lacrimal gland. a) Lysozyme. b) Lactoferrin. c) Rab3d. d) Rab27b. e) VAMP4. f ) VAMP8. g) Syntaxin-4. h) Syntaxin-6. Arrows, type T cells; arrowheads, type A cells; scale bars: 20 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
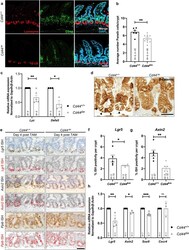
- Deletion of epithelial Cd44 reduces Wnt signaling in murine intestinal crypts. a Confocal image of IF staining on sections of the small intestine (SI) of Cd44 Deltaie and Cd44 +/+ mice using anti-lysozyme antibodies. Scale bar: 50 um. b Quantification of lysozyme-stained PCs in the crypts of Cd44 Deltaie and Cd44 +/+ mice. Error bars, +-SE. c qPCR of lysozyme and alpha-defensin5 (Lyz and Defa5) expression in intestinal crypts from Cd44 Deltaie and Cd44 +/+ mice. Gapdh and beta-Actin were used as reference genes. Error bars, +-SE. d Nuclear beta-catenin (arrowheads) IHC staining. Scale bar: 25 mum. e In situ hybridization (ISH) (brown, red) of Lgr5 and Axin2 expression in the SI. Positive control: Ppib . Error bars, +- SE. Scale bar: 50 um. f , g Quantification of ISH. Error bars, +-SE. h qPCR of Wnt target genes and stem cell marker expression in SI crypts isolated from Cd44 Deltaie and Cd44 +/+ mice. Non-Wnt-regulated control: Cxcr4 . Error bars, +-SE. Student's t test; * p < 0.05, ** p < 0.01, *** p < 0.001, ns not significant. N = number of mice, n = number of crypts.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
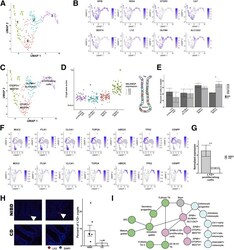
- Subcluster analysis of SPIB+ cells shows distinct lineages. ( A ) Uniform Manifold Approximation and Projection (UMAP) after subclustering of SPIB+ cells with labeling of the 4 identified subclusters. ( B ) UMAP of SPIB+ cell subclusters overlain with gene expression from genes marking the entire cluster or distinct subclusters. ( C ) SPIB+ cell subcluster UMAP following cell type assignment using highly enriched markers of the subclusters. ( D ) Crypt-axis scores (low near crypt bottom, high near crypt top) of subcluster-assigned cells uncovers a colonocyte signature in the OTOP2+/BEST4+ subcluster. Clusters are arranged on the x-axis by mean crypt-axis score. The size of the dot corresponds to the level of expression of SELENOP , a known marker of the top of the crypt. ( E ) Mean cluster cell abundances across NIBD and CD samples shows a significant increase in OTOP2+/BEST4+ subcluster in CD. Dots show abundances of individual samples. ( F ) UMAP of either all clusters ( top ) or SPIB+ cell subclusters ( bottom ) overlain with the expression of markers of secretory progenitor cells. ( G ) Mean normalized expression of LYZ in NIBD and CD samples in the LYZ+ proliferating subcluster. ( H ) LYZ-detected immunofluorescence in colonic crypts from either NIBD (n = 181 crypts, 5 patients) or CD (n = 76 crypts, 2 patients) patient examples ( left ) and quantified by sample ( right ). Mean percentage of crypts positive for LYZ in NIBD or CD. ( I ) Lineage reconstruction as determine
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot