Antibody data
- Antibody Data
- Antigen structure
- References [8]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Other assay [4]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 38-8200 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Claudin 12 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 0.25 mg/mL
- Storage
- -20°C
Submitted references Organic osmolytes increase expression of specific tight junction proteins in skin and alter barrier function in keratinocytes.
Circulating Ouabain Modulates Expression of Claudins in Rat Intestine and Cerebral Blood Vessels.
Glycocholic acid and butyrate synergistically increase vitamin D-induced calcium uptake in Caco-2 intestinal epithelial cell monolayers.
Claudin-12 is not required for blood-brain barrier tight junction function.
Vitamin D Receptor Deletion Leads to the Destruction of Tight and Adherens Junctions in Lungs.
Expression of Tight Junction Proteins and Cadherin 17 in the Small Intestine of Young Goats Offered a Reduced N and/or Ca Diet.
Vitamin D receptor knockout mice exhibit elongated intestinal microvilli and increased ezrin expression.
Distinct molecular composition of blood and lymphatic vascular endothelial cell junctions establishes specific functional barriers within the peripheral lymph node.
El-Chami C, Foster AR, Johnson C, Clausen RP, Cornwell P, Haslam IS, Steward MC, Watson REB, Young HS, O'Neill CA
The British journal of dermatology 2021 Mar;184(3):482-494
The British journal of dermatology 2021 Mar;184(3):482-494
Circulating Ouabain Modulates Expression of Claudins in Rat Intestine and Cerebral Blood Vessels.
Markov AG, Fedorova AA, Kravtsova VV, Bikmurzina AE, Okorokova LS, Matchkov VV, Cornelius V, Amasheh S, Krivoi II
International journal of molecular sciences 2020 Jul 17;21(14)
International journal of molecular sciences 2020 Jul 17;21(14)
Glycocholic acid and butyrate synergistically increase vitamin D-induced calcium uptake in Caco-2 intestinal epithelial cell monolayers.
Casselbrant A, Fändriks L, Wallenius V
Bone reports 2020 Dec;13:100294
Bone reports 2020 Dec;13:100294
Claudin-12 is not required for blood-brain barrier tight junction function.
Castro Dias M, Coisne C, Baden P, Enzmann G, Garrett L, Becker L, Hölter SM, German Mouse Clinic Consortium, Hrabě de Angelis M, Deutsch U, Engelhardt B
Fluids and barriers of the CNS 2019 Sep 12;16(1):30
Fluids and barriers of the CNS 2019 Sep 12;16(1):30
Vitamin D Receptor Deletion Leads to the Destruction of Tight and Adherens Junctions in Lungs.
Chen H, Lu R, Zhang YG, Sun J
Tissue barriers 2018;6(4):1-13
Tissue barriers 2018;6(4):1-13
Expression of Tight Junction Proteins and Cadherin 17 in the Small Intestine of Young Goats Offered a Reduced N and/or Ca Diet.
Elfers K, Marr I, Wilkens MR, Breves G, Langeheine M, Brehm R, Muscher-Banse AS
PloS one 2016;11(4):e0154311
PloS one 2016;11(4):e0154311
Vitamin D receptor knockout mice exhibit elongated intestinal microvilli and increased ezrin expression.
Kühne H, Hause G, Grundmann SM, Schutkowski A, Brandsch C, Stangl GI
Nutrition research (New York, N.Y.) 2016 Feb;36(2):184-92
Nutrition research (New York, N.Y.) 2016 Feb;36(2):184-92
Distinct molecular composition of blood and lymphatic vascular endothelial cell junctions establishes specific functional barriers within the peripheral lymph node.
Pfeiffer F, Kumar V, Butz S, Vestweber D, Imhof BA, Stein JV, Engelhardt B
European journal of immunology 2008 Aug;38(8):2142-55
European journal of immunology 2008 Aug;38(8):2142-55
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
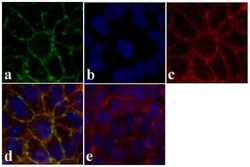
- Experimental details
- Immunofluorescent analysis of Claudin-12 was done on 90% confluent log phase Caco-2 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with Claudin-12 rabbit polyclonal Antibody (Product # 38-8200) at 2 µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing junctional localization. Panel e shows no primary antibody control. The images were captured at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
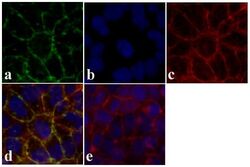
- Experimental details
- Immunofluorescent analysis of Claudin-12 was done on 90% confluent log phase Caco-2 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with Claudin-12 rabbit polyclonal Antibody (Product # 38-8200) at 2 µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing junctional localization. Panel e shows no primary antibody control. The images were captured at 20X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
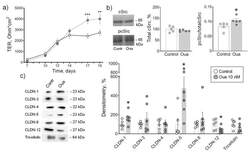
- Experimental details
- Figure 1 Epithelial barrier formation in IPEC-J2 cells grown in control medium and in the presence of 10 nM ouabain (Oua). After 7 days of culture, transepithelial resistance (TER) values were determined every 2-3 days using an EVOM volt-ohmmeter; after 19 days, cells were used for subsequent analysis. ( a ) Transepithelial resistance (TER) ( n = 6 for each group). ( b ) The expression level of total cSrc (central plot, the relative expression of cSrc protein shown as a percentage of average level in control samples taken for 100%) and the cSrc-kinase activation by phosphorylation (right plot, shown as a ratio between immunoblot intensity corresponding to phosphorylated pcSrc over total cSrc, as it is shown in the representative immunoblots in left panel) ( n = 5 for each group). ( c ) Western blot analysis of claudin (CLDN) and tricellulin expression ( n = 6 for each group); left panel shows representative immunoblots. Original images for Western blots using Stain-Free gels as a loading control are shown in Supplemental Materials . The number of symbols corresponds to the number of samples. One-way ANOVA with Dunnett correction: * p < 0.05, ** p < 0.01 and *** p < 0.001 compared with the corresponding control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
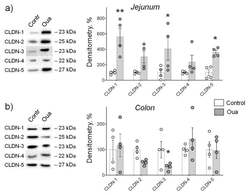
- Experimental details
- Figure 3 Chronic exposure to ouabain (Oua) differently alters claudin expression in rat jejunum ( a ) and colon ( b ). Rats were intraperitoneally injected with ouabain (1 µg/kg) for 4 days. Western blot analysis of claudins (CLDN) expression ( n = 4 for each group); left panel shows representative immunoblots. Original images for Western blots using Stain-Free gels as a loading control are shown in Supplemental Materials . The number of symbols corresponds to the number of samples. One-way ANOVA with Dunnett correction: * p < 0.05 and ** p < 0.01 compared with the corresponding control (vehicle treated group).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
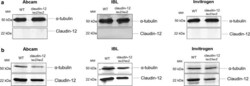
- Fig. 4 Lack of reagents allowing to detect expression of claudin-12 protein. Immunoblot analysis of claudin-12 protein levels in freshly isolated brain microvessels ( a ) and in skeletal muscle ( b ) from WT and claudin-12 lacZ/lacZ C57BL/6J mice, using three different polyclonal anti-claudin-12 antibodies. Three independent immunoblots for both skeletal muscle and brain microvessels were analysed
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
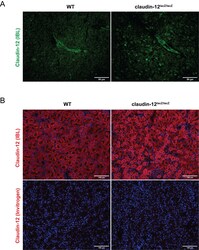
- Additional file 4. Lack of reagents allowing to localize expression of claudin-12 protein. (A) Immunofluorescence staining of frozen brain sections from WT and claudin-12 lacZ/lacZ C57BL/6J mice with the anti-claudin-12 antibody from IBL represented in green produces indistinguishable vascular and apparently junction associated staining in the brain tissues of both, WT and the claudin-12 lacZ/lacZ C57BL/6J mice. Scale bar = 50 mum. (B) Immunofluorescence staining of frozen liver sections from WT and claudin-12 lacZ/lacZ C57BL/6J mice, using two different antibodies for claudin-12, represented in red. Notice how the antibody from IBL stains WT and claudin-12 lacZ/lacZ tissue, while the anti-claudin-12 antibody from Invitrogen does not recognize claudin-12 in neither of the samples. Nuclei are stained with DAPI (blue). Three independent stainings were done. Scale bar = 100 mum.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry