Antibody data
- Antibody Data
- Antigen structure
- References [9]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunoprecipitation [2]
- Immunohistochemistry [2]
- Flow cytometry [2]
- Other assay [8]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 436400 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- PARP1 Monoclonal Antibody (123)
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Reactivity
- Human, Mouse, Rat, Canine
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 123
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- -20°C
Submitted references Infigratinib (BGJ 398), a Pan-FGFR Inhibitor, Targets P-Glycoprotein and Increases Chemotherapeutic-Induced Mortality of Multidrug-Resistant Tumor Cells.
Modulation of Epithelial Mesenchymal Transition after AGTR-1 Gene Edition by Crispr/Cas9 and Losartan Treatment in Mammary Tumor Cell Line: A Comparative Study between Human and Canine Species.
Inhibition of AKT-Signaling Sensitizes Soft Tissue Sarcomas (STS) and Gastrointestinal Stromal Tumors (GIST) to Doxorubicin via Targeting of Homology-Mediated DNA Repair.
Proteomics Profiling of KAIMRC1 in Comparison to MDA-MB231 and MCF-7.
Establishment and characterization of a triple negative basal-like breast cancer cell line with multi-drug resistance.
USP17-mediated deubiquitination and stabilization of HDAC2 in cigarette smoke extract-induced inflammation.
TIMELESS Forms a Complex with PARP1 Distinct from Its Complex with TIPIN and Plays a Role in the DNA Damage Response.
The Deubiquitinase USP17 Regulates the Stability and Nuclear Function of IL-33.
Deubiquitination and stabilization of IL-33 by USP21.
Boichuk S, Dunaev P, Mustafin I, Mani S, Syuzov K, Valeeva E, Bikinieva F, Galembikova A
Biomedicines 2022 Mar 3;10(3)
Biomedicines 2022 Mar 3;10(3)
Modulation of Epithelial Mesenchymal Transition after AGTR-1 Gene Edition by Crispr/Cas9 and Losartan Treatment in Mammary Tumor Cell Line: A Comparative Study between Human and Canine Species.
Moschetta-Pinheiro MG, Colombo J, Godoy BLV, Balan JF, Nascimento BC, Zuccari DAPC
Life (Basel, Switzerland) 2021 Dec 18;11(12)
Life (Basel, Switzerland) 2021 Dec 18;11(12)
Inhibition of AKT-Signaling Sensitizes Soft Tissue Sarcomas (STS) and Gastrointestinal Stromal Tumors (GIST) to Doxorubicin via Targeting of Homology-Mediated DNA Repair.
Boichuk S, Bikinieva F, Nurgatina I, Dunaev P, Valeeva E, Aukhadieva A, Sabirov A, Galembikova A
International journal of molecular sciences 2020 Nov 22;21(22)
International journal of molecular sciences 2020 Nov 22;21(22)
Proteomics Profiling of KAIMRC1 in Comparison to MDA-MB231 and MCF-7.
Alghanem B, Ali R, Nehdi A, Al Zahrani H, Altolayyan A, Shaibah H, Baz O, Alhallaj A, Moresco JJ, Diedrich JK, Yates JR 3rd, Boudjelal M
International journal of molecular sciences 2020 Jun 18;21(12)
International journal of molecular sciences 2020 Jun 18;21(12)
Establishment and characterization of a triple negative basal-like breast cancer cell line with multi-drug resistance.
Boichuk S, Galembikova A, Sitenkov A, Khusnutdinov R, Dunaev P, Valeeva E, Usolova N
Oncology letters 2017 Oct;14(4):5039-5045
Oncology letters 2017 Oct;14(4):5039-5045
USP17-mediated deubiquitination and stabilization of HDAC2 in cigarette smoke extract-induced inflammation.
Song H, Tao L, Chen C, Pan L, Hao J, Ni Y, Li D, Li B, Shi G
International journal of clinical and experimental pathology 2015;8(9):10707-15
International journal of clinical and experimental pathology 2015;8(9):10707-15
TIMELESS Forms a Complex with PARP1 Distinct from Its Complex with TIPIN and Plays a Role in the DNA Damage Response.
Young LM, Marzio A, Perez-Duran P, Reid DA, Meredith DN, Roberti D, Star A, Rothenberg E, Ueberheide B, Pagano M
Cell reports 2015 Oct 20;13(3):451-459
Cell reports 2015 Oct 20;13(3):451-459
The Deubiquitinase USP17 Regulates the Stability and Nuclear Function of IL-33.
Ni Y, Tao L, Chen C, Song H, Li Z, Gao Y, Nie J, Piccioni M, Shi G, Li B
International journal of molecular sciences 2015 Nov 24;16(11):27956-66
International journal of molecular sciences 2015 Nov 24;16(11):27956-66
Deubiquitination and stabilization of IL-33 by USP21.
Tao L, Chen C, Song H, Piccioni M, Shi G, Li B
International journal of clinical and experimental pathology 2014;7(8):4930-7
International journal of clinical and experimental pathology 2014;7(8):4930-7
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
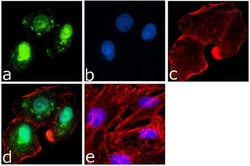
- Experimental details
- Immunofluorescence analysis of PARP was done on 70% confluent log phase A549 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with PARP Mouse Monoclonal Antibody (Product # 436400) at 1 µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing both nuclear and cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
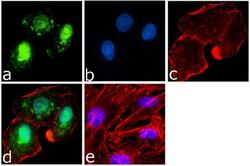
- Experimental details
- Immunofluorescence analysis of PARP was done on 70% confluent log phase A549 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with PARP Mouse Monoclonal Antibody (Product # 436400) at 1 µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing both nuclear and cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunoprecipitation of PARP1. Cells were seeded onto a 10 cm plate and allowed to grow overnight in DMEM/F12 + 10% FBS at 37°C to achieve ~80% confluence. Media was changed and menadione in ethanol was added to a final concentration of 100 µM. Cells were incubated at 37°C for 20 minutes then washed once in 1xPBS and moved to 4°C. Cells were collected in 500 µl PBS with 0.5% triton X-100 and 0.5 mM PMSF. Cells were sonicated on ice, sample was spun at 15,000 rpm at 4°C for 5 minutes and supernatant was collected. 30 µl was collected for input and 2x80 µl was collected for antibody vs IgG control (Bead). 1 µl monoclonal PARP1 antibody (Product # 436400) was added to rotated overnight at 4°C (no antibody added to bead lanes). The next day, 5 µl protein G beads in BSA was added to the antibody and IgG control tubes and rotated at 4°C for 1.5 hours. After incubation, four washes in ice cold 1xPBS with 0.5% triton X-100 were performed. Finally, 20 µl 1xSDS sample buffer was added to IP/bead and 10 µl 4xSDS sample buffer was added to input and all samples were heated to 95°C for 5 minutes. 10 µl of each sample with input diluted 1:5 were run on a 4-12% Bis-Tris gel and probed with rbPARP1 antibody. Data courtesy of Antibody Data Exchange Program.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
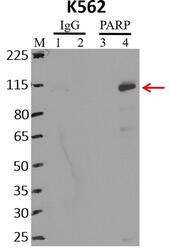
- Experimental details
- Immunoprecipitation of PARP was performed on K562 cells. Antigen-antibody complexes were formed by incubating approximately 500 µg whole cell lysate with 5 µg of PARP monoclonal antibody (Product # 436400) rotating 60 min at RT. The immune complexes were captured on 625 µg of anti-mouse coated Dynabeads (Product # 11202D), washed extensively, and eluted with NuPAGE™ LDS Sample Buffer (Product # NP0007). Samples were resolved onto NuPAGE™ 4-12% Bis-Tris gel (Product # NP0335BOX). Lanes 1 and 3 are input and lanes 2 and 4 are IP. Proteins were transferred to PVDF membrane (Product # IB23001). Membrane was blocked in 5% milk. Target was detected using a PARP monoclonal antibody (Product # 436400) at a dilution of 1:2000, followed by a 1:4000 dilution of secondary antibody. Chemiluminescent detection was performed using ECL Western Blotting Substrate (Product # 32106). Data courtesy of the Yeo lab as part of the ENCODE project (www.encodeproject.org).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
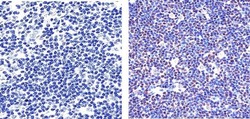
- Experimental details
- Immunohistochemistry analysis of PARP showing staining in the nucleus of paraffin-embedded human diffuse large B cell lymphoma tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a PARP Mouse Monoclonal Antibody (Product # 436400) diluted in 3% BSA-PBS at a dilution of 1:100 overnight at 4ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
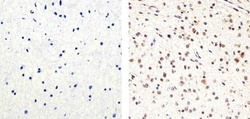
- Experimental details
- Immunohistochemistry analysis of PARP showing staining in the nucleus of paraffin-embedded human astroglioma (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a PARP Mouse Monoclonal Antibody (Product # 436400) diluted in 3% BSA-PBS at a dilution of 1:20 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
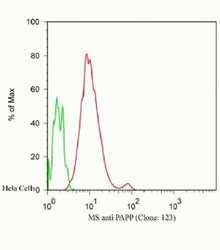
- Experimental details
- Flow Cytometric analysis of Hela cells, green: 0 µg/mL of primary antibody, red: 2.5 µg/mL of primary antibody using IgG (H+L), Goat F (ab´)2 Anti-Mouse, (R-PE) (Product # M350043) secondary antibody, and Product # GAS-003 for Fix and Perm.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
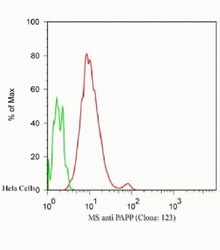
- Experimental details
- Flow Cytometric analysis of Hela cells, green: 0 µg/mL of primary antibody, red: 2.5 µg/mL of primary antibody using IgG (H+L), Goat F (ab´)2 Anti-Mouse, (R-PE) (Product # M350043) secondary antibody, and Product # GAS-003 for Fix and Perm.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
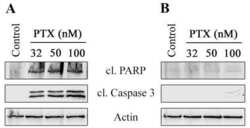
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
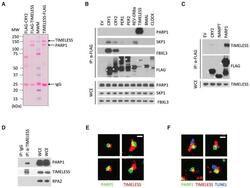
- Experimental details
- Figure 1 TIMELESS Physically Interacts with PARP1 (A) HEK293T cells were transfected with the indicated FLAG-tagged proteins. 24 hr post-transfection, cells were harvested and lysed. Cell extracts were subjected to immunoprecipitation with alpha-FLAG resin and stained with Ponceau S. MWM, molecular weight markers. (B) The experiment was performed as in (A), except that whole-cell extracts (WCEs) were subjected to immunoprecipitation (IP) with alpha-FLAG resin and immunoblotted as indicated. EV, empty vector. Asterisks denote FLAG-tagged proteins. (C) The experiment was performed as in (B). (D) Lysates of U2OS cells were immunoprecipitated with either an antibody against TIMELESS or a rabbit IgG and immunoblotted as indicated. (E) Super-resolution images of TIMELESS and PARP1 in U2OS cells selected from regions of the nucleus shown in Figure S1D . Scale bar, 400 nm. (F) Super-resolution images of TUNEL, TIMELESS, and PARP1 in neocarzinostatin treated U2OS cells showing that TIMELESS and PARP1 are found at DNA damage sites.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2 Identification of the PARP1 Binding Site in TIMELESS (A) HEK293T cells were transfected with either an empty vector (EV), FLAG-tagged TIMELESS, or FLAG-tagged TIMELESS mutants as indicated. Asterisks indicate the insertion of a STOP codon after the numerically specified codon. 24 hr post-transfection, WCEs were subjected to IP with alpha-FLAG resin and immunoblotted as indicated. (B) Schematic representation of TIMELESS mutants tested for binding to PARP1. TIMELESS mutants that interacted with endogenous PARP1 are designated with the symbol (+); those unable to co-precipitate PARP1 are designated with the symbol (-). (C) The experiment was performed as in (A), except that different TIMELESS mutants were used.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
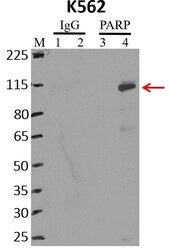
- Experimental details
- RNA immunoprecipitation (RIP) western of PARP was performed on K562 cells. Antigen-antibody complexes were formed by incubating approximately 500 µg whole cell lysate with 5 µg of PARP monoclonal antibody (Product # 436400) rotating 60 min at RT. The immune complexes were captured on 625 µg of anti-mouse coated Dynabeads (Product # 11202D), washed extensively, and eluted with NuPAGE™ LDS Sample Buffer (Product # NP0007). Samples were resolved onto NuPAGE™ 4-12% Bis-Tris gel (Product # NP0335BOX). Lanes 1 and 3 are input and lanes 2 and 4 are IP. Proteins were transferred to PVDF membrane (Product # IB23001). Membrane was blocked in 5% milk. Target was detected using a PARP monoclonal antibody (Product # 436400) at a dilution of 1:2000, followed by a 1:4000 dilution of secondary antibody. Chemiluminescent detection was performed using ECL Western Blotting Substrate (Product # 32106). Data courtesy of the Yeo lab as part of the ENCODE project (www.encodeproject.org).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunoprecipitation of PARP1. Cells were seeded onto a 10 cm plate and allowed to grow overnight in DMEM/F12 + 10% FBS at 37°C to achieve ~80% confluence. Media was changed and menadione in ethanol was added to a final concentration of 100 µM. Cells were incubated at 37°C for 20 minutes then washed once in 1xPBS and moved to 4°C. Cells were collected in 500 µl PBS with 0.5% triton X-100 and 0.5 mM PMSF. Cells were sonicated on ice, sample was spun at 15,000 rpm at 4°C for 5 minutes and supernatant was collected. 30 µl was collected for input and 2x80 µl was collected for antibody vs IgG control (Bead). 1 µl monoclonal PARP1 antibody (Product # 436400) was added to rotated overnight at 4°C (no antibody added to bead lanes). The next day, 5 µl protein G beads in BSA was added to the antibody and IgG control tubes and rotated at 4°C for 1.5 hours. After incubation, four washes in ice cold 1xPBS with 0.5% triton X-100 were performed. Finally, 20 µl 1xSDS sample buffer was added to IP/bead and 10 µl 4xSDS sample buffer was added to input and all samples were heated to 95°C for 5 minutes. 10 µl of each sample with input diluted 1:5 were run on a 4-12% Bis-Tris gel and probed with rbPARP1 antibody. Data courtesy of Antibody Data Exchange Program.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
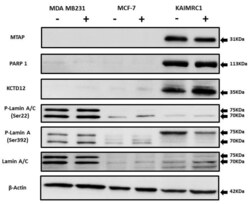
- Experimental details
- Figure 5 Expression of MTAP, PARP1, KCTD12, and Lamin A/C in cancer cell lines: Western blot analysis of protein expression of MTAP, PARP1, KCTD12, Lamin A/C, and phosphorylated Lamin A/C in MDA MB231, MCF-7, and KAIMRC1 cells. Preparation of cell lysates and western blot were performed as described under the Materials and Methods section. In both normal (+) and serum-starved (-) conditions, KAIMRC1 cells showed strong expression of MTAP, PARP1, and KCTD12 compared to MDA-MB231 and MCF-7 cells. Moreover, KAIMRC1 cells showed weak expression of Lamin A/C and phosphorylated Lamin A/C in comparison to MDA MB231 cells, thus validating our proteomics results.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
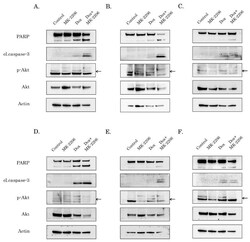
- Experimental details
- Figure 2 AKT inhibition potentiates pro-apoptotic activity of Dox in STS and GIST. Immunoblot analysis for apoptosis markers (cleaved forms of poly-(ADP)-ribose-polymerase (PARP) and caspase-3) in RD rhabdomyosarcoma ( A ), U2-OS osteosarcoma ( B ), HT-1080 fibrosarcoma ( C ), GIST T-1R ( D ), GIST 430 ( E ) and SK-LMS-1 leiomyosarcoma ( F ) cells after treatment with DMSO (control), Dox (0.25 g/mL) and MK-2206 (5 M) alone and in combination (e.g., Dox + MK-2206) for 72 h. The lysates were also probed for total and phosphorylated forms of AKT to illustrate AKT inhibition by MK-2206. pAKT expression is shown by arrows. Actin was used as a loading control.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry