Antibody data
- Antibody Data
- Antigen structure
- References [89]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [2]
- Other assay [55]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 37-4900 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Claudin 1 Monoclonal Antibody (2H10D10)
- Antibody type
- Monoclonal
- Antigen
- Synthetic peptide
- Description
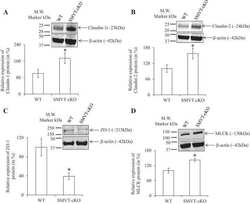
- Reactivity has been confirmed with human Caco-2 and canine MDCK cell lysates, mouse kidney and intestinal lysates, frozen mouse intestine (jejunum) tissue, and rat kidney homogenates. This antibody is specific for the C-terminal region of the claudin-1 protein. On western blots, it identifies the target band at ~22 kDa.
- Reactivity
- Human, Mouse, Rat, Canine
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 2H10D10
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- Maintain refrigerated at 2-8°C for up to 1 month. For long term storage store at -20°C
Submitted references SARS-CoV-2 spike S1 subunit protein-mediated increase of beta-secretase 1 (BACE1) impairs human brain vessel cells.
A mesenchymal to epithelial switch in Fgf10 expression specifies an evolutionary-conserved population of ionocytes in salivary glands.
Modeling alpha-synuclein pathology in a human brain-chip to assess blood-brain barrier disruption.
The Role of Processed Aloe vera Gel in Intestinal Tight Junction: An In Vivo and In Vitro Study.
The in ovo injection of methionine improves intestinal cell proliferation and differentiation in chick embryos by activating the JAK2/STAT3 signaling pathway.
Transport of Dietary Anti-Inflammatory Peptide, γ-Glutamyl Valine (γ-EV), across the Intestinal Caco-2 Monolayer.
Wnt/β-catenin-mediated heat exposure inhibits intestinal epithelial cell proliferation and stem cell expansion through endoplasmic reticulum stress.
Disease-associated KIF3A variants alter gene methylation and expression impacting skin barrier and atopic dermatitis risk.
Prevention of excessive exercise-induced adverse effects in rats with Bacillus subtilis BSB3.
Aeromonas sobria serine protease decreases epithelial barrier function in T84 cells and accelerates bacterial translocation across the T84 monolayer in vitro.
Effect of Bacillus subtilis Strains on Intestinal Barrier Function and Inflammatory Response.
Yeast fermentate prebiotic improves intestinal barrier integrity during heat stress by modulation of the gut microbiota in rats.
Temporal Effects of Quercetin on Tight Junction Barrier Properties and Claudin Expression and Localization in MDCK II Cells.
Improving Transient Transfection Efficiency in a Differentiated, Polar Epithelial Cell Layer.
Dietary Protein Intake Level Modulates Mucosal Healing and Mucosa-Adherent Microbiota in Mouse Model of Colitis.
The secretome of adipose-derived mesenchymal stem cells attenuates epithelial-mesenchymal transition in human corneal epithelium.
Epidermal development requires ninein for spindle orientation and cortical microtubule organization.
MicroRNA-203 Inhibits Long Noncoding RNA HOTAIR and Regulates Tumorigenesis through Epithelial-to-mesenchymal Transition Pathway in Renal Cell Carcinoma.
Dietary copper-fructose interactions alter gut microbial activity in male rats.
Enteropathogenic E. coli effectors EspF and Map independently disrupt tight junctions through distinct mechanisms involving transcriptional and post-transcriptional regulation.
Secreted Giardia intestinalis cysteine proteases disrupt intestinal epithelial cell junctional complexes and degrade chemokines.
Dopamine enhances duodenal epithelial permeability via the dopamine D(5) receptor in rodent.
Possible role of HIWI2 in modulating tight junction proteins in retinal pigment epithelial cells through Akt signaling pathway.
Chronic exposure to short-chain fatty acids modulates transport and metabolism of microbiome-derived phenolics in human intestinal cells.
Hepatitis C virus has a genetically determined lymphotropism through co-receptor B7.2.
Claudin-6 enhances cell invasiveness through claudin-1 in AGS human adenocarcinoma gastric cancer cells.
Astrocytic tight junctions control inflammatory CNS lesion pathogenesis.
Puerarin transport across a Calu-3 cell monolayer - an in vitro model of nasal mucosa permeability and the influence of paeoniflorin and menthol.
Role of the sodium-dependent multivitamin transporter (SMVT) in the maintenance of intestinal mucosal integrity.
Expression of claudins, occludin, junction adhesion molecule A and zona occludens 1 in canine organs.
ERK5 signalling rescues intestinal epithelial turnover and tumour cell proliferation upon ERK1/2 abrogation.
High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22.
Butyric acid increases transepithelial transport of ferulic acid through upregulation of the monocarboxylate transporters SLC16A1 (MCT1) and SLC16A3 (MCT4).
Candida albicans infection leads to barrier breakdown and a MAPK/NF-κB mediated stress response in the intestinal epithelial cell line C2BBe1.
Intestinal barrier analysis by assessment of mucins, tight junctions, and α-defensins in healthy C57BL/6J and BALB/cJ mice.
Epidermal RAF prevents allergic skin disease.
Claudin-1, -2, -4, and -5: comparison of expression levels and distribution in equine tissues.
Cinnamon extract reduces symptoms, inflammatory mediators and mast cell markers in murine IL-10(-/-) colitis.
Junctional abnormalities in human airway epithelial cells expressing F508del CFTR.
Selection of a hepatitis C virus with altered entry factor requirements reveals a genetic interaction between the E1 glycoprotein and claudins.
Diverse regulation of claudin-1 and claudin-4 in atopic dermatitis.
Biophysical characterization of interactions between the C-termini of peripheral nerve claudins and the PDZ₁ domain of zonula occludens.
Hepatitis C virus infects rhesus macaque hepatocytes and simianized mice.
Cell culture-derived HCV cannot infect synovial fibroblasts.
Quantitative Proteomics Identifies Serum Response Factor Binding Protein 1 as a Host Factor for Hepatitis C Virus Entry.
CaMKII regulates the strength of the epithelial barrier.
Nanotopography facilitates in vivo transdermal delivery of high molecular weight therapeutics through an integrin-dependent mechanism.
Tricellulin expression and its prognostic significance in primary liver carcinomas.
Amelioration of hypoxia and LPS-induced intestinal epithelial barrier dysfunction by emodin through the suppression of the NF-κB and HIF-1α signaling pathways.
Alteration of intestinal barrier function during activity-based anorexia in mice.
2,4,6-trinitrobenzene sulfonic acid-induced chronic colitis with fibrosis and modulation of TGF-β1 signaling.
Complete replication of hepatitis B virus and hepatitis C virus in a newly developed hepatoma cell line.
Occludin is involved in adhesion, apoptosis, differentiation and Ca2+-homeostasis of human keratinocytes: implications for tumorigenesis.
12/15-Lipoxygenase mediates high-fat diet-induced endothelial tight junction disruption and monocyte transmigration: a new role for 15(S)-hydroxyeicosatetraenoic acid in endothelial cell dysfunction.
Gastroesophageal reflux activates the NF-κB pathway and impairs esophageal barrier function in mice.
Behavior of tricellulin during destruction and formation of tight junctions under various extracellular calcium conditions.
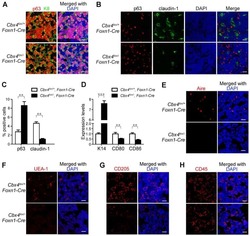
Cbx4 regulates the proliferation of thymic epithelial cells and thymus function.
The antiviral cytokines IFN-α and IFN-β modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration.
Contrasting roles of mitogen-activated protein kinases in cellular entry and replication of hepatitis C virus: MKNK1 facilitates cell entry.
Inflammation and disintegration of intestinal villi in an experimental model for Vibrio parahaemolyticus-induced diarrhea.
Evidence for phenotypic plasticity in aggressive triple-negative breast cancer: human biology is recapitulated by a novel model system.
Expression of myoferlin in human airway epithelium and its role in cell adhesion and zonula occludens-1 expression.
Characterization of hepatitis C virus particle subpopulations reveals multiple usage of the scavenger receptor BI for entry steps.
Expression and distribution of symplekin regulates the assembly and function of the epithelial tight junction.
Claudin-1, claudin-2 and claudin-11 genes differentially associate with distinct types of anti-inflammatory macrophages in vitro and with parasite- and tumour-elicited macrophages in vivo.
Modulation of tight junction proteins in the perineurium to facilitate peripheral opioid analgesia.
Hailey-Hailey disease and tight junctions: Claudins 1 and 4 are regulated by ATP2C1 gene encoding Ca(2+) /Mn(2+) ATPase SPCA1 in cultured keratinocytes.
Loss of claudin-1 in lipopolysaccharide-treated periodontal epithelium.
Dynamics of claudins expression in colitis and colitis-associated cancer in rat.
The tight junction scaffolding protein cingulin regulates neural crest cell migration.
Comparison of tight junction protein expression in the ciliary epithelia of mouse, rabbit, cat and human eyes.
Protein kinase Cα inhibitor enhances the sensitivity of human pancreatic cancer HPAC cells to Clostridium perfringens enterotoxin via claudin-4.
Impact of intra- and interspecies variation of occludin on its function as coreceptor for authentic hepatitis C virus particles.
Hepatitis C virus enters human peripheral neuroblastoma cells - evidence for extra-hepatic cells sustaining hepatitis C virus penetration.
Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders.
c-Jun N-terminal kinase is largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells.
The second extracellular loop dictates Occludin-mediated HCV entry.
Glucocorticosteroids increase cell entry by hepatitis C virus.
Increased B7-H1 expression on dendritic cells correlates with programmed death 1 expression on T cells in simian immunodeficiency virus-infected macaques and may contribute to T cell dysfunction and disease progression.
Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain.
Apolipoprotein E on hepatitis C virion facilitates infection through interaction with low-density lipoprotein receptor.
The mycotoxin patulin, modulates tight junctions in caco-2 cells.
Expression and localization of tricellulin in human nasal epithelial cells in vivo and in vitro.
Fatty acid synthase is up-regulated during hepatitis C virus infection and regulates hepatitis C virus entry and production.
Correlation of the tight junction-like distribution of Claudin-1 to the cellular tropism of hepatitis C virus.
Early events in HIV transmission through a human reconstructed vaginal mucosa.
Expression of Claudin-1, Claudin-3 and Claudin-5 in human blood-brain barrier mimicking cell line ECV304 is inducible by glioma-conditioned media.
Loss of claudins-1 and -7 and expression of claudins-3 and -4 correlate with prognostic variables in prostatic adenocarcinomas.
Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins.
Choi JY, Park JH, Jo C, Kim KC, Koh YH
Biochemical and biophysical research communications 2022 Oct 20;626:66-71
Biochemical and biophysical research communications 2022 Oct 20;626:66-71
A mesenchymal to epithelial switch in Fgf10 expression specifies an evolutionary-conserved population of ionocytes in salivary glands.
Mauduit O, Aure MH, Delcroix V, Basova L, Srivastava A, Umazume T, Mays JW, Bellusci S, Tucker AS, Hajihosseini MK, Hoffman MP, Makarenkova HP
Cell reports 2022 Apr 12;39(2):110663
Cell reports 2022 Apr 12;39(2):110663
Modeling alpha-synuclein pathology in a human brain-chip to assess blood-brain barrier disruption.
Pediaditakis I, Kodella KR, Manatakis DV, Le CY, Hinojosa CD, Tien-Street W, Manolakos ES, Vekrellis K, Hamilton GA, Ewart L, Rubin LL, Karalis K
Nature communications 2021 Oct 8;12(1):5907
Nature communications 2021 Oct 8;12(1):5907
The Role of Processed Aloe vera Gel in Intestinal Tight Junction: An In Vivo and In Vitro Study.
Le Phan TH, Park SY, Jung HJ, Kim MW, Cho E, Shim KS, Shin E, Yoon JH, Maeng HJ, Kang JH, Oh SH
International journal of molecular sciences 2021 Jun 17;22(12)
International journal of molecular sciences 2021 Jun 17;22(12)
The in ovo injection of methionine improves intestinal cell proliferation and differentiation in chick embryos by activating the JAK2/STAT3 signaling pathway.
Chen MJ, Zhou JY, Chen YJ, Wang XQ, Yan HC, Gao CQ
Animal nutrition (Zhongguo xu mu shou yi xue hui) 2021 Dec;7(4):1031-1038
Animal nutrition (Zhongguo xu mu shou yi xue hui) 2021 Dec;7(4):1031-1038
Transport of Dietary Anti-Inflammatory Peptide, γ-Glutamyl Valine (γ-EV), across the Intestinal Caco-2 Monolayer.
Guha S, Alvarez S, Majumder K
Nutrients 2021 Apr 24;13(5)
Nutrients 2021 Apr 24;13(5)
Wnt/β-catenin-mediated heat exposure inhibits intestinal epithelial cell proliferation and stem cell expansion through endoplasmic reticulum stress.
Zhou JY, Huang DG, Zhu M, Gao CQ, Yan HC, Li XG, Wang XQ
Journal of cellular physiology 2020 Jul;235(7-8):5613-5627
Journal of cellular physiology 2020 Jul;235(7-8):5613-5627
Disease-associated KIF3A variants alter gene methylation and expression impacting skin barrier and atopic dermatitis risk.
Stevens ML, Zhang Z, Johansson E, Ray S, Jagpal A, Ruff BP, Kothari A, He H, Martin LJ, Ji H, Wikenheiser-Brokamp K, Weirauch MT, Supp DM, Biagini Myers JM, Khurana Hershey GK
Nature communications 2020 Aug 14;11(1):4092
Nature communications 2020 Aug 14;11(1):4092
Prevention of excessive exercise-induced adverse effects in rats with Bacillus subtilis BSB3.
Ducray HAG, Globa L, Pustovyy O, Roberts MD, Rudisill M, Vodyanoy V, Sorokulova I
Journal of applied microbiology 2020 Apr;128(4):1163-1178
Journal of applied microbiology 2020 Apr;128(4):1163-1178
Aeromonas sobria serine protease decreases epithelial barrier function in T84 cells and accelerates bacterial translocation across the T84 monolayer in vitro.
Kobayashi H, Seike S, Yamaguchi M, Ueda M, Takahashi E, Okamoto K, Yamanaka H
PloS one 2019;14(8):e0221344
PloS one 2019;14(8):e0221344
Effect of Bacillus subtilis Strains on Intestinal Barrier Function and Inflammatory Response.
Rhayat L, Maresca M, Nicoletti C, Perrier J, Brinch KS, Christian S, Devillard E, Eckhardt E
Frontiers in immunology 2019;10:564
Frontiers in immunology 2019;10:564
Yeast fermentate prebiotic improves intestinal barrier integrity during heat stress by modulation of the gut microbiota in rats.
Ducray HAG, Globa L, Pustovyy O, Morrison E, Vodyanoy V, Sorokulova I
Journal of applied microbiology 2019 Oct;127(4):1192-1206
Journal of applied microbiology 2019 Oct;127(4):1192-1206
Temporal Effects of Quercetin on Tight Junction Barrier Properties and Claudin Expression and Localization in MDCK II Cells.
Gamero-Estevez E, Andonian S, Jean-Claude B, Gupta I, Ryan AK
International journal of molecular sciences 2019 Oct 2;20(19)
International journal of molecular sciences 2019 Oct 2;20(19)
Improving Transient Transfection Efficiency in a Differentiated, Polar Epithelial Cell Layer.
Rybakovsky E, Valenzano MC, DiGuilio KM, Buleza NB, Moskalenko DV, Harty RN, Mullin JM
Journal of biomolecular techniques : JBT 2019 Jul;30(2):19-24
Journal of biomolecular techniques : JBT 2019 Jul;30(2):19-24
Dietary Protein Intake Level Modulates Mucosal Healing and Mucosa-Adherent Microbiota in Mouse Model of Colitis.
Vidal-Lletjós S, Andriamihaja M, Blais A, Grauso M, Lepage P, Davila AM, Viel R, Gaudichon C, Leclerc M, Blachier F, Lan A
Nutrients 2019 Feb 28;11(3)
Nutrients 2019 Feb 28;11(3)
The secretome of adipose-derived mesenchymal stem cells attenuates epithelial-mesenchymal transition in human corneal epithelium.
Shibata S, Hayashi R, Okubo T, Kudo Y, Baba K, Honma Y, Nishida K
Regenerative therapy 2019 Dec;11:114-122
Regenerative therapy 2019 Dec;11:114-122
Epidermal development requires ninein for spindle orientation and cortical microtubule organization.
Lecland N, Hsu CY, Chemin C, Merdes A, Bierkamp C
Life science alliance 2019 Apr;2(2)
Life science alliance 2019 Apr;2(2)
MicroRNA-203 Inhibits Long Noncoding RNA HOTAIR and Regulates Tumorigenesis through Epithelial-to-mesenchymal Transition Pathway in Renal Cell Carcinoma.
Dasgupta P, Kulkarni P, Majid S, Shahryari V, Hashimoto Y, Bhat NS, Shiina M, Deng G, Saini S, Tabatabai ZL, Yamamura S, Tanaka Y, Dahiya R
Molecular cancer therapeutics 2018 May;17(5):1061-1069
Molecular cancer therapeutics 2018 May;17(5):1061-1069
Dietary copper-fructose interactions alter gut microbial activity in male rats.
Song M, Li X, Zhang X, Shi H, Vos MB, Wei X, Wang Y, Gao H, Rouchka EC, Yin X, Zhou Z, Prough RA, Cave MC, McClain CJ
American journal of physiology. Gastrointestinal and liver physiology 2018 Jan 1;314(1):G119-G130
American journal of physiology. Gastrointestinal and liver physiology 2018 Jan 1;314(1):G119-G130
Enteropathogenic E. coli effectors EspF and Map independently disrupt tight junctions through distinct mechanisms involving transcriptional and post-transcriptional regulation.
Singh AP, Sharma S, Pagarware K, Siraji RA, Ansari I, Mandal A, Walling P, Aijaz S
Scientific reports 2018 Feb 27;8(1):3719
Scientific reports 2018 Feb 27;8(1):3719
Secreted Giardia intestinalis cysteine proteases disrupt intestinal epithelial cell junctional complexes and degrade chemokines.
Liu J, Ma'ayeh S, Peirasmaki D, Lundström-Stadelmann B, Hellman L, Svärd SG
Virulence 2018 Dec 31;9(1):879-894
Virulence 2018 Dec 31;9(1):879-894
Dopamine enhances duodenal epithelial permeability via the dopamine D(5) receptor in rodent.
Feng XY, Zhang DN, Wang YA, Fan RF, Hong F, Zhang Y, Li Y, Zhu JX
Acta physiologica (Oxford, England) 2017 May;220(1):113-123
Acta physiologica (Oxford, England) 2017 May;220(1):113-123
Possible role of HIWI2 in modulating tight junction proteins in retinal pigment epithelial cells through Akt signaling pathway.
Sivagurunathan S, Palanisamy K, Arunachalam JP, Chidambaram S
Molecular and cellular biochemistry 2017 Mar;427(1-2):145-156
Molecular and cellular biochemistry 2017 Mar;427(1-2):145-156
Chronic exposure to short-chain fatty acids modulates transport and metabolism of microbiome-derived phenolics in human intestinal cells.
Van Rymenant E, Abrankó L, Tumova S, Grootaert C, Van Camp J, Williamson G, Kerimi A
The Journal of nutritional biochemistry 2017 Jan;39:156-168
The Journal of nutritional biochemistry 2017 Jan;39:156-168
Hepatitis C virus has a genetically determined lymphotropism through co-receptor B7.2.
Chen CL, Huang JY, Wang CH, Tahara SM, Zhou L, Kondo Y, Schechter J, Su L, Lai MM, Wakita T, Cosset FL, Jung JU, Machida K
Nature communications 2017 Jan 9;8:13882
Nature communications 2017 Jan 9;8:13882
Claudin-6 enhances cell invasiveness through claudin-1 in AGS human adenocarcinoma gastric cancer cells.
Torres-Martínez AC, Gallardo-Vera JF, Lara-Holguin AN, Montaño LF, Rendón-Huerta EP
Experimental cell research 2017 Jan 1;350(1):226-235
Experimental cell research 2017 Jan 1;350(1):226-235
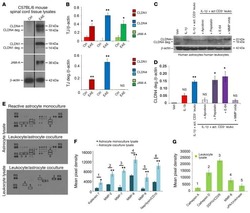
Astrocytic tight junctions control inflammatory CNS lesion pathogenesis.
Horng S, Therattil A, Moyon S, Gordon A, Kim K, Argaw AT, Hara Y, Mariani JN, Sawai S, Flodby P, Crandall ED, Borok Z, Sofroniew MV, Chapouly C, John GR
The Journal of clinical investigation 2017 Aug 1;127(8):3136-3151
The Journal of clinical investigation 2017 Aug 1;127(8):3136-3151
Puerarin transport across a Calu-3 cell monolayer - an in vitro model of nasal mucosa permeability and the influence of paeoniflorin and menthol.
Zhang L, Du SY, Lu Y, Liu C, Tian ZH, Yang C, Wu HC, Wang Z
Drug design, development and therapy 2016;10:2227-37
Drug design, development and therapy 2016;10:2227-37
Role of the sodium-dependent multivitamin transporter (SMVT) in the maintenance of intestinal mucosal integrity.
Sabui S, Bohl JA, Kapadia R, Cogburn K, Ghosal A, Lambrecht NW, Said HM
American journal of physiology. Gastrointestinal and liver physiology 2016 Sep 1;311(3):G561-70
American journal of physiology. Gastrointestinal and liver physiology 2016 Sep 1;311(3):G561-70
Expression of claudins, occludin, junction adhesion molecule A and zona occludens 1 in canine organs.
Ahn C, Shin DH, Lee D, Kang SM, Seok JH, Kang HY, Jeung EB
Molecular medicine reports 2016 Oct;14(4):3697-703
Molecular medicine reports 2016 Oct;14(4):3697-703
ERK5 signalling rescues intestinal epithelial turnover and tumour cell proliferation upon ERK1/2 abrogation.
de Jong PR, Taniguchi K, Harris AR, Bertin S, Takahashi N, Duong J, Campos AD, Powis G, Corr M, Karin M, Raz E
Nature communications 2016 May 17;7:11551
Nature communications 2016 May 17;7:11551
High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22.
Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang R, Kang A, Schreiber V, Wong KY, Magor G, Denman S, Begun J, Florin TH, Perkins A, Cuív PÓ, McGuckin MA, Hasnain SZ
Scientific reports 2016 Jun 28;6:28990
Scientific reports 2016 Jun 28;6:28990
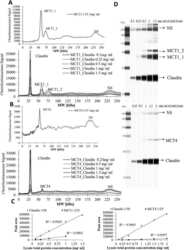
Butyric acid increases transepithelial transport of ferulic acid through upregulation of the monocarboxylate transporters SLC16A1 (MCT1) and SLC16A3 (MCT4).
Ziegler K, Kerimi A, Poquet L, Williamson G
Archives of biochemistry and biophysics 2016 Jun 1;599:3-12
Archives of biochemistry and biophysics 2016 Jun 1;599:3-12
Candida albicans infection leads to barrier breakdown and a MAPK/NF-κB mediated stress response in the intestinal epithelial cell line C2BBe1.
Böhringer M, Pohlers S, Schulze S, Albrecht-Eckardt D, Piegsa J, Weber M, Martin R, Hünniger K, Linde J, Guthke R, Kurzai O
Cellular microbiology 2016 Jul;18(7):889-904
Cellular microbiology 2016 Jul;18(7):889-904
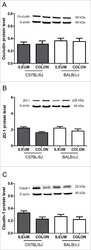
Intestinal barrier analysis by assessment of mucins, tight junctions, and α-defensins in healthy C57BL/6J and BALB/cJ mice.
Volynets V, Rings A, Bárdos G, Ostaff MJ, Wehkamp J, Bischoff SC
Tissue barriers 2016 Jul-Sep;4(3):e1208468
Tissue barriers 2016 Jul-Sep;4(3):e1208468
Epidermal RAF prevents allergic skin disease.
Raguz J, Jeric I, Niault T, Nowacka JD, Kuzet SE, Rupp C, Fischer I, Biggi S, Borsello T, Baccarini M
eLife 2016 Jul 19;5
eLife 2016 Jul 19;5
Claudin-1, -2, -4, and -5: comparison of expression levels and distribution in equine tissues.
Lee B, Kang HY, Lee DO, Ahn C, Jeung EB
Journal of veterinary science 2016 Dec 30;17(4):445-451
Journal of veterinary science 2016 Dec 30;17(4):445-451
Cinnamon extract reduces symptoms, inflammatory mediators and mast cell markers in murine IL-10(-/-) colitis.
Hagenlocher Y, Hösel A, Bischoff SC, Lorentz A
The Journal of nutritional biochemistry 2016 Apr;30:85-92
The Journal of nutritional biochemistry 2016 Apr;30:85-92
Junctional abnormalities in human airway epithelial cells expressing F508del CFTR.
Molina SA, Stauffer B, Moriarty HK, Kim AH, McCarty NA, Koval M
American journal of physiology. Lung cellular and molecular physiology 2015 Sep 1;309(5):L475-87
American journal of physiology. Lung cellular and molecular physiology 2015 Sep 1;309(5):L475-87
Selection of a hepatitis C virus with altered entry factor requirements reveals a genetic interaction between the E1 glycoprotein and claudins.
Hopcraft SE, Evans MJ
Hepatology (Baltimore, Md.) 2015 Oct;62(4):1059-69
Hepatology (Baltimore, Md.) 2015 Oct;62(4):1059-69
Diverse regulation of claudin-1 and claudin-4 in atopic dermatitis.
Gruber R, Börnchen C, Rose K, Daubmann A, Volksdorf T, Wladykowski E, Vidal-Y-Sy S, Peters EM, Danso M, Bouwstra JA, Hennies HC, Moll I, Schmuth M, Brandner JM
The American journal of pathology 2015 Oct;185(10):2777-89
The American journal of pathology 2015 Oct;185(10):2777-89
Biophysical characterization of interactions between the C-termini of peripheral nerve claudins and the PDZ₁ domain of zonula occludens.
Wu J, Peng D, Zhang Y, Lu Z, Voehler M, Sanders CR, Li J
Biochemical and biophysical research communications 2015 Mar 27;459(1):87-93
Biochemical and biophysical research communications 2015 Mar 27;459(1):87-93
Hepatitis C virus infects rhesus macaque hepatocytes and simianized mice.
Scull MA, Shi C, de Jong YP, Gerold G, Ries M, von Schaewen M, Donovan BM, Labitt RN, Horwitz JA, Gaska JM, Hrebikova G, Xiao JW, Flatley B, Fung C, Chiriboga L, Walker CM, Evans DT, Rice CM, Ploss A
Hepatology (Baltimore, Md.) 2015 Jul;62(1):57-67
Hepatology (Baltimore, Md.) 2015 Jul;62(1):57-67
Cell culture-derived HCV cannot infect synovial fibroblasts.
Nadeem AE, Thomas P, Ulf ML, Elena N, Anggakusuma A, Mohamed BM, Frank P, Patrick B
Scientific reports 2015 Dec 8;5:18043
Scientific reports 2015 Dec 8;5:18043
Quantitative Proteomics Identifies Serum Response Factor Binding Protein 1 as a Host Factor for Hepatitis C Virus Entry.
Gerold G, Meissner F, Bruening J, Welsch K, Perin PM, Baumert TF, Vondran FW, Kaderali L, Marcotrigiano J, Khan AG, Mann M, Rice CM, Pietschmann T
Cell reports 2015 Aug 4;12(5):864-78
Cell reports 2015 Aug 4;12(5):864-78
CaMKII regulates the strength of the epithelial barrier.
Shiomi R, Shigetomi K, Inai T, Sakai M, Ikenouchi J
Scientific reports 2015 Aug 18;5:13262
Scientific reports 2015 Aug 18;5:13262
Nanotopography facilitates in vivo transdermal delivery of high molecular weight therapeutics through an integrin-dependent mechanism.
Walsh L, Ryu J, Bock S, Koval M, Mauro T, Ross R, Desai T
Nano letters 2015 Apr 8;15(4):2434-41
Nano letters 2015 Apr 8;15(4):2434-41
Tricellulin expression and its prognostic significance in primary liver carcinomas.
Somorácz A, Korompay A, Törzsök P, Patonai A, Erdélyi-Belle B, Lotz G, Schaff Z, Kiss A
Pathology oncology research : POR 2014 Oct;20(4):755-64
Pathology oncology research : POR 2014 Oct;20(4):755-64
Amelioration of hypoxia and LPS-induced intestinal epithelial barrier dysfunction by emodin through the suppression of the NF-κB and HIF-1α signaling pathways.
Lei Q, Qiang F, Chao D, Di W, Guoqian Z, Bo Y, Lina Y
International journal of molecular medicine 2014 Dec;34(6):1629-39
International journal of molecular medicine 2014 Dec;34(6):1629-39
Alteration of intestinal barrier function during activity-based anorexia in mice.
Jésus P, Ouelaa W, François M, Riachy L, Guérin C, Aziz M, Do Rego JC, Déchelotte P, Fetissov SO, Coëffier M
Clinical nutrition (Edinburgh, Scotland) 2014 Dec;33(6):1046-53
Clinical nutrition (Edinburgh, Scotland) 2014 Dec;33(6):1046-53
2,4,6-trinitrobenzene sulfonic acid-induced chronic colitis with fibrosis and modulation of TGF-β1 signaling.
Loeuillard E, Bertrand J, Herranen A, Melchior C, Guérin C, Coëffier M, Aziz M, Déchelotte P, Savoye G, Marion-Letellier R
World journal of gastroenterology 2014 Dec 28;20(48):18207-15
World journal of gastroenterology 2014 Dec 28;20(48):18207-15
Complete replication of hepatitis B virus and hepatitis C virus in a newly developed hepatoma cell line.
Yang D, Zuo C, Wang X, Meng X, Xue B, Liu N, Yu R, Qin Y, Gao Y, Wang Q, Hu J, Wang L, Zhou Z, Liu B, Tan D, Guan Y, Zhu H
Proceedings of the National Academy of Sciences of the United States of America 2014 Apr 1;111(13):E1264-73
Proceedings of the National Academy of Sciences of the United States of America 2014 Apr 1;111(13):E1264-73
Occludin is involved in adhesion, apoptosis, differentiation and Ca2+-homeostasis of human keratinocytes: implications for tumorigenesis.
Rachow S, Zorn-Kruppa M, Ohnemus U, Kirschner N, Vidal-y-Sy S, von den Driesch P, Börnchen C, Eberle J, Mildner M, Vettorazzi E, Rosenthal R, Moll I, Brandner JM
PloS one 2013;8(2):e55116
PloS one 2013;8(2):e55116
12/15-Lipoxygenase mediates high-fat diet-induced endothelial tight junction disruption and monocyte transmigration: a new role for 15(S)-hydroxyeicosatetraenoic acid in endothelial cell dysfunction.
Kundumani-Sridharan V, Dyukova E, Hansen DE 3rd, Rao GN
The Journal of biological chemistry 2013 May 31;288(22):15830-42
The Journal of biological chemistry 2013 May 31;288(22):15830-42
Gastroesophageal reflux activates the NF-κB pathway and impairs esophageal barrier function in mice.
Fang Y, Chen H, Hu Y, Djukic Z, Tevebaugh W, Shaheen NJ, Orlando RC, Hu J, Chen X
American journal of physiology. Gastrointestinal and liver physiology 2013 Jul 1;305(1):G58-65
American journal of physiology. Gastrointestinal and liver physiology 2013 Jul 1;305(1):G58-65
Behavior of tricellulin during destruction and formation of tight junctions under various extracellular calcium conditions.
Takasawa A, Kojima T, Ninomiya T, Tsujiwaki M, Murata M, Tanaka S, Sawada N
Cell and tissue research 2013 Jan;351(1):73-84
Cell and tissue research 2013 Jan;351(1):73-84
Cbx4 regulates the proliferation of thymic epithelial cells and thymus function.
Liu B, Liu YF, Du YR, Mardaryev AN, Yang W, Chen H, Xu ZM, Xu CQ, Zhang XR, Botchkarev VA, Zhang Y, Xu GL
Development (Cambridge, England) 2013 Feb;140(4):780-8
Development (Cambridge, England) 2013 Feb;140(4):780-8
The antiviral cytokines IFN-α and IFN-β modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration.
Migliorini A, Angelotti ML, Mulay SR, Kulkarni OO, Demleitner J, Dietrich A, Sagrinati C, Ballerini L, Peired A, Shankland SJ, Liapis H, Romagnani P, Anders HJ
The American journal of pathology 2013 Aug;183(2):431-40
The American journal of pathology 2013 Aug;183(2):431-40
Contrasting roles of mitogen-activated protein kinases in cellular entry and replication of hepatitis C virus: MKNK1 facilitates cell entry.
Kim S, Ishida H, Yamane D, Yi M, Swinney DC, Foung S, Lemon SM
Journal of virology 2013 Apr;87(8):4214-24
Journal of virology 2013 Apr;87(8):4214-24
Inflammation and disintegration of intestinal villi in an experimental model for Vibrio parahaemolyticus-induced diarrhea.
Ritchie JM, Rui H, Zhou X, Iida T, Kodoma T, Ito S, Davis BM, Bronson RT, Waldor MK
PLoS pathogens 2012;8(3):e1002593
PLoS pathogens 2012;8(3):e1002593
Evidence for phenotypic plasticity in aggressive triple-negative breast cancer: human biology is recapitulated by a novel model system.
D'Amato NC, Ostrander JH, Bowie ML, Sistrunk C, Borowsky A, Cardiff RD, Bell K, Young LJ, Simin K, Bachelder RE, Delrow J, Dawson A, Yee LD, Mrózek K, Clay TM, Osada T, Seewaldt VL
PloS one 2012;7(9):e45684
PloS one 2012;7(9):e45684
Expression of myoferlin in human airway epithelium and its role in cell adhesion and zonula occludens-1 expression.
Leung C, Shaheen F, Bernatchez P, Hackett TL
PloS one 2012;7(7):e40478
PloS one 2012;7(7):e40478
Characterization of hepatitis C virus particle subpopulations reveals multiple usage of the scavenger receptor BI for entry steps.
Dao Thi VL, Granier C, Zeisel MB, Guérin M, Mancip J, Granio O, Penin F, Lavillette D, Bartenschlager R, Baumert TF, Cosset FL, Dreux M
The Journal of biological chemistry 2012 Sep 7;287(37):31242-57
The Journal of biological chemistry 2012 Sep 7;287(37):31242-57
Expression and distribution of symplekin regulates the assembly and function of the epithelial tight junction.
Chang H, Zhang C, Cao Y
Histochemistry and cell biology 2012 Mar;137(3):319-27
Histochemistry and cell biology 2012 Mar;137(3):319-27
Claudin-1, claudin-2 and claudin-11 genes differentially associate with distinct types of anti-inflammatory macrophages in vitro and with parasite- and tumour-elicited macrophages in vivo.
Van den Bossche J, Laoui D, Morias Y, Movahedi K, Raes G, De Baetselier P, Van Ginderachter JA
Scandinavian journal of immunology 2012 Jun;75(6):588-98
Scandinavian journal of immunology 2012 Jun;75(6):588-98
Modulation of tight junction proteins in the perineurium to facilitate peripheral opioid analgesia.
Rittner HL, Amasheh S, Moshourab R, Hackel D, Yamdeu RS, Mousa SA, Fromm M, Stein C, Brack A
Anesthesiology 2012 Jun;116(6):1323-34
Anesthesiology 2012 Jun;116(6):1323-34
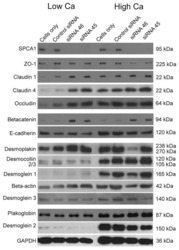
Hailey-Hailey disease and tight junctions: Claudins 1 and 4 are regulated by ATP2C1 gene encoding Ca(2+) /Mn(2+) ATPase SPCA1 in cultured keratinocytes.
Raiko L, Siljamäki E, Mahoney MG, Putaala H, Suominen E, Peltonen J, Peltonen S
Experimental dermatology 2012 Aug;21(8):586-91
Experimental dermatology 2012 Aug;21(8):586-91
Loss of claudin-1 in lipopolysaccharide-treated periodontal epithelium.
Fujita T, Firth JD, Kittaka M, Ekuni D, Kurihara H, Putnins EE
Journal of periodontal research 2012 Apr;47(2):222-7
Journal of periodontal research 2012 Apr;47(2):222-7
Dynamics of claudins expression in colitis and colitis-associated cancer in rat.
Arimura Y, Nagaishi K, Hosokawa M
Methods in molecular biology (Clifton, N.J.) 2011;762:409-25
Methods in molecular biology (Clifton, N.J.) 2011;762:409-25
The tight junction scaffolding protein cingulin regulates neural crest cell migration.
Wu CY, Jhingory S, Taneyhill LA
Developmental dynamics : an official publication of the American Association of Anatomists 2011 Oct;240(10):2309-23
Developmental dynamics : an official publication of the American Association of Anatomists 2011 Oct;240(10):2309-23
Comparison of tight junction protein expression in the ciliary epithelia of mouse, rabbit, cat and human eyes.
Karim MJ, Biswas S, Bhattacherjee P, Paterson CA
Biotechnic & histochemistry : official publication of the Biological Stain Commission 2011 Jun;86(3):161-7
Biotechnic & histochemistry : official publication of the Biological Stain Commission 2011 Jun;86(3):161-7
Protein kinase Cα inhibitor enhances the sensitivity of human pancreatic cancer HPAC cells to Clostridium perfringens enterotoxin via claudin-4.
Kyuno D, Kojima T, Ito T, Yamaguchi H, Tsujiwaki M, Takasawa A, Murata M, Tanaka S, Hirata K, Sawada N
Cell and tissue research 2011 Dec;346(3):369-81
Cell and tissue research 2011 Dec;346(3):369-81
Impact of intra- and interspecies variation of occludin on its function as coreceptor for authentic hepatitis C virus particles.
Ciesek S, Westhaus S, Wicht M, Wappler I, Henschen S, Sarrazin C, Hamdi N, Abdelaziz AI, Strassburg CP, Wedemeyer H, Manns MP, Pietschmann T, von Hahn T
Journal of virology 2011 Aug;85(15):7613-21
Journal of virology 2011 Aug;85(15):7613-21
Hepatitis C virus enters human peripheral neuroblastoma cells - evidence for extra-hepatic cells sustaining hepatitis C virus penetration.
Bürgel B, Friesland M, Koch A, Manns MP, Wedemeyer H, Weissenborn K, Schulz-Schaeffer WJ, Pietschmann T, Steinmann E, Ciesek S
Journal of viral hepatitis 2011 Aug;18(8):562-70
Journal of viral hepatitis 2011 Aug;18(8):562-70
Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders.
Lasagni L, Ballerini L, Angelotti ML, Parente E, Sagrinati C, Mazzinghi B, Peired A, Ronconi E, Becherucci F, Bani D, Gacci M, Carini M, Lazzeri E, Romagnani P
Stem cells (Dayton, Ohio) 2010 Sep;28(9):1674-85
Stem cells (Dayton, Ohio) 2010 Sep;28(9):1674-85
c-Jun N-terminal kinase is largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells.
Kojima T, Fuchimoto J, Yamaguchi H, Ito T, Takasawa A, Ninomiya T, Kikuchi S, Ogasawara N, Ohkuni T, Masaki T, Hirata K, Himi T, Sawada N
Journal of cellular physiology 2010 Nov;225(3):720-33
Journal of cellular physiology 2010 Nov;225(3):720-33
The second extracellular loop dictates Occludin-mediated HCV entry.
Liu S, Kuo W, Yang W, Liu W, Gibson GA, Dorko K, Watkins SC, Strom SC, Wang T
Virology 2010 Nov 10;407(1):160-70
Virology 2010 Nov 10;407(1):160-70
Glucocorticosteroids increase cell entry by hepatitis C virus.
Ciesek S, Steinmann E, Iken M, Ott M, Helfritz FA, Wappler I, Manns MP, Wedemeyer H, Pietschmann T
Gastroenterology 2010 May;138(5):1875-84
Gastroenterology 2010 May;138(5):1875-84
Increased B7-H1 expression on dendritic cells correlates with programmed death 1 expression on T cells in simian immunodeficiency virus-infected macaques and may contribute to T cell dysfunction and disease progression.
Xu H, Wang X, Pahar B, Moroney-Rasmussen T, Alvarez X, Lackner AA, Veazey RS
Journal of immunology (Baltimore, Md. : 1950) 2010 Dec 15;185(12):7340-8
Journal of immunology (Baltimore, Md. : 1950) 2010 Dec 15;185(12):7340-8
Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain.
Mullier A, Bouret SG, Prevot V, Dehouck B
The Journal of comparative neurology 2010 Apr 1;518(7):943-62
The Journal of comparative neurology 2010 Apr 1;518(7):943-62
Apolipoprotein E on hepatitis C virion facilitates infection through interaction with low-density lipoprotein receptor.
Owen DM, Huang H, Ye J, Gale M Jr
Virology 2009 Nov 10;394(1):99-108
Virology 2009 Nov 10;394(1):99-108
The mycotoxin patulin, modulates tight junctions in caco-2 cells.
McLaughlin J, Lambert D, Padfield PJ, Burt JP, O'Neill CA
Toxicology in vitro : an international journal published in association with BIBRA 2009 Feb;23(1):83-9
Toxicology in vitro : an international journal published in association with BIBRA 2009 Feb;23(1):83-9
Expression and localization of tricellulin in human nasal epithelial cells in vivo and in vitro.
Ohkuni T, Kojima T, Ogasawara N, Masaki T, Ninomiya T, Kikuchi S, Go M, Takano K, Himi T, Sawada N
Medical molecular morphology 2009 Dec;42(4):204-11
Medical molecular morphology 2009 Dec;42(4):204-11
Fatty acid synthase is up-regulated during hepatitis C virus infection and regulates hepatitis C virus entry and production.
Yang W, Hood BL, Chadwick SL, Liu S, Watkins SC, Luo G, Conrads TP, Wang T
Hepatology (Baltimore, Md.) 2008 Nov;48(5):1396-403
Hepatology (Baltimore, Md.) 2008 Nov;48(5):1396-403
Correlation of the tight junction-like distribution of Claudin-1 to the cellular tropism of hepatitis C virus.
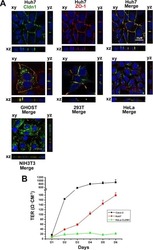
Yang W, Qiu C, Biswas N, Jin J, Watkins SC, Montelaro RC, Coyne CB, Wang T
The Journal of biological chemistry 2008 Mar 28;283(13):8643-53
The Journal of biological chemistry 2008 Mar 28;283(13):8643-53
Early events in HIV transmission through a human reconstructed vaginal mucosa.
Bouschbacher M, Bomsel M, Verronèse E, Gofflo S, Ganor Y, Dezutter-Dambuyant C, Valladeau J
AIDS (London, England) 2008 Jul 11;22(11):1257-66
AIDS (London, England) 2008 Jul 11;22(11):1257-66
Expression of Claudin-1, Claudin-3 and Claudin-5 in human blood-brain barrier mimicking cell line ECV304 is inducible by glioma-conditioned media.
Neuhaus W, Wirth M, Plattner VE, Germann B, Gabor F, Noe CR
Neuroscience letters 2008 Dec 3;446(2-3):59-64
Neuroscience letters 2008 Dec 3;446(2-3):59-64
Loss of claudins-1 and -7 and expression of claudins-3 and -4 correlate with prognostic variables in prostatic adenocarcinomas.
Sheehan GM, Kallakury BV, Sheehan CE, Fisher HA, Kaufman RP Jr, Ross JS
Human pathology 2007 Apr;38(4):564-9
Human pathology 2007 Apr;38(4):564-9
Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins.
Jacob C, Yang PC, Darmoul D, Amadesi S, Saito T, Cottrell GS, Coelho AM, Singh P, Grady EF, Perdue M, Bunnett NW
The Journal of biological chemistry 2005 Sep 9;280(36):31936-48
The Journal of biological chemistry 2005 Sep 9;280(36):31936-48
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
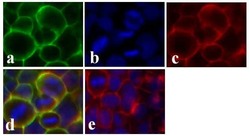
- Experimental details
- Immunofluorescent analysis of Claudin-1 was done on 90% confluent log phase CaCo-2 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes; permeabilized with 0.25% Triton X-100 for 10 minutes followed by blocking with 5% BSA for 1 hour at room temperature. The cells were incubated with Claudin-1 Mouse Monoclonal Antibody (Product # 37-4900) at 1 µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Rabbit Anti-Mouse IgG Secondary Antibody (Product # A-11059) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing junctional localization of Alexa Fluor 488 Rabbit Anti-Mouse IgG Secondary Antibody (Product # A-11059). and panel e is a no primary antibody control. The images were captured at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
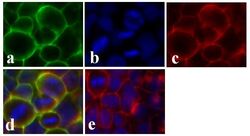
- Experimental details
- Immunofluorescent analysis of Claudin-1 was done on 90% confluent log phase CaCo-2 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes; permeabilized with 0.25% Triton X-100 for 10 minutes followed by blocking with 5% BSA for 1 hour at room temperature. The cells were incubated with Claudin-1 Mouse Monoclonal Antibody (Product # 37-4900) at 1 µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Rabbit Anti-Mouse IgG Secondary Antibody (Product # A-11059) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing junctional localization of Alexa Fluor 488 Rabbit Anti-Mouse IgG Secondary Antibody (Product # A-11059). and panel e is a no primary antibody control. The images were captured at 20X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
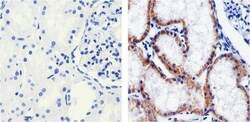
- Experimental details
- Immunohistochemistry analysis of Claudin 1 showing staining in the Membrane and Cytoplasm of paraffin-embedded human kidney tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Claudin 1 Mouse Monoclonal Antibody (Product # 37-9400) diluted in 3% BSA-PBS at a dilution of 1:100 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
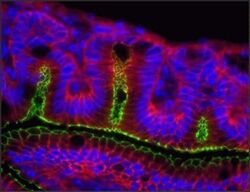
- Experimental details
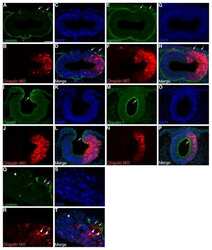
- Indirect immunofluorescence staining of frozen mouse jejunum tissue using Ms anti-Claudin-1 (red) (Product # 37-4900). ZO-1 is labeled in green; nuclei are stained with DAPI (blue). Image courtesy of Jennifer Holmes and Dr. James Anderson, University of North Carolina at Chapel Hill, NC.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
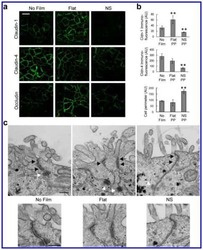
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
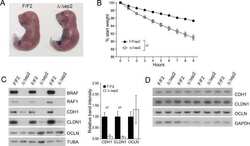
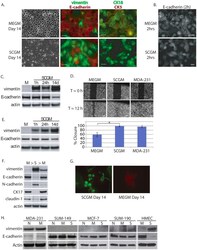
- Figure 4 Evidence for morphologic and phenotypic changes consistent with in vitro EMT. A. Phase contrast or immunofluorescence images of DKAT cells cultured in either MEGM (upper) or SCGM for 14 days (lower). Phase contrast images were acquired with a 20 x objective, scale bar = 40 um. IF images were acquired with a 40 x objective, scale bar = 20 um. Cells were stained simultaneously for vimentin (green) and E-cadherin (red), or CK18 (green) and CK5 (red). B. E-cadherin immunofluorescence images of DKAT cells cultured in MEGM or SCGM for 2 hours. C. Total cell lysates from DKAT cells cultured in MEGM or SCGM for 1 h, 24 h, or 14 days were analyzed by western blot for vimentin, E-cadherin and actin. D. Scratch wound healing assay comparing DKAT cells grown in MEGM or SCGM (14 days) and MDA-MB-231 cells. Images (upper panel) were taken at the time of the scratch (T = 0) and 12 hours later with a 5 x objective. Scale bar = 200 um. Results (lower panel) are expressed as the percentage of the scratch area closed at 12 hrs as measured by ImageJ software, and are an average of 12 scratches per sample. E. Total cell lysates from a clonal DKAT cell line cultured in MEGM or SCGM (14 days) were analyzed by western blot for vimentin, E-cadherin and actin. F. Total cell lysate from passage matched cultures of DKAT cells grown in MEGM, SCGM, or SCGM for 10 passages and then returned to MEGM for 5 passages were analyzed by western blot for epithelial and mesenchymal markers. G. Immunofluore
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
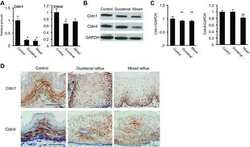
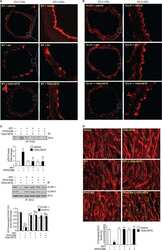
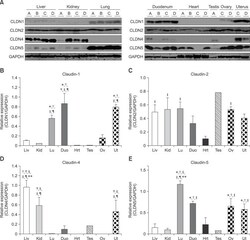
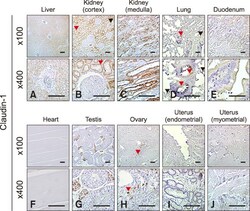
- Fig. 1 Protein levels of claudin-1, -2, -4 and -5 in equine tissues. Levels were measured by western blot (A) and were normalized against GAPDH protein (B-E; claudin-1, -2, -4 and -5, respectively). The signal intensity of each band was measured using the NIH Image J software (USA). Data are presented as the means +- standard error of the mean of four equine samples. Liv, Liver; Kid, Kidney; Lu, Lung; Duo, Duodenum; Hrt, Heart; Tes, Testis; Ov, Ovary; Ut, Uterus. * p < 0.01 vs . liver; + p < 0.01 vs . kidney; ++ p < 0.01 vs . lung; SS p < 0.01 vs . duodenum; p < 0.01 vs . heart; P p < 0.01 vs . ovary; ** p < 0.05 vs . uterus.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
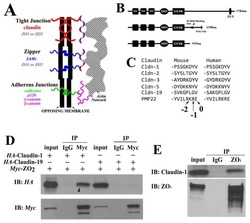
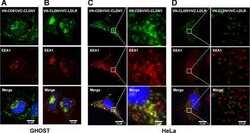
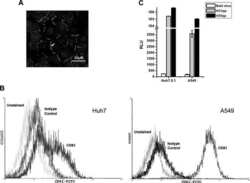
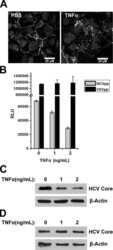
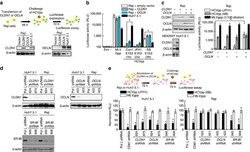
- Figure 4 HCV pseudo-particles (HCVpp) with envelope proteins of SB strain infected B lymphocytes, but not hepatic cells. ( a ) Claudin1 (CLDN1) or Occuludin (OCLN) expression confers weak susceptibility of Raji cells to JFH1 infection (Upper panel). Immunoblot analysis demonstrated that Claudin-1 and Occludin proteins were expressed by transfection of plasmids. ( b ) The respective expression vectors of CLDN1 and OCLN were transfected into both Raji and Huh7.5.1 cells. Luciferase-encoding pseudo-particles bearing the indicated envelope proteins were used to infect recipient Raji cells transfected with control vector (white), CLDN1-expressing vector (grey), or OCLN-expressing vector, or control Huh7.5.1 cells (black). For HCVpp, the respective E1E2 genes are indicated in parentheses. Luciferase assay was performed at 3 days after transduction (* P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
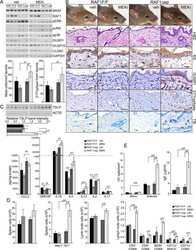
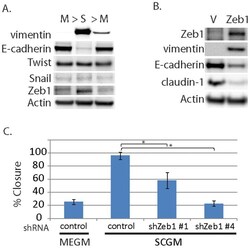
- Figure 5 Zeb1 regulates DKAT EMT/MET. A. Total cell lysate was prepared from DKAT-MEGM or DKAT-SCGM cells and subjected to western blot analysis for markers of EMT. B. Total cell lysate was prepared from DKAT-MEGM cells stably transfected with a control vector (V) or vector containing Zeb1 transcript (Zeb1) and subjected to western blot analysis for markers of EMT. C. Scratch wound healing assay comparing DKAT cells grown in MEGM or SCGM as indicated, stably expressing either a non-targeting shRNA sequence or one of two Zeb1-targeting sequences. Results are expressed as the percentage of the scratch area closed at 16 hrs as measured by ImageJ software, and are an average of 12 scratches per sample.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
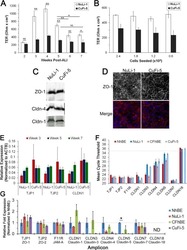
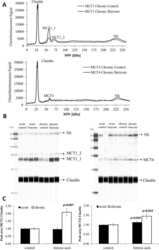
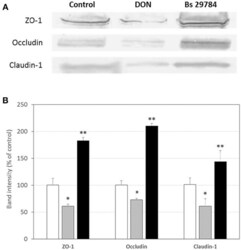
- Figure 2 Bs 29784 increases the expression of tight junction's proteins. Caco-2 cells were left untreated (white columns) or were treated apically for 16 h with DON at 100 muM (gray columns) or Bs 29784 at an initial bacterial density of 10 7 bacteria/ml (black columns). ZO-1, occludin, and claudin-1 proteins were visualized by Western-blot (A) . Band densities were measured using Image J software and subjected to statistical analysis (B) . * and ** Significant differences between the groups, p < 0.05 and p < 0.01, respectively.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
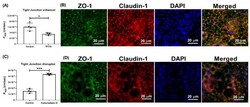
- Figure 5 Effect of TF3'G and cytochalasin D on the transport of gamma-EV across Caco-2 monolayer cells. ( A ) The transport of 1 mM gamma-EV, assessed over a period of 2 h, after the pretreatment of TF3'G (20 muM, 24 h). ( B ) Immunofluorescence staining of the tight-junction proteins ZO-1 and claudin-1 of the TF3'G-treated Caco-2 monolayer cells on the membrane, to ensure the maintenance of cell integrity after 2 h of transport. ( C ) The transport of 1 mM gamma-EV, assessed over a period of 2 h, after the pretreatment of cytochalasin D (0.5 mug/mL, 30 min). ( D ) Immunofluorescence staining of the tight-junction proteins ZO-1 and claudin-1 of the cytochalasin-treated Caco-2 monolayer cells on the membrane, to ensure the maintenance of cell integrity after 2 h of transport. Control immunofluorescence images are same as those mentioned in Figure 4 . Data are presented as the mean +- SD from at least n = 3 (TF3'G and cytochalasin D) and n = 4 (control) independent experiments; *, and *** indicate p < 0.05 and p < 0.001, respectively.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
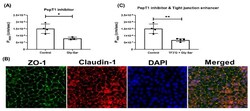
- Figure 6 Effect of Gly-Sar on the transport of gamma-EV across Caco-2 monolayer cells. ( A ) The transport of 1 mM gamma-EV, assessed over a period of 2 h, after the pretreatment of Gly-Sar (25 mM, 30 min). ( B ) Immunofluorescence staining of the tight-junction proteins ZO-1 and claudin-1 of the Gly-Sar-treated Caco-2 monolayer cells on the membrane, to ensure the maintenance of cell integrity after 2 h of transport. ( C ) The transport of 1 mM gamma-EV, assessed over a period of 2 h, after the pre-treatment of both TF3'G and Gly-Sar simultaneously. Control immunofluorescence images are same as those mentioned in Figure 4 . Data presented as the mean +- SD from at least n = 3 (Gly-Sar) and n = 4 (control and TF3'G/Gly-Sar) independent experiments; * and ** indicate p < 0.05 and p < 0.01, respectively.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
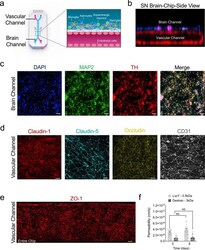
- Experimental details
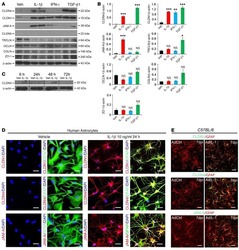
- Fig. 1 Reconstitution of the human substantia nigra Substantia Nigra Brain-Chip. a Schematic depiction of the Substantia Nigra Brain-Chip of a two-channel microengineered chip with iPSC-derived brain endothelial cells cultured on all surfaces of the lower vascular channel, and iPSC-derived dopaminergic neurons, primary human brain astrocytes, microglia, and pericytes on the upper surface of the central horizontal membrane in the upper brain channel. b 3D reconstruction of a confocal z-stack showing the organization of all five cell types in the Substantia Nigra (SN) Brain-Chip. c Representative image of iPSC-derived dopaminergic neurons that are stained with DAPI (blue), microtubule-associated protein 2 (green, MAP2), tyrosine hydroxylase (red, TH), and a merged image on day 8. Scale bars: 100 mum. d Immunofluorescence micrographs of the human brain endothelium cultured on the vascular channel of Brain-Chip for 7 days post-seeding (D8) labeled with Claudin-1 (red), Claudin-5 (cyan), Occludin (yellow), and CD31 (white). Scale bars: 100 mum. e Immunofluorescence micrographs demonstrate high levels of expression of zonula occludens-1 (red, ZO-1) across the entire endothelial monolayer. Scale bars: 100 mum. f Quantitative barrier function analysis via apparent permeability to 3 kDa fluorescent dextran, and 0.5 kDa lucifer yellow (LucY) crossing through the vascular to the neuronal channel on day 5 and 8. Statistical analysis is two-way ANOVA with Tukey's multiple comparisons test
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
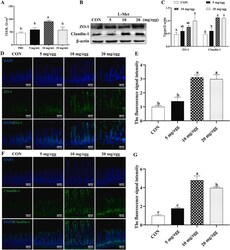
- Experimental details
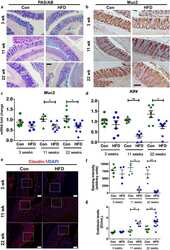
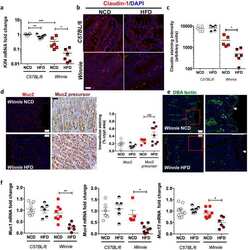
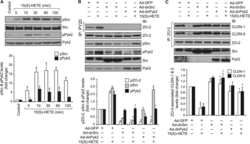
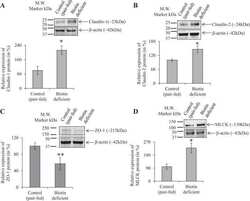
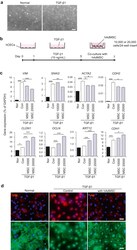
- Fig. 3 The in ovo injection of methionine enhances the intestinal barrier function of chicks. (A) Transepithelial cell resistance (TEER) of the jejuna of chicks injected with phosphate-buffered saline (PBS) or L-methionine (L-Met). (B to C) The expression of ZO-1 and claudin-1 in the jejunum of chicks injected with PBS or L-Met. (D) Representative images of immunofluorescence (IF) staining with a ZO-1 antibody in the chick jejunum are shown (200x magnification; scale bar, 100 mum). (E) Statistical analysis of the fluorescence signal intensity based on the images shown in (D). (F) Representative images of IF staining with a claudin 1 antibody in the chick jejunum are shown (200x magnification; scale bar, 100 mum). (G) Statistical analysis of the fluorescence signal intensity based on the images shown in (F). Bars without a common letter indicate significant differences ( P < 0.05); the values are means +- SEM. n = 3. CON = control. Fig. 3
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
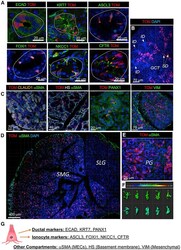
- Experimental details
- Figure 4. In adult SMGs and PGs, FGF10 pos cells are restricted to the ED, SD, and GCT ducts and express ionocyte markers (A) In adult glands, FGF10 pos cells (TOM, red) express E-cadherin (ECAD, green), KRT7 (green), ASCL3 (green), FOXI1 (green), NKCC1 (green), and CFTR (green), which are expressed at the apical membrane of FGF10 pos cells. Nuclei labeled with DAPI (blue). (B) In adult SMG FGF10 pos cells (TOM, red) were found in the GCTs (red arrows) and SDs (yellow arrows) but not in the IDs (white arrows). (C) FGF10 pos cells were labeled at P29/30 (TOM, red) and stained with different antibodies. Acini were labeled with claudin (orange); MECs were labeled with alphaSMA (green); the basement membrane was labeled with heparan sulfate (HS) (gray); ducts were labeled with pannexin-1 (PANX1); stromal cells were labeled with VIM (green); nuclei labeled with DAPI (blue). (D and E) FGF10 pos cells (TOM, red) were found within ducts of SMGs (D) and PGs (E), while not in SLGs (D). FGF10 pos cells were detected only within the mesenchyme. (F) IMARIS bounding box software was used to analyze shape and size of FGF10 pos cells. (G) Summary of immunostaining used to label different cell types and gland compartments. Also see Figures S4 and S5 , Data S6 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
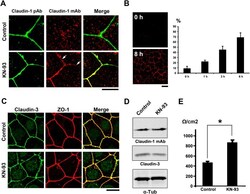
- Experimental details
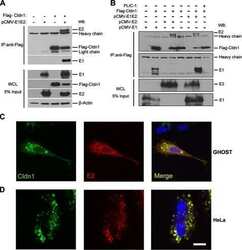
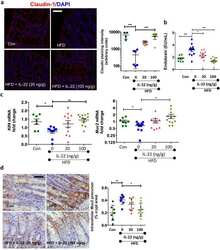
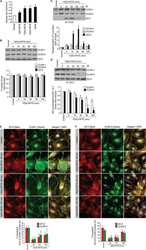
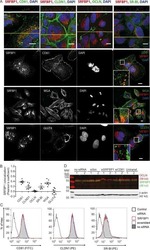
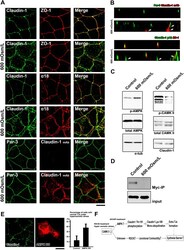
- Figure 2 KN-93 induces up-regulation of barrier function ( A ) Claudin-1 mAb (Clone 2H10D10) recognized claudin-1, which contributed to the formation of ectopic TJs induced by the treatment with KN-93 (arrows). Scale bar, 5 mum. ( B ) Quantification of the time-dependent effect of KN-93 on the enlargement of TJs. EpH4 cells treated with KN-93 for the indicated times were stained with DAPI and the anti-claudin-1 mAb (2H10D10). The number of cells with extended TJs was determined as a percentage of the total number of cells (based on DAPI staining) in the same field. Each experiment (n = 3) included 100 cells. Data are represented as the mean +- s.d. ( C ) EpH4 cells were treated with DMSO (control) or 10 muM KN-93 for 5 hours and stained with anti-claudin-3 pAb (green) and anti-ZO-1 mAb (red). Scale bar, 20 mum. ( D ) Immunoblotting of whole-cell lysates from EpH4 cells treated with DMSO (control) or 10 muM KN-93 with the indicated antibodies. ( E ) CSG1 cells were cultured in Transwell chambers and treated with DMSO (control) or 10 muM KN-93 for 5 hours and then analyzed for their TER. Analysis of TER showed about a 1.8-fold increase after the treatment with KN-93. Data are mean +- s.d.; *P < 0.05 (Student's t-test).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 5 TJs enlarge as an adaptation response to hyperosmotic stress ( A ) CSG1 cells were treated with hyperosmotic medium for 2 hours and doubly stained with anti-claudin-1 pAb (green) and anti-ZO-1 mAb (red) (upper panels), anti-claudin-1 pAb (green) and alpha18 antibody (red) (middle panels) and/or anti-Par-3 pAb (green) and anti-claudin-1 mAb (Clone 2H10D10) (red) (lower panels). Scale bar, 20 mum. ( B ) Z-sections of CSG1 cells treated with hyperosmotic medium. ( C ) Immunoblotting of whole-cell lysates of CSG1 cells treated with normal osmotic medium or 600 mOsm/L medium for 2 hours with the indicated antibodies. ( D ) CSG1 cells stably expressing myc-ubiquitin were cultured in normal medium or hyperosmotic medium for 2 hours. The cell lysates were subjected to immunoprecipitation with anti-myc antibody, followed by immunoblotting with anti-claudin-1 antibody. ( E ) Over-expression of dominant negative AMPK2alpha suppressed the formation of extra TJs, even under hyperosmotic conditions. CSG1 cells were transfected with mCherry only as a control or mCherry-AMPKalpha2 K45M. Then, cells were cultured in hyperosmotic medium for 2 hours. Then, cells were fixed and stained for anti-claudin-1 pAb (green). The percentages were calculated as (the number of cells with normal TJs under hyperosmotic stress)/(the number of cells transfected with mCherry only or mCherry-AMPKalpha2 K45M). In each experiment, the total cell number was 100 (n = 3). Data are mean +- s.d.; *P < 0.05 (St
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. 2 Localization of claudin-1 in equine tissues via immunohistochemistry. Scale bar = 100 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
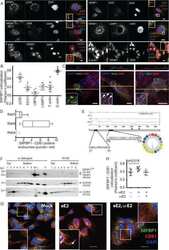
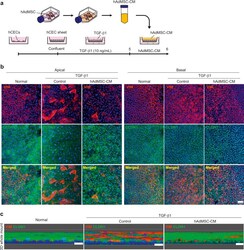
- Fig. 1 Co-culture with mesenchymal stem cells (MSCs) attenuates TGF-beta1-induced epithelial-mesenchymal transition (EMT) in corneal epithelial cells (CECs). (a) Phase contrast images of CECs with or without TGF-beta1 treatment. Scale bar, 100 mum. (b) Schematic of experimental method. (c) Gene expression analysis of EMT-related markers in CECs with or without co-culture with AdMSC (10,000 or 20,000 cells/insert). Data are expressed as the means +- SEM; n = 4 cell samples; *p < 0.05, **p < 0.01, and ***p < 0.001. (d) Immunostaining for VIM (red) and CLDN1 (green) in CECs. Nuclei, blue; scale bars, 100 mum. Fig. 1
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. 3 Conditioned medium from Adipose-derived mesenchymal stem cells (AdMSC-CM) attenuates TGF-beta1-induced epithelial-mesenchymal transition (EMT) in corneal epithelial cell (CEC) sheets. (a) Schematic of experimental method. (b) Immunostaining for VIM (red) and CLDN1 (green) in CEC sheets at the apical and basal region. Nuclei, blue. Scale bar, 50 mum. (c) Immunostaining for VIM (red) and CLDN1 (green) in whole-mounts of CEC sheets constructed as a three-dimensional image. Nuclei, blue. Scale bars, 20 mum. Fig. 3
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
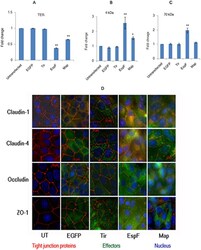
- Experimental details
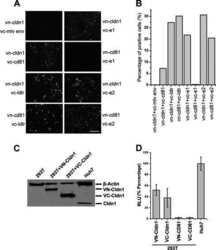
- Figure 1 Constitutive expression of EGFP-EspF and EGFP-Map in MDCK cells disrupts the tight junction barrier. ( A ) Untransfected cells or cells constitutively expressing EGFP vector alone, EGFP-Tir, EGFP-EspF and EGFP-Map were cultured on polycarbonate Transwell filters and TER was measured daily to monitor the integrity of tight junctions. The maximum values of TER for each cell line are indicated. The paracellular flux of 4 kDa dextran ( B ) and 70 kDa dextran ( C ) was measured and plotted as fold change with respect to untransfected MDCK cells (normalized to 1). Error bars represent means +- s.e.m from three independent experiments; *indicates p-value < 0.01 and **indicates p-value < 0.001. ( D ) The cellular localization of TJ proteins was examined using the indicated primary antibodies and Cy3-conjugated secondary antibodies. Nucleus was labeled with DAPI (4',6-Diamidino-2-phenylindole dihydrochloride). Constitutive expression of EGFP-EspF and EGFP-Map caused the reorganization of claudin-1, -4, occludin and ZO-1. Scale: 10 um; UT: Untransfected.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
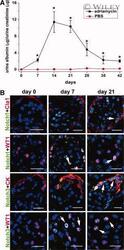
- Experimental details
- 6 Upregulation of the Notch signaling components in podocytes and/or renal progenitors of SCID mice with adriamycin nephropathy. ( A ): Time course assessment of albumin/creatinine ratio as measured in mice with adriamycin nephropathy (black square) in comparison with PBS-treated mice (red circle). Data are expressed as mean +- SEM as obtained in three independent experiments ( n = 12 mice at each time point for each group of treatment); *, p < .05. ( B ): Double label immunofluorescence for Notch1 (green) and Claudin-1 (Cla1, red); Notch1 (green) and WT1 (red); Notch3 (green) and cytokeratin (CK, red); Notch3 (green) and WT1 (red) in SCID mice affected by adriamycin nephropathy at different time points. Topro-3 counterstains nuclei (blue). One representative of three independent experiments performed on a total of 12 mice is shown. Scale bar = 20 mum. Abbreviations: PBS, phosphate buffered saline; SCID, severe combined immunodeficient.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
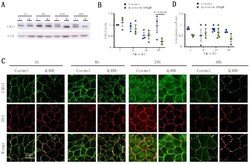
- Experimental details
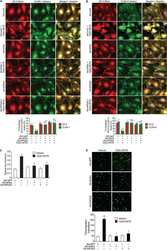
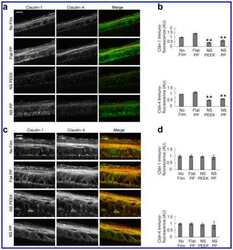
- Figure 2 Analysis of Cldn1 expression and localization in MDCK II cells following quercetin treatment. ( A ) Western blot analysis of Cldn1 expression in cell lysates from control and 400 uM quercetin-treated MDCK II cells. Actin was used as a loading control. ( B ) Densitometry measurements of Cldn1 and actin intensity on western blot were normalized to expression at 1h. Normalized Cldn1 expression relative to normalized actin expression is plotted (P int = 0.11; P time = 0.022; P treat = 0.16). ( C ) Immunofluorescence of control and 400 uM of quercetin-treated MDCK II cells at 1, 6, 24, and 48 h. Cldn1 is shown in green and ZO1 is shown in red. ( D ) Localization of claudin at the tight junction was assessed by determining the amount of ZO1 that was co-localized with claudin expression (P int = 0.24; P time = 0.35; P treat = 0.055). For all graphs, each point corresponds to an independent experiment; mean and SEM are shown. C = Control and Q = Quercetin. Scale bar, 20 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 2 Expression of TJ proteins in the intestine of rats from different experimental groups: ES--rats were orally gavaged with Saccharomyces cerevisiae fermentate and exposed to heat stress; EC--rats were orally gavaged with Saccharomyces cerevisiae fermentate and kept at room temperature; PS--rats were orally gavaged with PBS and exposed to heat stress; PC--rats were orally gavaged with PBS and kept at room temperature; * P < 0*05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
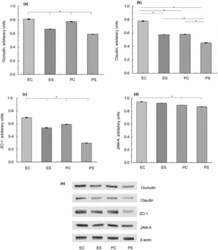
- Experimental details
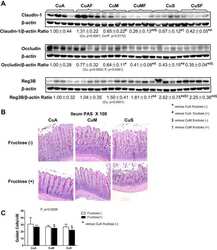
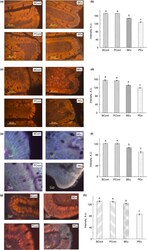
- Figure 3 Immunofluorescence staining of TJ proteins in the intestine of rats from different experimental groups. (a) Images of occludin immunostaining, Bar 50 um; (b) Analysis of occludin expression with immunostaining; (c) Images of ZO-1 immunostaining, bar 50 um; (d) Analysis of ZO-1 expression with immunostaining; (e) Images of claudin immunostaining, bar 10 um; (f) Analysis of claudin expression with immunostaining; (g) Images of JAM-A immunostaining, bar 10 um; (h) Analysis of JAM-A expression with immunostaining. A.U., arbitrary units. Rats were pretreated by oral gavage with B. subtilis BSB3 (BSB3) or with PBS and were either exposed forced running or remained sedentary ( n = 6 rats per condition). BEx, pretreated with BSB3, undergoing forced running; BCont, pretreated with BSB3 remaining sedentary; PEx, pretreated with PBS, undergoing forced running; PCont, pretreated with PBS remaining sedentary. Data with different superscript letters signify groups are significantly different ( P < 0*05). [Colour figure can be viewed at https://www.wileyonlinelibrary.com ]
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry