Antibody data
- Antibody Data
- Antigen structure
- References [53]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Other assay [33]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 352588 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Claudin 5 Monoclonal Antibody (4C3C2), Alexa Fluor™ 488
- Antibody type
- Monoclonal
- Antigen
- Synthetic peptide
- Description
- Reactivity has been confirmed with rat, human and mouse Claudin-5 using rat lung, mouse kidney, mouse small intestine, mouse lung homogenates, human colon tissue, and CACO-2 human cell line. This antibody reacts specifically with the ~ 22-24 kDa endogenous Claudin-5 protein. Excitation/Peak Emission (nm): 495/519
- Reactivity
- Human, Mouse, Rat
- Host
- Mouse
- Conjugate
- Green dye
- Isotype
- IgG
- Antibody clone number
- 4C3C2
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- 4°C
Submitted references Genome Editing with AAV-BR1-CRISPR in Postnatal Mouse Brain Endothelial Cells.
Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier.
Endothelial Unc5B controls blood-brain barrier integrity.
Vascular Sema3E-Plexin-D1 Signaling Reactivation Promotes Post-stroke Recovery through VEGF Downregulation in Mice.
Mfsd2b and Spns2 are essential for maintenance of blood vessels during development and in anaphylactic shock.
Retinoid signaling regulates angiogenesis and blood-retinal barrier integrity in neonatal mouse retina.
Cryopreservation of human cerebral microvascular endothelial cells and astrocytes in suspension and monolayers.
Phosphorylated cingulin localises GEF-H1 at tight junctions to protect vascular barriers in blood endothelial cells.
Erythrocytes, a New Contributor to Age-Associated Loss of Blood-Brain Barrier Integrity.
A cellular and spatial map of the choroid plexus across brain ventricles and ages.
A Norrin/Wnt surrogate antibody stimulates endothelial cell barrier function and rescues retinopathy.
Hepatocyte growth factor-regulated tyrosine kinase substrate is essential for endothelial cell polarity and cerebrovascular stability.
Increased Catecholamine Levels and Inflammatory Mediators Alter Barrier Properties of Brain Microvascular Endothelial Cells in vitro.
Burns Impair Blood-Brain Barrier and Mesenchymal Stem Cells Can Reverse the Process in Mice.
Mapping endothelial-cell diversity in cerebral cavernous malformations at single-cell resolution.
A mouse model for kinesin family member 11 (Kif11)-associated familial exudative vitreoretinopathy.
3D brain angiogenesis model to reconstitute functional human blood-brain barrier in vitro.
Sphingosine 1-Phosphate Receptor Signaling Establishes AP-1 Gradients to Allow for Retinal Endothelial Cell Specialization.
Single-cell profiling reveals an endothelium-mediated immunomodulatory pathway in the eye choroid.
MEK/MELK inhibition and blood-brain barrier deficiencies in atypical teratoid/rhabdoid tumors.
Sphingosine 1-phosphate-regulated transcriptomes in heterogenous arterial and lymphatic endothelium of the aorta.
Fgfbp1 promotes blood-brain barrier development by regulating collagen IV deposition and maintaining Wnt/β-catenin signaling.
Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation.
Dlg1 activates beta-catenin signaling to regulate retinal angiogenesis and the blood-retina and blood-brain barriers.
Blood-retina barrier failure and vision loss in neuron-specific degeneration.
Brain Endothelial Cells Maintain Lactate Homeostasis and Control Adult Hippocampal Neurogenesis.
Regional Differences in Tight Junction Protein Expression in the Blood-DRG Barrier and Their Alterations after Nerve Traumatic Injury in Rats.
Mixed Vehicle Emissions Induces Angiotensin II and Cerebral Microvascular Angiotensin Receptor Expression in C57Bl/6 Mice and Promotes Alterations in Integrity in a Blood-Brain Barrier Coculture Model.
Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures.
CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature.
Role of ADTRP (Androgen-Dependent Tissue Factor Pathway Inhibitor Regulating Protein) in Vascular Development and Function.
Interplay of the Norrin and Wnt7a/Wnt7b signaling systems in blood-brain barrier and blood-retina barrier development and maintenance.
Glial scars are permeable to the neurotoxic environment of chronic stroke infarcts.
Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis.
Gradual Suppression of Transcytosis Governs Functional Blood-Retinal Barrier Formation.
Transdifferentiated Human Vascular Smooth Muscle Cells are a New Potential Cell Source for Endothelial Regeneration.
Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown.
Vascular Pericyte Impairment and Connexin43 Gap Junction Deficit Contribute to Vasomotor Decline in Diabetic Retinopathy.
Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation.
Size-selective opening of the blood-brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1.
Appearance of claudin-5(+) leukocytes in the central nervous system during neuroinflammation: a novel role for endothelial-derived extracellular vesicles.
Presymptomatic activation of the PDGF-CC pathway accelerates onset of ALS neurodegeneration.
CD8+ T Cells Induce Fatal Brainstem Pathology during Cerebral Malaria via Luminal Antigen-Specific Engagement of Brain Vasculature.
Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina.
Real-time estimation of paracellular permeability of cerebral endothelial cells by capacitance sensor array.
Structural and functional features of central nervous system lymphatic vessels.
Canonical WNT signaling components in vascular development and barrier formation.
Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling.
The role of the hypoxia response in shaping retinal vascular development in the absence of Norrin/Frizzled4 signaling.
Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease.
EndMT contributes to the onset and progression of cerebral cavernous malformations.
Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity.
Wnt signaling mediates pathological vascular growth in proliferative retinopathy.
Song X, Cui Y, Wang Y, Zhang Y, He Q, Yu Z, Xu C, Ning H, Han Y, Cai Y, Cheng X, Wang J, Teng Y, Yang X, Wang J
International journal of biological sciences 2022;18(2):652-660
International journal of biological sciences 2022;18(2):652-660
Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier.
Ayloo S, Lazo CG, Sun S, Zhang W, Cui B, Gu C
Neuron 2022 May 18;110(10):1641-1655.e6
Neuron 2022 May 18;110(10):1641-1655.e6
Endothelial Unc5B controls blood-brain barrier integrity.
Boyé K, Geraldo LH, Furtado J, Pibouin-Fragner L, Poulet M, Kim D, Nelson B, Xu Y, Jacob L, Maissa N, Agalliu D, Claesson-Welsh L, Ackerman SL, Eichmann A
Nature communications 2022 Mar 4;13(1):1169
Nature communications 2022 Mar 4;13(1):1169
Vascular Sema3E-Plexin-D1 Signaling Reactivation Promotes Post-stroke Recovery through VEGF Downregulation in Mice.
Yu R, Kim NS, Li Y, Jeong JY, Park SJ, Zhou B, Oh WJ
Translational stroke research 2022 Feb;13(1):142-159
Translational stroke research 2022 Feb;13(1):142-159
Mfsd2b and Spns2 are essential for maintenance of blood vessels during development and in anaphylactic shock.
Le TNU, Nguyen TQ, Kalailingam P, Nguyen YTK, Sukumar VK, Tan CKH, Tukijan F, Couty L, Hasan Z, Del Gaudio I, Wenk MR, Cazenave-Gassiot A, Camerer E, Nguyen LN
Cell reports 2022 Aug 16;40(7):111208
Cell reports 2022 Aug 16;40(7):111208
Retinoid signaling regulates angiogenesis and blood-retinal barrier integrity in neonatal mouse retina.
Engelbrecht E, Metzler MA, Sandell LL
Microcirculation (New York, N.Y. : 1994) 2022 Apr;29(3):e12752
Microcirculation (New York, N.Y. : 1994) 2022 Apr;29(3):e12752
Cryopreservation of human cerebral microvascular endothelial cells and astrocytes in suspension and monolayers.
Marquez-Curtis LA, Bokenfohr R, McGann LE, Elliott JAW
PloS one 2021;16(4):e0249814
PloS one 2021;16(4):e0249814
Phosphorylated cingulin localises GEF-H1 at tight junctions to protect vascular barriers in blood endothelial cells.
Holzner S, Bromberger S, Wenzina J, Neumüller K, Holper TM, Petzelbauer P, Bauer W, Weber B, Schossleitner K
Journal of cell science 2021 Sep 1;134(17)
Journal of cell science 2021 Sep 1;134(17)
Erythrocytes, a New Contributor to Age-Associated Loss of Blood-Brain Barrier Integrity.
Amiri P, DeCastro J, Littig J, Lu HW, Liu C, Conboy I, Aran K
Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2021 Oct;8(20):e2101912
Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2021 Oct;8(20):e2101912
A cellular and spatial map of the choroid plexus across brain ventricles and ages.
Dani N, Herbst RH, McCabe C, Green GS, Kaiser K, Head JP, Cui J, Shipley FB, Jang A, Dionne D, Nguyen L, Rodman C, Riesenfeld SJ, Prochazka J, Prochazkova M, Sedlacek R, Zhang F, Bryja V, Rozenblatt-Rosen O, Habib N, Regev A, Lehtinen MK
Cell 2021 May 27;184(11):3056-3074.e21
Cell 2021 May 27;184(11):3056-3074.e21
A Norrin/Wnt surrogate antibody stimulates endothelial cell barrier function and rescues retinopathy.
Chidiac R, Abedin M, Macleod G, Yang A, Thibeault PE, Blazer LL, Adams JJ, Zhang L, Roehrich H, Jo HN, Seshagiri S, Sidhu SS, Junge HJ, Angers S
EMBO molecular medicine 2021 Jul 7;13(7):e13977
EMBO molecular medicine 2021 Jul 7;13(7):e13977
Hepatocyte growth factor-regulated tyrosine kinase substrate is essential for endothelial cell polarity and cerebrovascular stability.
Yu Z, Zeng J, Wang J, Cui Y, Song X, Zhang Y, Cheng X, Hou N, Teng Y, Lan Y, Chen Y, Yang X
Cardiovascular research 2021 Jan 21;117(2):533-546
Cardiovascular research 2021 Jan 21;117(2):533-546
Increased Catecholamine Levels and Inflammatory Mediators Alter Barrier Properties of Brain Microvascular Endothelial Cells in vitro.
Ittner C, Burek M, Störk S, Nagai M, Förster CY
Frontiers in cardiovascular medicine 2020;7:73
Frontiers in cardiovascular medicine 2020;7:73
Burns Impair Blood-Brain Barrier and Mesenchymal Stem Cells Can Reverse the Process in Mice.
Yang J, Ma K, Zhang C, Liu Y, Liang F, Hu W, Bian X, Yang S, Fu X
Frontiers in immunology 2020;11:578879
Frontiers in immunology 2020;11:578879
Mapping endothelial-cell diversity in cerebral cavernous malformations at single-cell resolution.
Orsenigo F, Conze LL, Jauhiainen S, Corada M, Lazzaroni F, Malinverno M, Sundell V, Cunha SI, Brännström J, Globisch MA, Maderna C, Lampugnani MG, Magnusson PU, Dejana E
eLife 2020 Nov 3;9
eLife 2020 Nov 3;9
A mouse model for kinesin family member 11 (Kif11)-associated familial exudative vitreoretinopathy.
Wang Y, Smallwood PM, Williams J, Nathans J
Human molecular genetics 2020 May 8;29(7):1121-1131
Human molecular genetics 2020 May 8;29(7):1121-1131
3D brain angiogenesis model to reconstitute functional human blood-brain barrier in vitro.
Lee S, Chung M, Lee SR, Jeon NL
Biotechnology and bioengineering 2020 Mar;117(3):748-762
Biotechnology and bioengineering 2020 Mar;117(3):748-762
Sphingosine 1-Phosphate Receptor Signaling Establishes AP-1 Gradients to Allow for Retinal Endothelial Cell Specialization.
Yanagida K, Engelbrecht E, Niaudet C, Jung B, Gaengel K, Holton K, Swendeman S, Liu CH, Levesque MV, Kuo A, Fu Z, Smith LEH, Betsholtz C, Hla T
Developmental cell 2020 Mar 23;52(6):779-793.e7
Developmental cell 2020 Mar 23;52(6):779-793.e7
Single-cell profiling reveals an endothelium-mediated immunomodulatory pathway in the eye choroid.
Lehmann GL, Hanke-Gogokhia C, Hu Y, Bareja R, Salfati Z, Ginsberg M, Nolan DJ, Mendez-Huergo SP, Dalotto-Moreno T, Wojcinski A, Ochoa F, Zeng S, Cerliani JP, Panagis L, Zager PJ, Mullins RF, Ogura S, Lutty GA, Bang J, Zippin JH, Romano C, Rabinovich GA, Elemento O, Joyner AL, Rafii S, Rodriguez-Boulan E, Benedicto I
The Journal of experimental medicine 2020 Jun 1;217(6)
The Journal of experimental medicine 2020 Jun 1;217(6)
MEK/MELK inhibition and blood-brain barrier deficiencies in atypical teratoid/rhabdoid tumors.
Meel MH, Guillén Navarro M, de Gooijer MC, Metselaar DS, Waranecki P, Breur M, Lagerweij T, Wedekind LE, Koster J, van de Wetering MD, Schouten-van Meeteren N, Aronica E, van Tellingen O, Bugiani M, Phoenix TN, Kaspers GJL, Hulleman E
Neuro-oncology 2020 Jan 11;22(1):58-69
Neuro-oncology 2020 Jan 11;22(1):58-69
Sphingosine 1-phosphate-regulated transcriptomes in heterogenous arterial and lymphatic endothelium of the aorta.
Engelbrecht E, Levesque MV, He L, Vanlandewijck M, Nitzsche A, Niazi H, Kuo A, Singh SA, Aikawa M, Holton K, Proia RL, Kono M, Pu WT, Camerer E, Betsholtz C, Hla T
eLife 2020 Feb 24;9
eLife 2020 Feb 24;9
Fgfbp1 promotes blood-brain barrier development by regulating collagen IV deposition and maintaining Wnt/β-catenin signaling.
Cottarelli A, Corada M, Beznoussenko GV, Mironov AA, Globisch MA, Biswas S, Huang H, Dimberg A, Magnusson PU, Agalliu D, Lampugnani MG, Dejana E
Development (Cambridge, England) 2020 Aug 24;147(16)
Development (Cambridge, England) 2020 Aug 24;147(16)
Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation.
Niu J, Tsai HH, Hoi KK, Huang N, Yu G, Kim K, Baranzini SE, Xiao L, Chan JR, Fancy SPJ
Nature neuroscience 2019 May;22(5):709-718
Nature neuroscience 2019 May;22(5):709-718
Dlg1 activates beta-catenin signaling to regulate retinal angiogenesis and the blood-retina and blood-brain barriers.
Cho C, Wang Y, Smallwood PM, Williams J, Nathans J
eLife 2019 May 8;8
eLife 2019 May 8;8
Blood-retina barrier failure and vision loss in neuron-specific degeneration.
Ivanova E, Alam NM, Prusky GT, Sagdullaev BT
JCI insight 2019 Mar 19;5(8)
JCI insight 2019 Mar 19;5(8)
Brain Endothelial Cells Maintain Lactate Homeostasis and Control Adult Hippocampal Neurogenesis.
Wang J, Cui Y, Yu Z, Wang W, Cheng X, Ji W, Guo S, Zhou Q, Wu N, Chen Y, Chen Y, Song X, Jiang H, Wang Y, Lan Y, Zhou B, Mao L, Li J, Yang H, Guo W, Yang X
Cell stem cell 2019 Dec 5;25(6):754-767.e9
Cell stem cell 2019 Dec 5;25(6):754-767.e9
Regional Differences in Tight Junction Protein Expression in the Blood-DRG Barrier and Their Alterations after Nerve Traumatic Injury in Rats.
Lux TJ, Hu X, Ben-Kraiem A, Blum R, Chen JT, Rittner HL
International journal of molecular sciences 2019 Dec 31;21(1)
International journal of molecular sciences 2019 Dec 31;21(1)
Mixed Vehicle Emissions Induces Angiotensin II and Cerebral Microvascular Angiotensin Receptor Expression in C57Bl/6 Mice and Promotes Alterations in Integrity in a Blood-Brain Barrier Coculture Model.
Suwannasual U, Lucero J, Davis G, McDonald JD, Lund AK
Toxicological sciences : an official journal of the Society of Toxicology 2019 Aug 1;170(2):525-535
Toxicological sciences : an official journal of the Society of Toxicology 2019 Aug 1;170(2):525-535
Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures.
Wang Y, Sabbagh MF, Gu X, Rattner A, Williams J, Nathans J
eLife 2019 Apr 1;8
eLife 2019 Apr 1;8
CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature.
Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, Da Mesquita S, Frost EL, Gaultier A, Harris TH, Cao R, Hu S, Lukens JR, Smirnov I, Overall CC, Oliver G, Kipnis J
Nature neuroscience 2018 Oct;21(10):1380-1391
Nature neuroscience 2018 Oct;21(10):1380-1391
Role of ADTRP (Androgen-Dependent Tissue Factor Pathway Inhibitor Regulating Protein) in Vascular Development and Function.
Patel MM, Behar AR, Silasi R, Regmi G, Sansam CL, Keshari RS, Lupu F, Lupu C
Journal of the American Heart Association 2018 Nov 20;7(22):e010690
Journal of the American Heart Association 2018 Nov 20;7(22):e010690
Interplay of the Norrin and Wnt7a/Wnt7b signaling systems in blood-brain barrier and blood-retina barrier development and maintenance.
Wang Y, Cho C, Williams J, Smallwood PM, Zhang C, Junge HJ, Nathans J
Proceedings of the National Academy of Sciences of the United States of America 2018 Dec 11;115(50):E11827-E11836
Proceedings of the National Academy of Sciences of the United States of America 2018 Dec 11;115(50):E11827-E11836
Glial scars are permeable to the neurotoxic environment of chronic stroke infarcts.
Zbesko JC, Nguyen TV, Yang T, Frye JB, Hussain O, Hayes M, Chung A, Day WA, Stepanovic K, Krumberger M, Mona J, Longo FM, Doyle KP
Neurobiology of disease 2018 Apr;112:63-78
Neurobiology of disease 2018 Apr;112:63-78
Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis.
Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C
Neuron 2017 May 3;94(3):581-594.e5
Neuron 2017 May 3;94(3):581-594.e5
Gradual Suppression of Transcytosis Governs Functional Blood-Retinal Barrier Formation.
Chow BW, Gu C
Neuron 2017 Mar 22;93(6):1325-1333.e3
Neuron 2017 Mar 22;93(6):1325-1333.e3
Transdifferentiated Human Vascular Smooth Muscle Cells are a New Potential Cell Source for Endothelial Regeneration.
Hong X, Margariti A, Le Bras A, Jacquet L, Kong W, Hu Y, Xu Q
Scientific reports 2017 Jul 17;7(1):5590
Scientific reports 2017 Jul 17;7(1):5590
Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown.
Ogura S, Kurata K, Hattori Y, Takase H, Ishiguro-Oonuma T, Hwang Y, Ahn S, Park I, Ikeda W, Kusuhara S, Fukushima Y, Nara H, Sakai H, Fujiwara T, Matsushita J, Ema M, Hirashima M, Minami T, Shibuya M, Takakura N, Kim P, Miyata T, Ogura Y, Uemura A
JCI insight 2017 Feb 9;2(3):e90905
JCI insight 2017 Feb 9;2(3):e90905
Vascular Pericyte Impairment and Connexin43 Gap Junction Deficit Contribute to Vasomotor Decline in Diabetic Retinopathy.
Ivanova E, Kovacs-Oller T, Sagdullaev BT
The Journal of neuroscience : the official journal of the Society for Neuroscience 2017 Aug 9;37(32):7580-7594
The Journal of neuroscience : the official journal of the Society for Neuroscience 2017 Aug 9;37(32):7580-7594
Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation.
Cho C, Smallwood PM, Nathans J
Neuron 2017 Aug 30;95(5):1056-1073.e5
Neuron 2017 Aug 30;95(5):1056-1073.e5
Size-selective opening of the blood-brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1.
Yanagida K, Liu CH, Faraco G, Galvani S, Smith HK, Burg N, Anrather J, Sanchez T, Iadecola C, Hla T
Proceedings of the National Academy of Sciences of the United States of America 2017 Apr 25;114(17):4531-4536
Proceedings of the National Academy of Sciences of the United States of America 2017 Apr 25;114(17):4531-4536
Appearance of claudin-5(+) leukocytes in the central nervous system during neuroinflammation: a novel role for endothelial-derived extracellular vesicles.
Paul D, Baena V, Ge S, Jiang X, Jellison ER, Kiprono T, Agalliu D, Pachter JS
Journal of neuroinflammation 2016 Nov 16;13(1):292
Journal of neuroinflammation 2016 Nov 16;13(1):292
Presymptomatic activation of the PDGF-CC pathway accelerates onset of ALS neurodegeneration.
Lewandowski SA, Nilsson I, Fredriksson L, Lönnerberg P, Muhl L, Zeitelhofer M, Adzemovic MZ, Nichterwitz S, Lawrence DA, Hedlund E, Eriksson U
Acta neuropathologica 2016 Mar;131(3):453-64
Acta neuropathologica 2016 Mar;131(3):453-64
CD8+ T Cells Induce Fatal Brainstem Pathology during Cerebral Malaria via Luminal Antigen-Specific Engagement of Brain Vasculature.
Swanson PA 2nd, Hart GT, Russo MV, Nayak D, Yazew T, Peña M, Khan SM, Janse CJ, Pierce SK, McGavern DB
PLoS pathogens 2016 Dec;12(12):e1006022
PLoS pathogens 2016 Dec;12(12):e1006022
Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina.
Zhou Y, Williams J, Smallwood PM, Nathans J
PloS one 2015;10(12):e0143650
PloS one 2015;10(12):e0143650
Real-time estimation of paracellular permeability of cerebral endothelial cells by capacitance sensor array.
Hyun Jo D, Lee R, Hyoung Kim J, Oh Jun H, Geol Lee T, Hun Kim J
Scientific reports 2015 Jun 5;5:11014
Scientific reports 2015 Jun 5;5:11014
Structural and functional features of central nervous system lymphatic vessels.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J
Nature 2015 Jul 16;523(7560):337-41
Nature 2015 Jul 16;523(7560):337-41
Canonical WNT signaling components in vascular development and barrier formation.
Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Nathans J
The Journal of clinical investigation 2014 Sep;124(9):3825-46
The Journal of clinical investigation 2014 Sep;124(9):3825-46
Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling.
Zhou Y, Nathans J
Developmental cell 2014 Oct 27;31(2):248-56
Developmental cell 2014 Oct 27;31(2):248-56
The role of the hypoxia response in shaping retinal vascular development in the absence of Norrin/Frizzled4 signaling.
Rattner A, Wang Y, Zhou Y, Williams J, Nathans J
Investigative ophthalmology & visual science 2014 Nov 20;55(12):8614-25
Investigative ophthalmology & visual science 2014 Nov 20;55(12):8614-25
Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease.
Wu H, Luo J, Yu H, Rattner A, Mo A, Wang Y, Smallwood PM, Erlanger B, Wheelan SJ, Nathans J
Neuron 2014 Jan 8;81(1):103-19
Neuron 2014 Jan 8;81(1):103-19
EndMT contributes to the onset and progression of cerebral cavernous malformations.
Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, Tournier-Lasserve E, Chapon F, Richichi C, Retta SF, Lampugnani MG, Dejana E
Nature 2013 Jun 27;498(7455):492-6
Nature 2013 Jun 27;498(7455):492-6
Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity.
Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J
Cell 2012 Dec 7;151(6):1332-44
Cell 2012 Dec 7;151(6):1332-44
Wnt signaling mediates pathological vascular growth in proliferative retinopathy.
Chen J, Stahl A, Krah NM, Seaward MR, Dennison RJ, Sapieha P, Hua J, Hatton CJ, Juan AM, Aderman CM, Willett KL, Guerin KI, Mammoto A, Campbell M, Smith LE
Circulation 2011 Oct 25;124(17):1871-81
Circulation 2011 Oct 25;124(17):1871-81
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
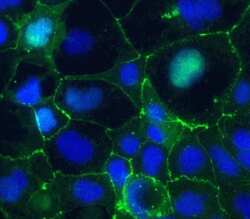
- Experimental details
- Immunofluorescent staining of Claudin-5 in human Caco-2 cells using mouse monoclonal antibody - Alexa Fluor® 488 (Product # 352588). DNA is counter stained with blue Hoechst 33258 (Product # H3569).
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
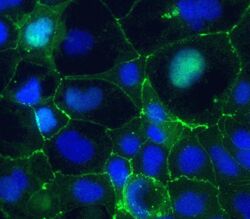
- Experimental details
- Immunofluorescent staining of Claudin-5 in human Caco-2 cells using mouse monoclonal antibody - Alexa Fluor® 488 (Product # 352588). DNA is counter stained with blue Hoechst 33258 (Product # H3569).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
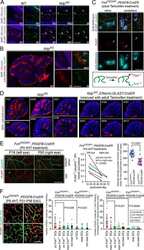
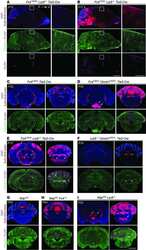
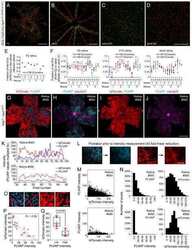
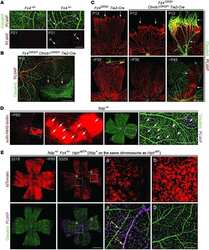
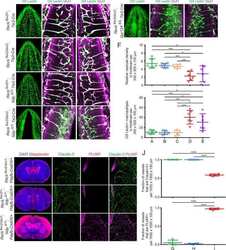
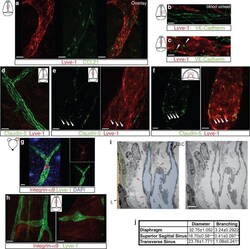
- Extended Figure 5 Initial lymphatic features of meningeal lymphatic vessels a. Representative images of CCL21 and Lyve-1 labeling of the meningeal lymphatic vessels (scale bar = 10 mum). b-c . Representative images of VE-Cadherin and Lyve-1 staining on meningeal blood vessels (b) and meningeal lymphatic vessels (c), arrowheads point to the VE-Cadherin aggregates; scale bar = 10 mum). d-f . Representative images of Claudin-5 and Lyve-1 staining on meningeal blood (d) and lymphatic (e) vessels, and diaphragm lymphatic vessels (f); arrowheads point to Claudin-5 aggregates (scale bar = 10 mum). g-h. Representative images of integrin-alpha9 and Lyve-1 labeling on skin (g; ear) and meninges whole mount (h). Scale bar = 40 mum. No integrin-alpha9 expressing valves were detected in the meningeal lymphatic vessels. i . Representative low power micrographs (transmission EM) of the meningeal lymphatic vessels (scale bar = 2 mum); (L = lumen; SC = supporting cell; LEC = lymphatic endothelial cell; BEC = sinusal endothelial cell). Red arrowheads point to anchoring filaments. j . Table summarizing morphological features of the lymphatic network in different regions of the meninges and the diaphragm. Diameters are expressed in mum and branching as number of branches per mm of vessel; (mean +- SEM; n = 4 animals each group, *p
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
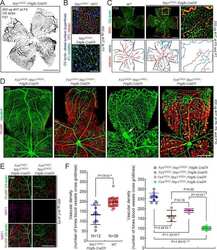
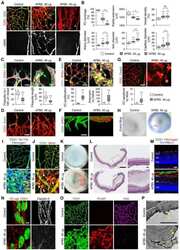
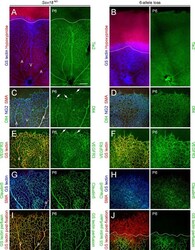
- Fig 5 Excess tip cell production in the Sox7, Sox17, and Sox18 EC-specific triple KO ('6-allele loss') at P6. (A-J) Retina flat mounts from control Sox18 +/- (A, C, E, G, I) and 6-allele loss Sox7 CKO/- ;Sox17 CKO/CKO ;Sox18 -/- ;Pdgfb-CreER (B, D, F, H, J) mice at P6. In (I) and (J), retinas were labeled with Alexa488 GS-lectin (green) via intracardiac injection and Alexa595 GS-lectin (red) after postfixation and permeabilization. The dashed lines delineate the boundary between hypoxia/normoxia in (A and B), and perfused/non-perfused vessels in (J). In control retinas, most tip cells (arrows) express higher levels of Dll4 (C) and VEGFR3 (E) than adjacent capillaries. The larger zone of hypoxia in (B) compared to (A) reflects the retarded growth of the vascular plexus. 50-100 mug 4HT was given at P0 and P2. A, artery; V, vein. Scale bar, 200 mum.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
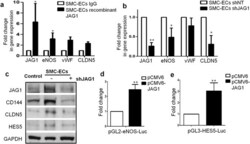
- Figure 7 HES5 and JAG1 are implicated in endothelial lineage generation from PR-SMCs (II). ( a ) Real-time PCR revealed that after stimulating the cells with immobilized recombinant JAG1 from day 3 during differentiation, eNOS, vWF and Claudin-5 could be further upregulated (*p < 0.05 by Student's t test, n = 3). ( b ) Knockdown JAG1 expression led to the downregulation of eNOS and Claudin-5 (*p < 0.05 by Student's t test, n = 3). ( c ) Western blot analysis confirmed the downregulation of endothelial markers in concert with the JAG1 suppression. Control group refers to the SMC transfected with empty lentiviral vector that underwent the same reprogramming and differentiation protocol. Bands were cropped for the clarity. ( d , e ) Luciferase assay showed the activation of eNOS and HES5 promoter area with the transient overexpression of JAG1 (**p < 0.01 by Student's t test, n = 3).
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
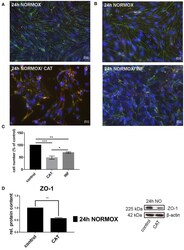
- Figure 1 Effects of catecholamines and inflammatory mediators under normoxia conditions on cEND cells. Cells were grown to confluence with subsequent differentiation. Catecholamines (CAT) or inflammatory mediators (INF) were applied for 24 h under normoxia conditions (24 h NORMOX). (A,B) Immunofluorescence staining of tight junction proteins claudin-5 (green) and ZO-1 (red) as markers of morphological changes of the endothelial cell monolayer. DAPI (blue) was used for staining of nuclei. Magnification 400 times, scale bar 20 mum. (C) Cell number after CAT and INF treatment normalized to control. (D) CAT induced changes of ZO-1 protein expression in cEND cells investigated by Western blot. Data is presented as the means (+- SEM) of n = 5 independent experiments. Altered protein expression was normalized to beta-actin. ZO-1 protein level changes are expressed as fold over untreated control, which was set 1. * P < 0.05, ** P < 0.01, *** P < 0.001.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
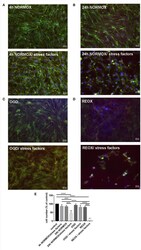
- Figure 2 Loss of morphological integrity of cEND cells exposed to elevated catecholamine levels and inflammatory mediators. Immunofluorescence staining of tight junction proteins claudin-5 (green) and ZO-1 (red) as markers of morphological changes of the endothelial cell monolayer. DAPI (blue) was used for staining nuclei. Cells were grown on cover slips to confluence. After differentiation cEND cells were treated with combination of catecholamines and inflammatory mediators (stress factors). Stress factors were administered under different incubation conditions. Magnification 400 times, scale bar 20 mum. (A) Stress factor application for 4h under normoxia conditions (4 h NORMOX). (B) Stress factor application for 24 h under normoxia conditions (24h NORMOX). (C) . Stress factor application for 4 h under oxygen glucose deprivation conditions (OGD). (D) Stress factor application for 4 h under OGD conditions with 20 h of subsequent reoxygenation under normoxia conditions (REOX). (E) Cell number in treatments shown in (A-D) normalized to control. *** P < 0.001, **** P < 0.0001.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
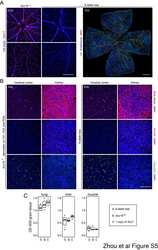
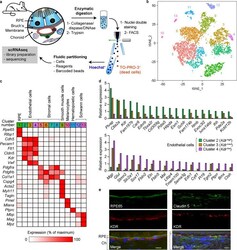
- Figure 1. Characterization of mouse RPE/choroid by scRNAseq and identification of transcriptionally distinct choroidal EC subtypes. (a) Scheme of the preparation of RPE/choroid single-cell suspensions by sequential enzymatic digestion and cell sorting. Hoechst + /TO-PRO-3 - live cells were subjected to scRNAseq. (b) Principal-component analysis of 7,723 single-cell expression profiles obtained from RPE/choroid tissue from four mice (C57BL/6J and B6129PF1/J strains, one male and one female per strain). Data are shown in two dimensions using tSNE. Unsupervised analysis clustered cells into 13 transcriptionally distinct cell populations, each plotted in a different color. (c) Identification of cell types in RPE/choroid tissue from B6129PF1/J mice according to the average expression of known markers in each cluster. Cluster number colors are the same as in panel b. Relative gene expression among cell types was calculated using the average normalized UMIs in each cluster and represented as the percentage of the cluster with maximum expression. White, 0%; red, 100%. (d) Genes with at least fivefold differential expression between Kdr high (green bars) and Kdr low (purple bars) ECs (Benjamini-Hochberg adjusted likelihood-ratio test, adj P < 0.05). Only genes with maximum average normalized UMIs >=1 are shown. Relative gene expression in Kdr med ECs (brown bars) is also included. Top: Kdr high expression at least fivefold higher than Kdr low . Bottom, Kdr low expression at least five
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
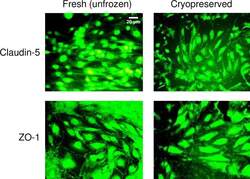
- Fig 7 Junction protein expression in fresh and cryopreserved hCMEC/D3 monolayers. The expression of claudin-5 and zonula occludens (ZO)-1 in hCMEC/D3 monolayers that were non-cryopreserved (fresh, unfrozen control), or were loaded with 5% DMSO, 6% HES, and 2% chondroitin sulfate, subjected to cooling at 1degC/min to -40degC, plunging and storage in liquid nitrogen, rapid thawing, and immediate cryoprotectant removal. Fresh and cryopreserved monolayers were stained with the corresponding antibody conjugated to Alexa Fluor 488. The scale bar for claudin-5-stained fresh, unfrozen cells applies to all images.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
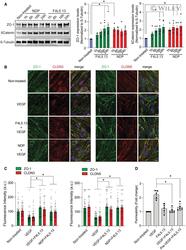
- 3 Figure F4L5.13 promotes endothelial cell barrier function Expression of endothelial junction protein ZO-1 and stabilization of betacatenin in bEnd.3 cells treated with 30 nM F4L5.13 or 30 nM recombinant NDP for the indicated times. Blots are representative of five different experiments. Histogram represents the ratio of ZO-1 and betacatenin levels over beta-Tubulin measured by densitometry of five independent experiments. Data are presented as mean +- SEM. Significance was calculated by one-way ANOVA with Bonferroni's multiple comparisons test (* P < 0.05 as compared with NT). Immunofluorescence of ZO-1, CLDN3, and CLDN5 localization at bEnd.3 cell junctions. bEnd.3 cells were treated or not with 30 nM F4L5.13 or 30 nM Norrin (NDP) with or without 100 ng/ml VEGF for 1 h. ZO-1 is shown in green, CLDN3/5 in red, and DAPI in blue. Scale bars: 15 um. Quantification of ZO-1, CLDN3, and CLDN5 fluorescence intensity. Each column represents 40 measurements of fluorescence intensity per condition (y-axis). Data are presented as mean +- SEM. Significance was calculated by one-way ANOVA with Bonferroni's multiple comparisons test (* P < 0.05 as compared to VEGF treatment). Transendothelial permeability assay quantifying the passage of FITC-dextran through a monolayer of bEnd.3 cells. Passage of FITC-dextran was measured after exposure of bEnd.3 cells to 100 ng/ml VEGF, 30 nM F4L5.13 or both or pretreated with VEGF for 1 h before treating with F4L5.13 for 1 h. Data are presented as mea
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
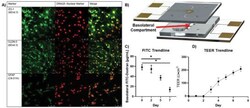
- Figure 3 Validation of uE-BBB. A) Excised membranes were stained for cell line specific markers. The bEnd.3 cells were stained for TJ markers, ZO-1 and CLDN-5, after 5 days of growth and the C8-D1A cells were stained for astrocyte marker, GFAP, after 7 days of growth. DRAQ5 was used as a nuclear stain (Biological replicate: n = 1; technical replicates: n = 3). B) Schematic of the uE-BBB lumen channel to the basolateral compartment where resistance of large molecule passage to the basolateral compartment was evaluated after 2, 5, and 7 days of growth. C8-D1A cells were incorporated after day 0 measurements and bEnd.3 cells were incorporated after day 2 measurements. C) Large particle transport assays through uE-BBB were conducted by passing 100 ug mL -1 of 10 kDa FITC-dextran solution through the lumen compartment of the system for 1 h and quantifying the amount of FITC-dextran that had accumulated in the basolateral compartment ( n = 4; Mann-Whitney two-tailed test; * signifies p < 0.05). Initially, there is little resistance to the diffusion of FITC-dextran into the basolateral compartment, but as the device matures, the permeability of the system decreases. D) Utilizing the integrated electrodes of the system, TEER measurements were collected daily. During the first 2 days of culture, the astrocytes alone do not induce any change in resistance. However, the resistance begins to increase following incorporation of bEnd.3 cells after day 2 ( n = 8). All data represented as me
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
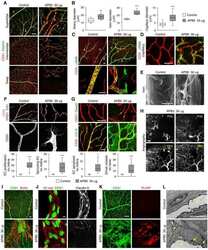
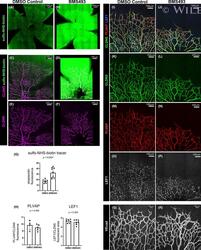
- 4 FIGURE Inhibition of RA signaling disrupts vascular barrier function in the postnatal mouse retina. (A-F) Pups treated with BMS493 or DMSO were systemically injected with sulfo-NHS-biotin 1 h before euthanasia at P4.5. Streptavidin staining reveals leakage of the sulfo-NHS-biotin tracer into retinal tissue of BMS493-treated pups (B) and confinement of sulfo-NHS-biotin to vasculature of control pups (A). (C-F) Extravascular sulfo-NHS-biotin in BMS493-treated pups is apparent in images co-stained for sulfo-NHS-biotin and CLDN5. (G) Quantification of streptavidin fluorescence per retina (DMSO n = 7, BMS493 n = 9). (I-P) Immunostaining for CLDN5, PLVAP, and LEF1 in retinas from P4.5 control and BMS493-treated pups was not remarkably different. (H) Quantification of PLVAP fluorescence (DMSO n = 4, BMS493 n = 5) and LEF1 fluorescence (DMSO n = 7, BMS493 n = 7) normalized to vascular content per field (CLDN5 signal) in the nascent vascular network of BMS493-treated and control pups (average of 2 fields per retina). (Q,R) VEcad immunostaining in retinas of P4.5 BMS493-treated and control pups (representative of DMSO n = 3, BMS493 n = 3; see also Figure S6). Error bars indicate StDev. Significance was assessed by unpaired unequal variance t test (Welch's t test)
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
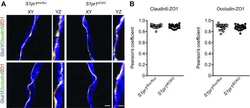
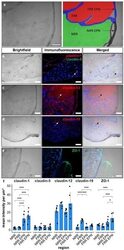
- Figure 1 Claudin-1 immunoreactivity (IR) and ZO-1 IR are preferentially found in epi-/perineurium in rats' dorsal root ganglia (DRGs), while claudin-19 IR is most abundant in the fiber-rich region. Classification into neuron rich region (NRR, green), NRR epi-/perineurium (EPN, black), fiber rich region (FRR, red), and FRR-EPN (blue) is shown in ( a ). Control DRG sections (CL) from Wistar rats were immunostained. IRs of claudin-1, claudin-5, and ZO-1 were quantified and compared between NRR and FRR, together with their putative EPN. Representative stainings for claudin-1 (red), claudin-5 (green) ( b ), claudin-12 ( c ), claudin-19 ( d ), and ZO-1 ( e ) are shown. Arrows point to structures identified as vessels ( b ), Schwann cells ( c ), and paranodes of Schwann cells ( d ). Quantification of the signal intensity in the specified areas ( f ). (Scale bars = 100 um; n = 5 or 6; claudin-1: NRR versus NRR-EPN, NRR versus FRR-EPN, FRR versus NRR-EPN, and FRR versus FRR-EPN: p < 0.0001; ZO-1: FRR-EPN versus NRR: p = 0.0003; FRR-EPN versus NRR-EPN: p = 0.0138; FRR-EPN versus NRR: p = 0.00018. No normal distribution: ZO-1 FRR. No variance homogeneity: claudin-1. Two-way ANOVA, Tukey HSD. * p < 0.05, *** p < 0.001).
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
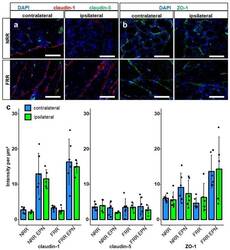
- Figure 4 Claudin-1 and ZO-1 IR are unchanged after CCI. DRG sections from Wistar rats with 7 d CCI and naive controls were immunostained. IRs of claudin-1, claudin-5, and ZO-1 were quantified and compared between IL and CL DRGs after CCI in the neuron rich region (NRR) and fiber rich region (FRR), and their putative EPN are presented as light intensity per um 2 . Representative sections of claudin-1 (red) and claudin-5 (green) ( a ), and ZO-1 (green) ( b ) are shown. Quantification of the signal intensity in the specified areas: significant differences between regions were not indicated, as they are already analyzed in Figure 1 ( c ). (Scale bars = 100 um. n = 5 or 6; claudin-1: NRR versus NRR-EPN, NRR versus FRR-EPN, FRR versus NRR-EPN, and FRR versus FRR-EPN: p < 0.0001; ZO-1: FRR-EPN versus NRR: p = 0.0003; FRR-EPN versus NRR-EPN: p = 0.0138; FRR-EPN versus NRR: p = 0.00018. No normal distribution: ZO-1 CL FRR. No variance homogeneity: claudin-1 CL. Two-way ANOVA, Tukey HSD).
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
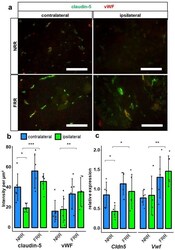
- Figure 6 Claudin-5 IR within vessels and Cldn5 mRNA in the NRR are reduced in rat DRGs. DRG sections from Wistar rats after CCI were immunostained. Quantification of claudin-5 and van Willebrand factor (vWF) IRs and comparison between IL and CL DRGs after CCI in the NRR and FRR as light intensity per um 2 . Tissue samples from the NRR and vessels of the FRR were obtained using laser microdissection (LMD). Claudin-5 and vWF mRNA were quantified as relative expression (to GAPDH) via RT-qPCR. Representative sections of co-stainings with claudin-5 (green) and vWF (red) ( a ). Quantification of the claudin-5 and vWF IR ( b ) n = 6; claudin-5: FRR versus NRR, p = 0.000261; NRR: CL versus CCI, p = 0.02985; vWF: NRR versus FRR, p = 0.00515. No normal distribution: claudin-5 FRR CCI, vWF NRR CL. No variance homogeneity: claudin-5 FRR. Two-way ANOVA, Tukey HSD.) And quantification of mRNA expression ( c ) n = 5; Cldn5 : NRR versus FRR, p = 0.0136; CL versus IL: p = 0.0448; Vwf : NRR versus FRR, p = 0.00838; two-way ANOVA, Tukey HSD) in the specified areas. (Scale bars = 100 um. * p < 0.05, ** p < 0.01, *** p < 0.001.) All results are summarized in Table 1 .
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
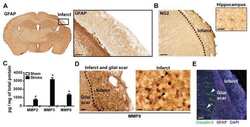
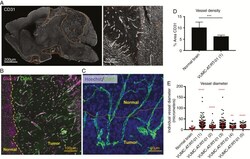
- Fig. 4 Abnormal intratumoral blood vessels in AT/RT xenografts. (A) Confocal microscopy images of a sagittal section of the brain of a mouse carrying a VUMC-AT/RT-01 xenograft, stained for the endothelial cell marker CD31, showing reduced density but increased diameter of intratumoral blood vessels. (B) Confocal microscopy image of the brain of a mouse carrying a VUMC-AT/RT-01 xenograft, stained for the blood-brain barrier endothelial cell markers GLUT1 and CLDN5, showing reduced GLUT1 expression in intratumoral blood vessels. (C) Confocal microscopy image of the brain of a mouse carrying a VUMC-AT/RT-01 xenograft, stained for the tight junction protein CLDN5, showing disorganization of tight junctions between endothelial cells of intratumoral blood vessels. (D) Quantification of vessel density, as determined by area of tissue staining positive for CD31, in VUMC-AT/RT-01 xenografts compared with normal brain. *** P < 0.001 (Mann-Whitney U -test). (E) Quantification of vessel diameter of intratumoral blood vessels in VUMC-AT/RT-01 xenografts compared with blood vessels in normal brain tissue. ** P < 0.01, **** P < 0.0001 (Dunnett's multiple comparisons test).
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
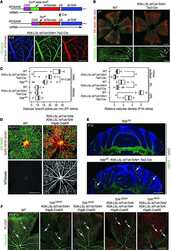
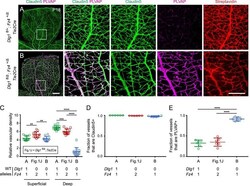
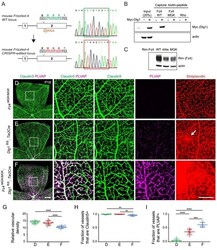
- Figure 2. Dlg1 genetically interacts with Fz4 to regulate retinal angiogenesis and the BRB. ( A-B ) Maximum projection of the superficial, intermediate, and deep vascular plexuses of P18 retinas from the indicated genotypes (column 1), with boxed regions displayed at higher magnification in columns 2-5. Mice were injected IP with 2-3 mg of sulfo-NHS-biotin 1-2 hr before sacrifice. Scale bar for column 1, 400 mum. Scale bar for columns 2-5, 200 mum. ( C-E ) Quantification of vascular density in the superficial and deep plexuses ( C ), the fraction of vessels that immunostain for Claudin5 ( D ), and the fraction of vessels that immunostain for PLVAP ( E ), for the genotypes shown in ( A ), ( B ), and Figure 1J . In this Figure and Figures 3 and 4 , the numbers of WT alleles for the genes of interest are listed below each set of data points.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
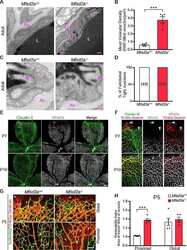
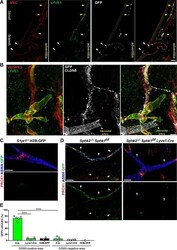
- Figure 8. Mutation of the Fz4 C-terminal PDZ-binding domain enhances the severity of retinal vascular defects in a Dlg1 mutant background. ( A ) Diagram of the Fz4 allele produced by CRISPR/Cas9 editing ( Fz4 MGK ). Numbers indicate exons. TVV, the C-terminal three amino acids, were substituted with MGK. Right, sequencing chromatograms from mouse tail PCR products. Boxes encompass the three altered codons. Top, WT allele. Bottom, Fz4 MGK allele. ( B ) Protein extracts from HEK/293T cells transfected with WT Myc-Dlg1 (+) or an empty expression vector (-) were subjected to affinity capture using a biotin-Fz4 WT C-terminal peptide, a biotin-Fz4 peptide with the C-terminal three amino acids substituted by MGK, or a biotin-rhodopsin C-terminal peptide. Myc-Dlg1 binds only to the Fz4 WT C-terminal peptide. ( C ) Immunoblot of protein extracts from HEK/293T cells transfected with Rim-Fz4 WT, Rim-Fz4(4A) or Rim-Fz4(MGK), i.e., Fz4 with the C-terminal three amino acids changed to MGK. ( D-F ) Maximum projection of the superficial, intermediate, and deep vascular plexuses of P18 retinas from the indicated genotypes (column 1) with boxed regions enlarged in columns 2-5. Mice were injected IP with 2-3 mg of sulfo-NHS-biotin 1-2 hr before sacrifice. Arrow in ( E ) indicates biotin leakage. Scale bar for column 1, 400 mum. Scale bar for columns 2-5, 200 mum. ( G-I ) Quantification of summed vascular density ( G ), the fraction of vessels that immunostain for Claudin5 ( H ), and the
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
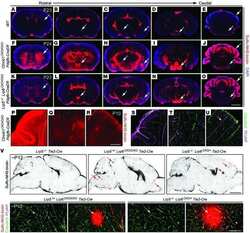
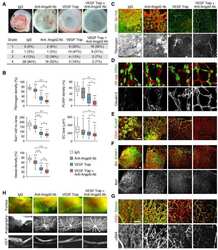
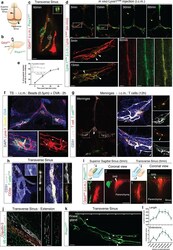
- Figure 1: Meningeal lymphatic sub-arachnoid extensions uptake molecules and immune cells from the cerebrospinal fluid. a, General scheme of meningeal lymphatic vascular organization. b, Scheme of the experiment presented in (c). Prox1 GFP mice were injected into the cisterna magna (i.c.m.) with 5ul of Qdot 655 . The transverse sinus was imaged through a thinned skull. c, Representative image of meningeal lymphatics adjacent to the transverse sinus (Prox1 GFP - green) filled with the i.c.m. injected Qdot 655 (red) 60min after injection. The inset represents the coronal view of the lymphatic vessel filled with Qdot 655 . Scale bar = 65 um. Representative of 3 independent animals. d, Representative images of the lymphatic vessels co-labeled with i.c.m. injected anti-Lyve-1 A488 and exogenously applied anti-Lyve-1 A660 at different time points after i.c.m. injection. Arrows in inset at 5 and 15 min illustrate the initial points where the i.c.m. injected anti-Lyve-1 A488 labelled the meningeal lymphatics. Scale bar = 1000 um (upper panel); 250 um (bottom left panel) 60 um (bottom right panel). e, Quantification of the percentage of lymphatic vessels labeled by the i.c.m. injected antibody and total lymphatic area at different time points post injection (mean +- s.e.m.; n=4 mice/group). f, Representative images of OVA 594 and fluorescent bead accumulation along the lymphatics (Lyve-1 - red) are shown. Arrows point to lymphatic extensions in the area of microbeads
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 8. S1PR1/ss-arrestin coupling in aorta-associated lymphatic vessels and S1P-dependent signaling in LECs. ( A ) Representative confocal image of a sagittal cryosection (14 uM) of a S1RP1-GS mouse aorta immunostained for VEC and lymphatic vessel endothelial hyaluronic acid receptor 1 (LYVE1) (N = 2). White arrows indicate GFP+ arterial ECs at a branch point orifices and yellow arrows indicate adventitia-associated GFP+ LECs. Scale bar is 50 uM. ( B ) Confocal image of a S1RP1-GS mouse thoracic aorta whole-mount preparation immunostained for vascular endothelial growth factor receptor 3 (VEGFR3; Flt4 ), LYVE1, and CLDN5 with the tunica intima in contact with coverslip. Orange stars indicate VEGFR3+LYVE1+GFP+ areas, cyan stars indicate VEGFR3+LYVE1 low GFP+ areas, and magenta stars indicate VEGFR3+LYVE1+GFP- areas. Image is representative of observations from three mice (see also Figure 8--figure supplement 1B ). Scale bar is 100 uM. ( C-D ) Representative confocal images of mesenteric lymphatics from H2B-GFP control ( C ) and S1PR1-GS mice bearing Sphk2 -/- : Sphk1 f/f (Cre-) or Sphk2 -/- : Sphk1 f/f : Lyve1-Cre (Lyve1-Cre+) alleles whole-mounted and immunostained for PROX1 and ASMA. White arrows indicate ASMA+PROX1+GFP+, yellow arrows indicate PROX1+GFP-, cyan arrows indicate ASMA+PROX1+GFP-. Scale bars are 100 uM. ( E ) Quantification of GFP+ signal over ASMA+PROX1+ and ASMA-PROX1+ areas from Cre- (n = 5), Lyve1-Cre (n = 4), and H2B-GFP control (n = 2) mice. One-way ANO
- Conjugate
- Green dye
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry