Antibody data
- Antibody Data
- Antigen structure
- References [2]
- Comments [0]
- Validations
- Immunocytochemistry [1]
- Flow cytometry [1]
- Other assay [1]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 39-7500 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- c-Jun Monoclonal Antibody (ZC008)
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Reactivity
- Human, Mouse, Rabbit
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- ZC008
- Vial size
- 100 µg
- Concentration
- 0.5 mg/mL
- Storage
- -20°C
Submitted references The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression.
Quantifying antibody binding on protein microarrays using microarray nonlinear calibration.
Tyagi AM, Yu M, Darby TM, Vaccaro C, Li JY, Owens JA, Hsu E, Adams J, Weitzmann MN, Jones RM, Pacifici R
Immunity 2018 Dec 18;49(6):1116-1131.e7
Immunity 2018 Dec 18;49(6):1116-1131.e7
Quantifying antibody binding on protein microarrays using microarray nonlinear calibration.
Yu X, Wallstrom G, Magee DM, Qiu J, Mendoza DE, Wang J, Bian X, Graves M, LaBaer J
BioTechniques 2013 May;54(5):257-64
BioTechniques 2013 May;54(5):257-64
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
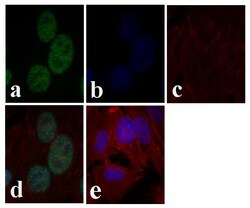
- Experimental details
- Immunofluorescent analysis of cJUN was done on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with cJUN Mouse Monoclonal Antibody (Product # 39-7500) at 1 µg/mL and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Rabbit Anti-Mouse IgG Secondary Antibody (Product # A-11059) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing nuclear localization. Panel e shows no primary antibody control. The images were captured at 20X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
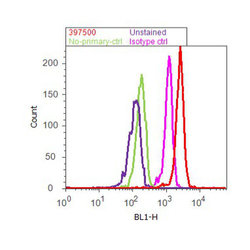
- Experimental details
- Flow cytometry analysis of cJun was done on HeLa cells treated with EGF (100µg/mL, 10 minutes). Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Tritonª X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with c-Jun Mouse Monoclonal Antibody (397500, red histogram) or with mouse isotype control (pink histogram) at 3-5 µg/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor¨ 488 Rabbit Anti-Mouse Secondary Antibody (A11059) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune¨ Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
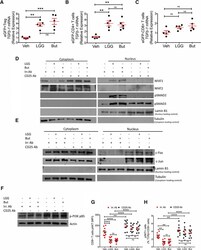
- Experimental details
- Figure 7. Effects of LGG and Butyrate (But) on TGFb1 Production by BM T Cells, onNFAT1/2 and SMAD2/3 Activation, and on PI3K and Akt Signaling in BMCD8 + T Cells (A-C) TGFb1 mRNA expression by FACS-sorted BM conventionaleGFPCD4 + T cells, eGFPCD8 + T cells, andeGFP + Treg cells. DEREG (eGFP.Foxp3) reporter mice were treatedwith vehicle, LGG, or butyrate for 4 weeks. BM cells were sorted at the end ofthe treatment period. (D) Immunoblot analysis for the detection of NFAT1, NFAT2, pSMAD2, andpSMAD3 in purified BM CD8 + T cells. (E) Immunoblot analysis for the detection of c-Jun and c-Fos inpurified BM CD8 + T cells. (D and E) Fresh BM CD8 + T cells were pooled together andthen used for obtaining nuclear and cytoplasmic fractions. Laminin B1 was usedas nuclear loading control. Tubulin was used as cytoplasmic loading control. (F) Immunoblot analysis for the detection of Phospho-PI3K p85 in thewhole lysate from BM CD8 + T cells. (G and H) pAKT levels in BM CD8 + T cells and percent ofpAKT + BM CD8 + T cells, as determined by flowcytometry. (D-F) Conventionally raised WT mice were treated with vehicle,LGG, or butyrate for 4 weeks. BM CD8 + T cells were purified at theend of the treatment period using the EasySep Mouse CD8 + T CellIsolation Kit. One representative experiment of 3 experiments. Data were expressed as mean +- SEM, n = 5-10 mice pergroup in (A)-(C), (G), and (H). Data were analyzed by two-way ANOVA andpost hoc tests applying the Bonferroni correction for mu
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry