Antibody data
- Antibody Data
- Antigen structure
- References [27]
- Comments [0]
- Validations
- Immunocytochemistry [4]
- Immunohistochemistry [4]
- Flow cytometry [1]
- Other assay [22]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 44-618G - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Phospho-PYK2 (Tyr402) Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- This product contains enough material for 10 mini-blots. Antibody has been negatively preadsorbed using a nonphosphopeptide then purified by epitope-specific affinity chromatography. The antibody has been used in western blotting and immunohistochemistry. Other applications may work but have not been tested. Positive controls used were CEF cell extracts +/- Pyk2 transfection or A431 whole cell lysate.
- Reactivity
- Human, Mouse, Rat, Chicken/Avian
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- lot-specific
- Storage
- -20°C
Submitted references PYK2 senses calcium through a disordered dimerization and calmodulin-binding element.
PYK2 Is Involved in Premalignant Acinar Cell Reprogramming and Pancreatic Ductal Adenocarcinoma Maintenance by Phosphorylating β-Catenin(Y654).
BDNF increases synaptic NMDA receptor abundance by enhancing the local translation of Pyk2 in cultured hippocampal neurons.
The human ion channel TRPM2 modulates neuroblastoma cell survival and mitochondrial function through Pyk2, CREB, and MCU activation.
Adhesion structures in leukemia cells and their regulation by Src family kinases.
Atypical Endocannabinoid Signaling Initiates a New Form of Memory-Related Plasticity at a Cortical Input to Hippocampus.
Pyk2 inhibition promotes contractile differentiation in arterial smooth muscle.
Pyk2 is essential for astrocytes mobility following brain lesion.
A role for the tyrosine kinase Pyk2 in depolarization-induced contraction of vascular smooth muscle.
PYK2 selectively mediates signals for growth versus differentiation in response to stretch of spontaneously active vascular smooth muscle.
CD28 and CD3 have complementary roles in T-cell traction forces.
Integrin dynamics produce a delayed stage of long-term potentiation and memory consolidation.
Biophysical stimulation induces demyelination via an integrin-dependent mechanism.
Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation.
ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration.
ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration.
The role of proline-rich protein tyrosine kinase 2 in differentiation-dependent signaling in human epidermal keratinocytes.
Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells.
Activation of FAK is necessary for the osteogenic differentiation of human mesenchymal stem cells on laminin-5.
Analyzing FAK and Pyk2 in early integrin signaling events.
Role of PYK2 in the development of obesity and insulin resistance.
Focal adhesion kinase plays a pivotal role in herpes simplex virus entry.
Proline-rich tyrosine kinase 2 (Pyk2) mediates vascular endothelial-cadherin-based cell-cell adhesion by regulating beta-catenin tyrosine phosphorylation.
PYK2 regulates SERCA2 gene expression in neonatal rat ventricular myocytes.
Glucose activates protein kinase C-zeta /lambda through proline-rich tyrosine kinase-2, extracellular signal-regulated kinase, and phospholipase D: a novel mechanism for activating glucose transporter translocation.
Salicylate Inhibits Phosphorylation of the Nonreceptor Tyrosine Kinases, Proline-Rich Tyrosine Kinase 2 and c-Src.
Salicylate Inhibits Phosphorylation of the Nonreceptor Tyrosine Kinases, Proline-Rich Tyrosine Kinase 2 and c-Src.
Momin AA, Mendes T, Barthe P, Faure C, Hong S, Yu P, Kadaré G, Jaremko M, Girault JA, Jaremko Ł, Arold ST
Communications biology 2022 Aug 9;5(1):800
Communications biology 2022 Aug 9;5(1):800
PYK2 Is Involved in Premalignant Acinar Cell Reprogramming and Pancreatic Ductal Adenocarcinoma Maintenance by Phosphorylating β-Catenin(Y654).
Gao C, Chen G, Zhang DH, Zhang J, Kuan SF, Hu W, Esni F, Gao X, Guan JL, Chu E, Hu J
Cellular and molecular gastroenterology and hepatology 2019;8(4):561-578
Cellular and molecular gastroenterology and hepatology 2019;8(4):561-578
BDNF increases synaptic NMDA receptor abundance by enhancing the local translation of Pyk2 in cultured hippocampal neurons.
Afonso P, De Luca P, Carvalho RS, Cortes L, Pinheiro P, Oliveiros B, Almeida RD, Mele M, Duarte CB
Science signaling 2019 Jun 18;12(586)
Science signaling 2019 Jun 18;12(586)
The human ion channel TRPM2 modulates neuroblastoma cell survival and mitochondrial function through Pyk2, CREB, and MCU activation.
Hirschler-Laszkiewicz I, Chen SJ, Bao L, Wang J, Zhang XQ, Shanmughapriya S, Keefer K, Madesh M, Cheung JY, Miller BA
American journal of physiology. Cell physiology 2018 Oct 1;315(4):C571-C586
American journal of physiology. Cell physiology 2018 Oct 1;315(4):C571-C586
Adhesion structures in leukemia cells and their regulation by Src family kinases.
Röselová P, Obr A, Holoubek A, Grebeňová D, Kuželová K
Cell adhesion & migration 2018 May 4;12(3):286-298
Cell adhesion & migration 2018 May 4;12(3):286-298
Atypical Endocannabinoid Signaling Initiates a New Form of Memory-Related Plasticity at a Cortical Input to Hippocampus.
Wang W, Jia Y, Pham DT, Palmer LC, Jung KM, Cox CD, Rumbaugh G, Piomelli D, Gall CM, Lynch G
Cerebral cortex (New York, N.Y. : 1991) 2018 Jul 1;28(7):2253-2266
Cerebral cortex (New York, N.Y. : 1991) 2018 Jul 1;28(7):2253-2266
Pyk2 inhibition promotes contractile differentiation in arterial smooth muscle.
Grossi M, Bhattachariya A, Nordström I, Turczyńska KM, Svensson D, Albinsson S, Nilsson BO, Hellstrand P
Journal of cellular physiology 2017 Nov;232(11):3088-3102
Journal of cellular physiology 2017 Nov;232(11):3088-3102
Pyk2 is essential for astrocytes mobility following brain lesion.
Giralt A, Coura R, Girault JA
Glia 2016 Apr;64(4):620-34
Glia 2016 Apr;64(4):620-34
A role for the tyrosine kinase Pyk2 in depolarization-induced contraction of vascular smooth muscle.
Mills RD, Mita M, Nakagawa J, Shoji M, Sutherland C, Walsh MP
The Journal of biological chemistry 2015 Apr 3;290(14):8677-92
The Journal of biological chemistry 2015 Apr 3;290(14):8677-92
PYK2 selectively mediates signals for growth versus differentiation in response to stretch of spontaneously active vascular smooth muscle.
Bhattachariya A, Turczyńska KM, Grossi M, Nordström I, Buckbinder L, Albinsson S, Hellstrand P
Physiological reports 2014 Jul 16;2(7)
Physiological reports 2014 Jul 16;2(7)
CD28 and CD3 have complementary roles in T-cell traction forces.
Bashour KT, Gondarenko A, Chen H, Shen K, Liu X, Huse M, Hone JC, Kam LC
Proceedings of the National Academy of Sciences of the United States of America 2014 Feb 11;111(6):2241-6
Proceedings of the National Academy of Sciences of the United States of America 2014 Feb 11;111(6):2241-6
Integrin dynamics produce a delayed stage of long-term potentiation and memory consolidation.
Babayan AH, Kramár EA, Barrett RM, Jafari M, Häettig J, Chen LY, Rex CS, Lauterborn JC, Wood MA, Gall CM, Lynch G
The Journal of neuroscience : the official journal of the Society for Neuroscience 2012 Sep 12;32(37):12854-61
The Journal of neuroscience : the official journal of the Society for Neuroscience 2012 Sep 12;32(37):12854-61
Biophysical stimulation induces demyelination via an integrin-dependent mechanism.
Lin MY, Frieboes LS, Forootan M, Palispis WA, Mozaffar T, Jafari M, Steward O, Gall CM, Gupta R
Annals of neurology 2012 Jul;72(1):112-23
Annals of neurology 2012 Jul;72(1):112-23
Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation.
Lim ST, Chen XL, Tomar A, Miller NL, Yoo J, Schlaepfer DD
The Journal of biological chemistry 2010 Jul 9;285(28):21526-36
The Journal of biological chemistry 2010 Jul 9;285(28):21526-36
ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration.
Allingham MJ, van Buul JD, Burridge K
Journal of immunology (Baltimore, Md. : 1950) 2007 Sep 15;179(6):4053-64
Journal of immunology (Baltimore, Md. : 1950) 2007 Sep 15;179(6):4053-64
ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration.
Allingham MJ, van Buul JD, Burridge K
Journal of immunology (Baltimore, Md. : 1950) 2007 Sep 15;179(6):4053-64
Journal of immunology (Baltimore, Md. : 1950) 2007 Sep 15;179(6):4053-64
The role of proline-rich protein tyrosine kinase 2 in differentiation-dependent signaling in human epidermal keratinocytes.
Schindler EM, Baumgartner M, Gribben EM, Li L, Efimova T
The Journal of investigative dermatology 2007 May;127(5):1094-106
The Journal of investigative dermatology 2007 May;127(5):1094-106
Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells.
Salasznyk RM, Klees RF, Williams WA, Boskey A, Plopper GE
Experimental cell research 2007 Jan 1;313(1):22-37
Experimental cell research 2007 Jan 1;313(1):22-37
Activation of FAK is necessary for the osteogenic differentiation of human mesenchymal stem cells on laminin-5.
Salasznyk RM, Klees RF, Boskey A, Plopper GE
Journal of cellular biochemistry 2007 Feb 1;100(2):499-514
Journal of cellular biochemistry 2007 Feb 1;100(2):499-514
Analyzing FAK and Pyk2 in early integrin signaling events.
Bernard-Trifilo JA, Lim ST, Hou S, Schlaepfer DD, Ilic D
Current protocols in cell biology 2006 Apr;Chapter 14:Unit 14.7
Current protocols in cell biology 2006 Apr;Chapter 14:Unit 14.7
Role of PYK2 in the development of obesity and insulin resistance.
Yu Y, Ross SA, Halseth AE, Hollenbach PW, Hill RJ, Gulve EA, Bond BR
Biochemical and biophysical research communications 2005 Sep 9;334(4):1085-91
Biochemical and biophysical research communications 2005 Sep 9;334(4):1085-91
Focal adhesion kinase plays a pivotal role in herpes simplex virus entry.
Cheshenko N, Liu W, Satlin LM, Herold BC
The Journal of biological chemistry 2005 Sep 2;280(35):31116-25
The Journal of biological chemistry 2005 Sep 2;280(35):31116-25
Proline-rich tyrosine kinase 2 (Pyk2) mediates vascular endothelial-cadherin-based cell-cell adhesion by regulating beta-catenin tyrosine phosphorylation.
van Buul JD, Anthony EC, Fernandez-Borja M, Burridge K, Hordijk PL
The Journal of biological chemistry 2005 Jun 3;280(22):21129-36
The Journal of biological chemistry 2005 Jun 3;280(22):21129-36
PYK2 regulates SERCA2 gene expression in neonatal rat ventricular myocytes.
Heidkamp MC, Scully BT, Vijayan K, Engman SJ, Szotek EL, Samarel AM
American journal of physiology. Cell physiology 2005 Aug;289(2):C471-82
American journal of physiology. Cell physiology 2005 Aug;289(2):C471-82
Glucose activates protein kinase C-zeta /lambda through proline-rich tyrosine kinase-2, extracellular signal-regulated kinase, and phospholipase D: a novel mechanism for activating glucose transporter translocation.
Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert ML, Quon MJ, Reed BC, Dikic I, Farese RV
The Journal of biological chemistry 2001 Sep 21;276(38):35537-45
The Journal of biological chemistry 2001 Sep 21;276(38):35537-45
Salicylate Inhibits Phosphorylation of the Nonreceptor Tyrosine Kinases, Proline-Rich Tyrosine Kinase 2 and c-Src.
Wang Z, Brecher P
Hypertension (Dallas, Tex. : 1979) 2001 Jan;37(1):148-153
Hypertension (Dallas, Tex. : 1979) 2001 Jan;37(1):148-153
Salicylate Inhibits Phosphorylation of the Nonreceptor Tyrosine Kinases, Proline-Rich Tyrosine Kinase 2 and c-Src.
Wang Z, Brecher P
Hypertension (Dallas, Tex. : 1979) 2001 Jan;37(1):148-153
Hypertension (Dallas, Tex. : 1979) 2001 Jan;37(1):148-153
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
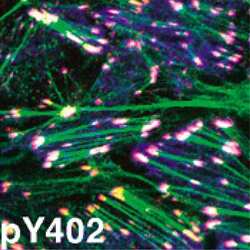
- Pyk2 (pY402) phosphospecific antibody. Pyk2 (pY402) phosphospecific antibody. Image shows auto-phosphorylation in TGF-beta treated NMµMG cells using Pyk2 (pY402) PSSA. (Product # 44-618G)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
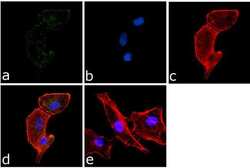
- Immunofluorescence analysis of PYK2 (pY402) was done on 70% confluent log phase A549 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with PYK2 (pY402) Rabbit Polyclonal Antibody (Product # 44-618G) at 1:250 dilution in 1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
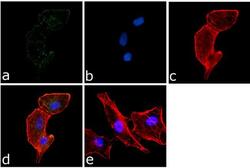
- Immunofluorescence analysis of PYK2 (pY402) was done on 70% confluent log phase A549 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with PYK2 (pY402) Rabbit Polyclonal Antibody (Product # 44-618G) at 1:250 dilution in 1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
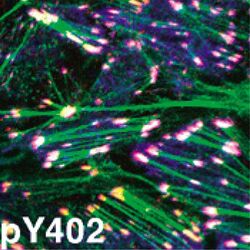
- Pyk2 (pY402) phosphospecific antibody. Pyk2 (pY402) phosphospecific antibody. Image shows auto-phosphorylation in TGF-beta treated NMµMG cells using Pyk2 (pY402) PSSA. (Product # 44-618G)
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
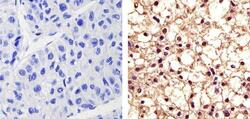
- Immunohistochemistry analysis of Phospho PYK2 [pY402] showing staining in the nucleus and cytoplasm of paraffin-embedded human hepatocarcinoma (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with Phospho PYK2 [pY402] Rabbit Polyclonal Antibody Product # (44-618G) diluted in 3% BSA-PBS at a dilution of 1:50 overnight at 4ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
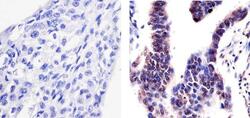
- Immunohistochemistry analysis of PYK2 (PY402) showing staining in the cytoplasm and nucleus of paraffin-embedded human lung adenocarcinoma (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a PYK2 (PY402) Rabbit Polyclonal Antibody (Product # 44-618G) diluted in 3% BSA-PBS at a dilution of 1:20 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
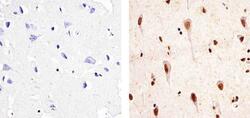
- Immunohistochemistry analysis of Phospho PYK2 [pY402] showing staining in the nucleus and cytoplasm of paraffin-embedded human brain tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Phospho PYK2 [pY402] Rabbit Polyclonal Antibody (Product # 44-618G) diluted in 3% BSA-PBS at a dilution of 1:50 overnight at 4ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
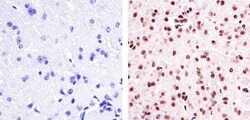
- Immunohistochemistry analysis of Phospho PYK2 [pY402] showing staining in the nucleus and cytoplasm of paraffin-embedded Mouse brain tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Phospho PYK2 [pY402] Rabbit Polyclonal Antibody (Product # 44-618G) diluted in 3% BSA-PBS at a dilution of 1:20 overnight at 4ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
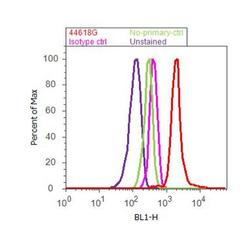
- Experimental details
- Flow cytometry analysis of FAK2 / PYK2 [pTyr402] was done on PC-3 cells treated with Vanadate (100uM, 1 hour). Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with FAK2 / PYK2 [pTyr402] Rabbit Polyclonal Antibody (44618G, red histogram) or with rabbit isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
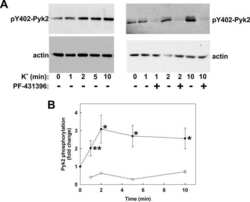
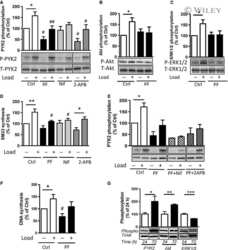
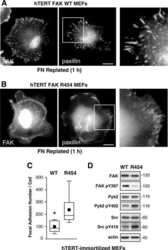
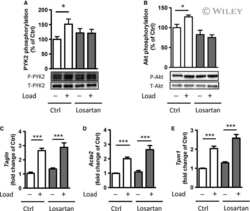
- Figure 1. Proline-rich tyrosine kinase 2 phosphorylation induced by acute stretch is inhibited by PF-4594755 (PF). Rat portal veins were cultured with either DMSO (0.08%) or PF (1 mu mol/L) overnight (A-D). Following overnight equilibration, portal vein strips were stretched by a hanging weight for 10 min and quickly frozen in liquid nitrogen. Phosphorylation of PYK2 (A), Akt (B), FAK (C), and ERK1/2 (D) was determined with phospho-specific antibodies and the signal intensity normalized to the signal intensity of the respective total protein ( n = 7-10). * P < 0.05, ** P < 0.01, *** P < 0.001, n.s., not significant, for comparison with unloaded strips.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
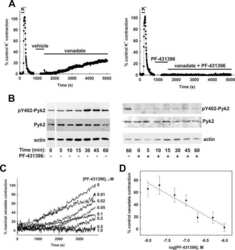
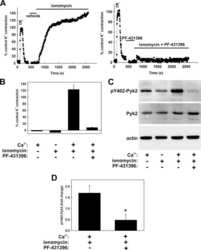
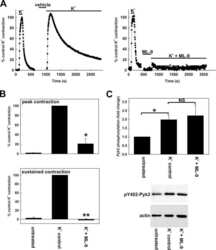
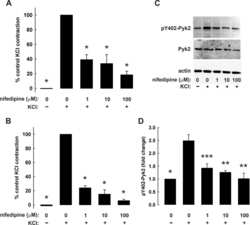
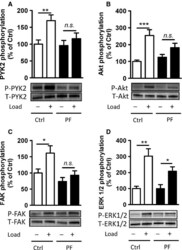
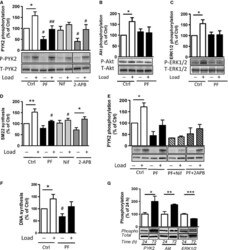
- Figure 4. Interactions between PF-4594755 and inhibitors of voltage/non-voltage-dependent Ca 2+ entry. Portal vein strips were incubated in organ culture with or without load for 3 days in the presence of [ 35 S]-methionine for the last 24 h (A-D). Effect of PF (0.5 mu mol/L), nifedipine (Nif, 1 mu mol/L), and 2-APB (30 mu mol/L) on PYK2 phosphorylation (A), and effect of PF on Akt (B) and ERK1/2 (C) phosphorylation was evaluated by western blot ( n = 5-6). Summarized data of SM22 alpha synthesis as measured by autoradiography (D; n = 6). Effects of the combined action of PF and nifedipine or 2-APB on PYK2 phosphorylation compared with that of PF alone, normalized to phosphorylation of untreated control as in panel A (E; n = 3-4). DNA synthesis as measured by [ 3 H]-thymidine incorporation in portal veins cultured unloaded/loaded for 3 days with or without PF (F; n = 6). PYK2, Akt and ERK1/2 phosphorylation of portal vein cultured unloaded for 24 h or 72 h as indicated. (F, n = 5). In each panel, all blots shown are from the same gels for phosphorylated or total protein, respectively. * P < 0.05, ** P < 0.01, *** P < 0.001 for comparison with unloaded strip, # P < 0.05, ## P < 0.01 for comparison with untreated control (unloaded/loaded).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
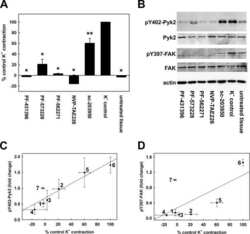
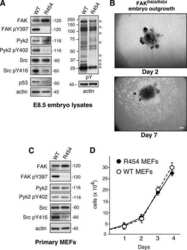
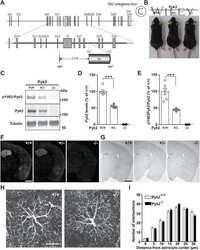
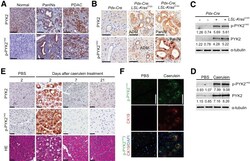
- Figure 1 Hyperactivation of PYK2 in ADM and PanIN lesions. ( A ) Human PDAC tissue was stained with antibodies against PYK2 and p-PYK2 Y402 . Representative IHC staining is shown. Scale bars : 100 mum. ( B ) IHC staining of PYK2 and p-PYK2 Y402 in pancreas from 6-month-old Pdx-Cre mice and Pdx-Cre ; LSL-Kras G12D mice. Scale bars : 50 mum. ( C ) Immunoblotting analysis of PYK2 and p-PYK2 Y402 levels in pancreatic tissues from Pdx-Cre control mice and Pdx-Cre ; LSL-Kras G12D mice. Each lane represents a single mouse. ( D ) C57BL/6 mice were injected with cerulein (to induce pancreatitis) or PBS (control) for 2 consecutive days. The pancreatic tissues were collected 2 days after injection and prepared for immunoblotting analysis with indicated antibodies. ( E ) Six-week-old C57BL/6 mice were treated with PBS or cerulein for 2 consecutive days. The pancreas was harvested at the indicated time points after injection for H&E staining and IHC staining. Scale bars : 50 mum. ( F ) Co-immunofluorescence staining for p-PYK2 Y402 and CK19 in pancreatic sections from cerulein- and PBS-treated mice. Scale bars : 50 mum. For all panels, representative results from 3 independent experiments are shown. ( C and D ) The numbers under each blot represent band intensity normalized to alpha-tubulin and relative to expression of target proteins in Pdx-Cre mice or PBS-treated C57BL/6 mice.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
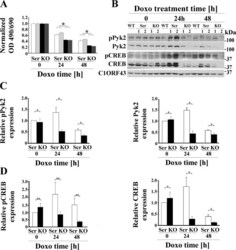
- Fig. 1. TRPM2 depletion significantly increases doxorubicin sensitivity and reduces Pyk2 and CREB phosphorylation and expression. A : two different SH-SY5Y clones in which TRPM2 was depleted with CRISPR (KO) or scrambled control cells (Scr) were studied. Cells were untreated or treated with 0.3 muM doxorubicin for 24 or 48 h. Cell proliferation was measured by 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay. Results are expressed as OD reading of plated cells (5 x 10 4 cells) normalized to time 0 for each group. Values are means +- SE for one representative experiment analyzed in triplicate. Four experiments were performed. *Significantly different at P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
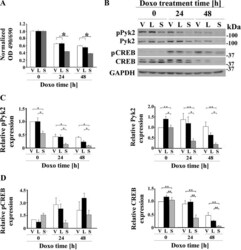
- Fig. 2. TRPM2 inhibition with TRPM2-S significantly increases doxorubicin sensitivity and reduces Pyk2 phosphorylation and Pyk2 and CREB expression. A : SH-SY5Y cells expressing empty vector (V), TRPM2-L (L) or TRPM2-S (S) were untreated or treated with 0.5 muM doxorubicin for 24 or 48 h. Cell proliferation was measured by 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay. Results are expressed as OD reading of plated cells (5 x 10 4 cells) normalized to control at time 0 for each group. Values are means +- SE for one representative experiment analyzed in triplicate of two performed. *Significant differences at P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
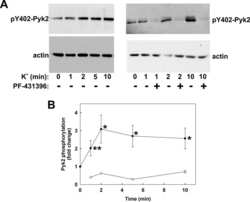
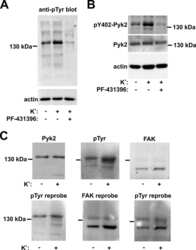
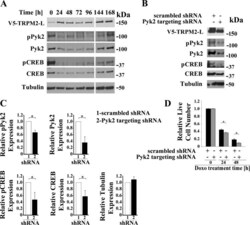
- Fig. 4. Pyk2 regulates CREB expression and cell viability. A : time course. SH-SY5Y cells stably expressing V5-TRPM2-L were transiently transfected with shRNA targeting Pyk2, and samples for Western blotting removed from culture at 24-h intervals for 168 h. Western blotting was performed with antibodies to V5, pPyk2, Pyk2, pCREB, and CREB. Tubulin was probed to confirm equivalent loading. B : SH-SY5Y cells expressing V5-TRPM2-L were stably transfected with shRNA targeting Pyk2 or control scrambled shRNA. Western blotting was performed on lysates from untreated cells with antibodies to V5, pPyk2, Pyk2, pCREB, and total CREB and confirmed that down modulation of Pyk2 resulted in reduced pPyk2, pCREB, and CREB. A representative Western blot from one of three experiments is shown. C : expression of pPyk2, Pyk2, pCREB, CREB, and tubulin was normalized by comparison of expression in cells transfected with Pyk2 targeted shRNA to that in scrambled shRNA for each densitometry measurement in the three experiments in B . The Student's t -test was used for analysis of differences. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
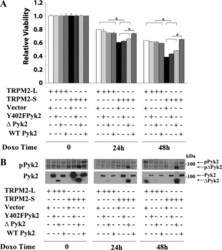
- Experimental details
- Fig. 5. Pyk2 rescues viability of TRPM2-S expressing cells. SH-SY5Y cells expressing V5-TRPM2-L or TRPM2-S were transfected with vector, Y402F Pyk2, Pyk2, or wild-type Pyk2. Cells were then treated with 0.3 muM doxorubicin for 24 or 48 h. A : cell proliferation was measured by 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay. Results are expressed as relative viability calculated by OD reading of plated cells (5 x 10 4 cells) normalized to time 0 for each group. Values are means +- SE for one representative experiment of three done in six replicates. Results were analyzed with one-way ANOVA. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
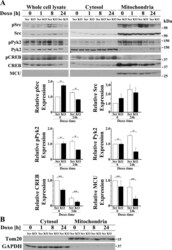
- Experimental details
- Fig. 6. Depletion of TRPM2 reduces mitochondrial phosphorylation of Src and Pyk2 and mitochondrial expression of Pyk2, CREB, and MCU. A : TRPM2-depleted and scrambled SH-SY5Y cells were separated into cytosol and mitochondrial fractions and Src, Pyk2, and CREB phosphorylation and expression examined. In both whole cell lysates and mitochondria, phosphorylation of Src and Pyk2 was decreased after doxorubicin treatment of TRPM2-depleted cells. Mitochondrial Pyk2 was also decreased after doxorubicin application. Levels of CREB and the mitochondrial calcium uniporter MCU were reduced in the mitochondrial fraction of KO cells. Similar results were observed in three experiments and representative blots are shown. Densitometry measurements of mitochondrial protein for three experiments were standardized to results for each experiment's scrambled mitochondrial control at time 0 , and the means +- SE of phosphorylated or total Src, Pyk2, CREB, or MCU calculated from three experiments are shown. ** P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
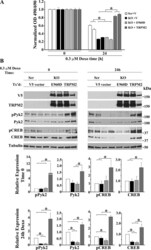
- Experimental details
- Fig. 10. Reconstitution of TRPM2-depleted cells with wild-type TRPM2 but not the Ca 2+ -impermeable mutant E960D restored viability in TRPM2-depleted cells and phosphorylation and expression of Pyk2 and CREB. TRPM2-depleted SH-SY5Y cells (KO) were stably transfected with empty vector, V5-tagged wild-type TRPM2 or the V5-tagged Ca 2+ -impermeable TRPM2 mutant E960D. Scrambled control cells (Scr) were transfected with empty vector. Two different single cells clones from each group of transfected cells (Scr, KO) were untreated or treated with doxorubicin for 24 h and cell lysates prepared. A : reconstitution of cell viability with wild-type TRPM2 but not empty vector or the E960D pore mutant is shown. Viability was measured in three experiments with XTT. Measurements were standardized to results for untreated cells in each group, and the means +- SE of six replicates from one representative experiment are shown. * P
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry