Antibody data
- Antibody Data
- Antigen structure
- References [100]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [1]
- Other assay [60]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 51-6100 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Claudin 2 Polyclonal Antibody (MH44)
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- This antibody is specific for the ~ 22 kDa Claudin-2 protein and does not appear to cross-react with related endogenous proteins. Reactivity was confirmed by western blotting with rat kidney and by immunostaining of rat small intestine. This antibody is confirmed reactive with human, rat, and dog Claudin-2. The reactivity of this antibody with other species has not been determined.
- Reactivity
- Human, Rat, Canine
- Host
- Rabbit
- Isotype
- IgG
- Antibody clone number
- MH44
- Vial size
- 100 μg
- Concentration
- 0.25 mg/mL
- Storage
- -20°C
Submitted references MarvelD3 Is Upregulated in Ulcerative Colitis and Has Attenuating Effects during Colitis Indirectly Stabilizing the Intestinal Barrier.
Nanoscale segregation of channel and barrier claudins enables paracellular ion flux.
miR-195-5p Regulates Tight Junctions Expression via Claudin-2 Downregulation in Ulcerative Colitis.
Altered Mucus Barrier Integrity and Increased Susceptibility to Colitis in Mice upon Loss of Telocyte Bone Morphogenetic Protein Signalling.
Claudin-2 and claudin-12 form independent, complementary pores required to maintain calcium homeostasis.
Chromogranin A regulates gut permeability via the antagonistic actions of its proteolytic peptides.
Elevation of Chemosensitivity of Lung Adenocarcinoma A549 Spheroid Cells by Claudin-2 Knockdown through Activation of Glucose Transport and Inhibition of Nrf2 Signal.
Down-Regulation of Claudin-2 Expression by Cyanidin-3-Glucoside Enhances Sensitivity to Anticancer Drugs in the Spheroid of Human Lung Adenocarcinoma A549 Cells.
Smoothelin-like 2 Inhibits Coronin-1B to Stabilize the Apical Actin Cortex during Epithelial Morphogenesis.
Carbachol protects the intestinal barrier in severe acute pancreatitis by regulating Cdc42/F-actin cytoskeleton.
E7 oncoprotein from human papillomavirus 16 alters claudins expression and the sealing of epithelial tight junctions.
Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice.
Altered Structural Expression and Enzymatic Activity Parameters in Quiescent Ulcerative Colitis: Are These Potential Normalization Criteria?
A Low Cost Antibody Signal Enhancer Improves Immunolabeling in Cell Culture, Primate Brain and Human Cancer Biopsy.
Glycocholic acid and butyrate synergistically increase vitamin D-induced calcium uptake in Caco-2 intestinal epithelial cell monolayers.
Doxorubicin increases permeability of murine small intestinal epithelium and cultured T84 monolayers.
Expression of Tight Junction Proteins According to Functional Dyspepsia Subtype and Sex.
TLR3 deficiency exacerbates the loss of epithelial barrier function during genital tract Chlamydia muridarum infection.
Syncytiotrophoblast of Placentae from Women with Zika Virus Infection Has Altered Tight Junction Protein Expression and Increased Paracellular Permeability.
Temporal Effects of Quercetin on Tight Junction Barrier Properties and Claudin Expression and Localization in MDCK II Cells.
Toll-like receptor 5-mediated IL-17C expression in intestinal epithelial cells enhances epithelial host defense against F4(+) ETEC infection.
Apical Membrane Alterations in Non-intestinal Organs in Microvillus Inclusion Disease.
Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells.
The role of human fibronectin- or placenta basement membrane extract-based gels in favouring the formation of polarized salivary acinar-like structures.
Chloride channel ClC- 2 enhances intestinal epithelial tight junction barrier function via regulation of caveolin-1 and caveolar trafficking of occludin.
Inflammatory cytokines down-regulate the barrier-protective prostasin-matriptase proteolytic cascade early in experimental colitis.
Inhibition of Autophagic Degradation Process Contributes to Claudin-2 Expression Increase and Epithelial Tight Junction Dysfunction in TNF-α Treated Cell Monolayers.
Claudin-6 enhances cell invasiveness through claudin-1 in AGS human adenocarcinoma gastric cancer cells.
Down-regulation of Claudin-2 Expression and Proliferation by Epigenetic Inhibitors in Human Lung Adenocarcinoma A549 Cells.
Claudin-2 Expression Levels in Ulcerative Colitis: Development and Validation of an In-Situ Hybridisation Assay for Therapeutic Studies.
T-Lymphocytes Traffic into the Brain across the Blood-CSF Barrier: Evidence Using a Reconstituted Choroid Plexus Epithelium.
The serine protease-mediated increase in intestinal epithelial barrier function is dependent on occludin and requires an intact tight junction.
Gata4 is critical to maintain gut barrier function and mucosal integrity following epithelial injury.
Hypotonic Stress-induced Down-regulation of Claudin-1 and -2 Mediated by Dephosphorylation and Clathrin-dependent Endocytosis in Renal Tubular Epithelial Cells.
Identification of Proteins Bound to Dengue Viral RNA In Vivo Reveals New Host Proteins Important for Virus Replication.
Modeling immune functions of the mouse blood-cerebrospinal fluid barrier in vitro: primary rather than immortalized mouse choroid plexus epithelial cells are suited to study immune cell migration across this brain barrier.
Claudin-1, -2, -4, and -5: comparison of expression levels and distribution in equine tissues.
Shear Stress-Induced Alteration of Epithelial Organization in Human Renal Tubular Cells.
Alterations of intercellular junctions in peritoneal mesothelial cells from patients undergoing dialysis: effect of retinoic Acid.
Mode of action of claudin peptidomimetics in the transient opening of cellular tight junction barriers.
A non-tight junction function of claudin-7-Interaction with integrin signaling in suppressing lung cancer cell proliferation and detachment.
Tumor necrosis factor-α induces a biphasic change in claudin-2 expression in tubular epithelial cells: role in barrier functions.
EGF regulates claudin-2 and -4 expression through Src and STAT3 in MDCK cells.
Expression of tight-junction proteins in human proximal small intestinal mucosa before and after Roux-en-Y gastric bypass surgery.
Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease.
Loss of EP2 receptor subtype in colonic cells compromise epithelial barrier integrity by altering claudin-4.
Impaired intestinal mucosal barrier upon ischemia-reperfusion: "patching holes in the shield with a simple surgical method".
Diets high in fermentable protein and fibre alter tight junction protein composition with minor effects on barrier function in piglet colon.
Ouabain induces endocytosis and degradation of tight junction proteins through ERK1/2-dependent pathways.
Transmigration of neural stem cells across the blood brain barrier induced by glioma cells.
Guanylate cyclase C limits systemic dissemination of a murine enteric pathogen.
Cholera toxin disrupts barrier function by inhibiting exocyst-mediated trafficking of host proteins to intestinal cell junctions.
H. pylori-encoded CagA disrupts tight junctions and induces invasiveness of AGS gastric carcinoma cells via Cdx2-dependent targeting of Claudin-2.
Role of tight junction proteins in gastroesophageal reflux disease.
Phosphorylation of Rab11-FIP2 regulates polarity in MDCK cells.
Effects of proinflammatory cytokines on the claudin-19 rich tight junctions of human retinal pigment epithelium.
Functional characterization and localization of a gill-specific claudin isoform in Atlantic salmon.
Complexity and developmental changes in the expression pattern of claudins at the blood-CSF barrier.
Claudin-2 promotes breast cancer liver metastasis by facilitating tumor cell interactions with hepatocytes.
A novel microfluidics-based method for probing weak protein-protein interactions.
Dynamics of claudins expression in colitis and colitis-associated cancer in rat.
Matrigel improves functional properties of primary human salivary gland cells.
Ouabain modulates ciliogenesis in epithelial cells.
Loss of Smad5 leads to the disassembly of the apical junctional complex and increased susceptibility to experimental colitis.
Ovarian hormones control the changing expression of claudins and occludin in rat uterine epithelial cells during early pregnancy.
Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells.
Tight junction proteins in human Schwann cell autotypic junctions.
Distribution of tight junction proteins in adult human salivary glands.
Cadmium causes delayed effects on renal function in the offspring of cadmium-contaminated pregnant female rats.
Differences in claudin synthesis in primary cultures of acinar cells from rat salivary gland are correlated with the specific three-dimensional organization of the cells.
Tight and adherens junctions in the ovine uterus: differential regulation by pregnancy and progesterone.
Zinc protects renal function during cadmium intoxication in the rat.
An optimized in vitro model of the respiratory tract wall to study particle cell interactions.
Impairment of blood-cerebrospinal fluid barrier properties by retrovirus-activated T lymphocytes: reduction in cerebrospinal fluid-to-blood efflux of prostaglandin E2.
Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells.
Inducible expression of Snail selectively increases paracellular ion permeability and differentially modulates tight junction proteins.
Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells.
Amphiregulin causes functional downregulation of adherens junctions in psoriasis.
Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells.
A three-dimensional cellular model of the human respiratory tract to study the interaction with particles.
Cultured monolayers of the dog jejunum with the structural and functional properties resembling the normal epithelium.
Paracellular Cl- permeability is regulated by WNK4 kinase: insight into normal physiology and hypertension.
Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms.
Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells.
Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation.
Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells.
Tight junction proteins ZO-1, occludin, and claudins in developing and adult human perineurium.
Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium.
The specific fates of tight junction proteins in apoptotic epithelial cells.
Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation.
Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins.
Bryostatin-1 enhances barrier function in T84 epithelia through PKC-dependent regulation of tight junction proteins.
Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells.
E-Cadherin and tight junctions between epithelial cells of different animal species.
Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells.
Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells.
The renal segmental distribution of claudins changes with development.
Mechanisms of diarrhea in collagenous colitis.
Mechanisms of diarrhea in collagenous colitis.
Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions.
Weiß F, Czichos C, Knobe L, Voges L, Bojarski C, Michel G, Fromm M, Krug SM
Cells 2022 May 4;11(9)
Cells 2022 May 4;11(9)
Nanoscale segregation of channel and barrier claudins enables paracellular ion flux.
Gonschior H, Schmied C, Van der Veen RE, Eichhorst J, Himmerkus N, Piontek J, Günzel D, Bleich M, Furuse M, Haucke V, Lehmann M
Nature communications 2022 Aug 25;13(1):4985
Nature communications 2022 Aug 25;13(1):4985
miR-195-5p Regulates Tight Junctions Expression via Claudin-2 Downregulation in Ulcerative Colitis.
Scalavino V, Piccinno E, Lacalamita A, Tafaro A, Armentano R, Giannelli G, Serino G
Biomedicines 2022 Apr 16;10(4)
Biomedicines 2022 Apr 16;10(4)
Altered Mucus Barrier Integrity and Increased Susceptibility to Colitis in Mice upon Loss of Telocyte Bone Morphogenetic Protein Signalling.
Reyes Nicolás V, Allaire JM, Alfonso AB, Pupo Gómez D, Pomerleau V, Giroux V, Boudreau F, Perreault N
Cells 2021 Oct 29;10(11)
Cells 2021 Oct 29;10(11)
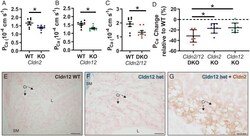
Claudin-2 and claudin-12 form independent, complementary pores required to maintain calcium homeostasis.
Beggs MR, Young K, Pan W, O'Neill DD, Saurette M, Plain A, Rievaj J, Doschak MR, Cordat E, Dimke H, Alexander RT
Proceedings of the National Academy of Sciences of the United States of America 2021 Nov 30;118(48)
Proceedings of the National Academy of Sciences of the United States of America 2021 Nov 30;118(48)
Chromogranin A regulates gut permeability via the antagonistic actions of its proteolytic peptides.
Muntjewerff EM, Tang K, Lutter L, Christoffersson G, Nicolasen MJT, Gao H, Katkar GD, Das S, Ter Beest M, Ying W, Ghosh P, El Aidy S, Oldenburg B, van den Bogaart G, Mahata SK
Acta physiologica (Oxford, England) 2021 Jun;232(2):e13655
Acta physiologica (Oxford, England) 2021 Jun;232(2):e13655
Elevation of Chemosensitivity of Lung Adenocarcinoma A549 Spheroid Cells by Claudin-2 Knockdown through Activation of Glucose Transport and Inhibition of Nrf2 Signal.
Ito A, Nasako H, Akizuki R, Takashina Y, Eguchi H, Matsunaga T, Yoshino Y, Endo S, Ikari A
International journal of molecular sciences 2021 Jun 19;22(12)
International journal of molecular sciences 2021 Jun 19;22(12)
Down-Regulation of Claudin-2 Expression by Cyanidin-3-Glucoside Enhances Sensitivity to Anticancer Drugs in the Spheroid of Human Lung Adenocarcinoma A549 Cells.
Eguchi H, Matsunaga H, Onuma S, Yoshino Y, Matsunaga T, Ikari A
International journal of molecular sciences 2021 Jan 6;22(2)
International journal of molecular sciences 2021 Jan 6;22(2)
Smoothelin-like 2 Inhibits Coronin-1B to Stabilize the Apical Actin Cortex during Epithelial Morphogenesis.
Hachimi M, Grabowski C, Campanario S, Herranz G, Baonza G, Serrador JM, Gomez-Lopez S, Barea MD, Bosch-Fortea M, Gilmour D, Bagnat M, Rodriguez-Fraticelli AE, Martin-Belmonte F
Current biology : CB 2021 Feb 22;31(4):696-706.e9
Current biology : CB 2021 Feb 22;31(4):696-706.e9
Carbachol protects the intestinal barrier in severe acute pancreatitis by regulating Cdc42/F-actin cytoskeleton.
Wang H, Jiang Y, Li H, Wang J, Li C, Zhang D
Experimental and therapeutic medicine 2020 Sep;20(3):2828-2837
Experimental and therapeutic medicine 2020 Sep;20(3):2828-2837
E7 oncoprotein from human papillomavirus 16 alters claudins expression and the sealing of epithelial tight junctions.
Uc PY, Miranda J, Raya-Sandino A, Alarcón L, Roldán ML, Ocadiz-Delgado R, Cortés-Malagón EM, Chávez-Munguía B, Ramírez G, Asomoza R, Shoshani L, Gariglio P, González-Mariscal L
International journal of oncology 2020 Oct;57(4):905-924
International journal of oncology 2020 Oct;57(4):905-924
Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice.
Raju P, Shashikanth N, Tsai PY, Pongkorpsakol P, Chanez-Paredes S, Steinhagen PR, Kuo WT, Singh G, Tsukita S, Turner JR
The Journal of clinical investigation 2020 Oct 1;130(10):5197-5208
The Journal of clinical investigation 2020 Oct 1;130(10):5197-5208
Altered Structural Expression and Enzymatic Activity Parameters in Quiescent Ulcerative Colitis: Are These Potential Normalization Criteria?
Kjærgaard S, Damm MMB, Chang J, Riis LB, Rasmussen HB, Hytting-Andreasen R, Krug SM, Schulzke JD, Bindslev N, Hansen MB
International journal of molecular sciences 2020 Mar 10;21(5)
International journal of molecular sciences 2020 Mar 10;21(5)
A Low Cost Antibody Signal Enhancer Improves Immunolabeling in Cell Culture, Primate Brain and Human Cancer Biopsy.
Flores-Maldonado C, Albino-Sánchez ME, Rodríguez-Callejas JD, Estrada-Mondragon A, León-Galicia I, Maqueda-Alfaro R, Perez-Cruz C, Fuchs E, García-Carrancá A, Contreras RG, Missirlis F, Rosas-Arellano A
Neuroscience 2020 Jul 15;439:275-286
Neuroscience 2020 Jul 15;439:275-286
Glycocholic acid and butyrate synergistically increase vitamin D-induced calcium uptake in Caco-2 intestinal epithelial cell monolayers.
Casselbrant A, Fändriks L, Wallenius V
Bone reports 2020 Dec;13:100294
Bone reports 2020 Dec;13:100294
Doxorubicin increases permeability of murine small intestinal epithelium and cultured T84 monolayers.
Cray P, Sheahan BJ, Cortes JE, Dekaney CM
Scientific reports 2020 Dec 8;10(1):21486
Scientific reports 2020 Dec 8;10(1):21486
Expression of Tight Junction Proteins According to Functional Dyspepsia Subtype and Sex.
Lee JY, Kim N, Choi YJ, Park JH, Ashktorab H, Smoot DT, Lee DH
Journal of neurogastroenterology and motility 2020 Apr 30;26(2):248-258
Journal of neurogastroenterology and motility 2020 Apr 30;26(2):248-258
TLR3 deficiency exacerbates the loss of epithelial barrier function during genital tract Chlamydia muridarum infection.
Kumar R, Gong H, Liu L, Ramos-Solis N, Seye CI, Derbigny WA
PloS one 2019;14(1):e0207422
PloS one 2019;14(1):e0207422
Syncytiotrophoblast of Placentae from Women with Zika Virus Infection Has Altered Tight Junction Protein Expression and Increased Paracellular Permeability.
Miranda J, Martín-Tapia D, Valdespino-Vázquez Y, Alarcón L, Espejel-Nuñez A, Guzmán-Huerta M, Muñoz-Medina JE, Shibayama M, Chávez-Munguía B, Estrada-Gutiérrez G, Lievano S, Ludert JE, González-Mariscal L
Cells 2019 Sep 29;8(10)
Cells 2019 Sep 29;8(10)
Temporal Effects of Quercetin on Tight Junction Barrier Properties and Claudin Expression and Localization in MDCK II Cells.
Gamero-Estevez E, Andonian S, Jean-Claude B, Gupta I, Ryan AK
International journal of molecular sciences 2019 Oct 2;20(19)
International journal of molecular sciences 2019 Oct 2;20(19)
Toll-like receptor 5-mediated IL-17C expression in intestinal epithelial cells enhances epithelial host defense against F4(+) ETEC infection.
Luo Y, Xu J, Zhang C, Jiang C, Ma Y, He H, Wu Y, Devriendt B, Cox E, Zhang H
Veterinary research 2019 Jun 20;50(1):48
Veterinary research 2019 Jun 20;50(1):48
Apical Membrane Alterations in Non-intestinal Organs in Microvillus Inclusion Disease.
Schlegel C, Weis VG, Knowles BC, Lapierre LA, Martin MG, Dickman P, Goldenring JR, Shub MD
Digestive diseases and sciences 2018 Feb;63(2):356-365
Digestive diseases and sciences 2018 Feb;63(2):356-365
Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells.
Samuel W, Jaworski C, Postnikova OA, Kutty RK, Duncan T, Tan LX, Poliakov E, Lakkaraju A, Redmond TM
Molecular vision 2017;23:60-89
Molecular vision 2017;23:60-89
The role of human fibronectin- or placenta basement membrane extract-based gels in favouring the formation of polarized salivary acinar-like structures.
Maria OM, Liu Y, El-Hakim M, Zeitouni A, Tran SD
Journal of tissue engineering and regenerative medicine 2017 Sep;11(9):2643-2657
Journal of tissue engineering and regenerative medicine 2017 Sep;11(9):2643-2657
Chloride channel ClC- 2 enhances intestinal epithelial tight junction barrier function via regulation of caveolin-1 and caveolar trafficking of occludin.
Nighot PK, Leung L, Ma TY
Experimental cell research 2017 Mar 1;352(1):113-122
Experimental cell research 2017 Mar 1;352(1):113-122
Inflammatory cytokines down-regulate the barrier-protective prostasin-matriptase proteolytic cascade early in experimental colitis.
Buzza MS, Johnson TA, Conway GD, Martin EW, Mukhopadhyay S, Shea-Donohue T, Antalis TM
The Journal of biological chemistry 2017 Jun 30;292(26):10801-10812
The Journal of biological chemistry 2017 Jun 30;292(26):10801-10812
Inhibition of Autophagic Degradation Process Contributes to Claudin-2 Expression Increase and Epithelial Tight Junction Dysfunction in TNF-α Treated Cell Monolayers.
Zhang C, Yan J, Xiao Y, Shen Y, Wang J, Ge W, Chen Y
International journal of molecular sciences 2017 Jan 17;18(1)
International journal of molecular sciences 2017 Jan 17;18(1)
Claudin-6 enhances cell invasiveness through claudin-1 in AGS human adenocarcinoma gastric cancer cells.
Torres-Martínez AC, Gallardo-Vera JF, Lara-Holguin AN, Montaño LF, Rendón-Huerta EP
Experimental cell research 2017 Jan 1;350(1):226-235
Experimental cell research 2017 Jan 1;350(1):226-235
Down-regulation of Claudin-2 Expression and Proliferation by Epigenetic Inhibitors in Human Lung Adenocarcinoma A549 Cells.
Hichino A, Okamoto M, Taga S, Akizuki R, Endo S, Matsunaga T, Ikari A
The Journal of biological chemistry 2017 Feb 10;292(6):2411-2421
The Journal of biological chemistry 2017 Feb 10;292(6):2411-2421
Claudin-2 Expression Levels in Ulcerative Colitis: Development and Validation of an In-Situ Hybridisation Assay for Therapeutic Studies.
Randall K, Henderson N, Reens J, Eckersley S, Nyström AC, South MC, Balendran CA, Böttcher G, Hughes G, Price SA
PloS one 2016;11(9):e0162076
PloS one 2016;11(9):e0162076
T-Lymphocytes Traffic into the Brain across the Blood-CSF Barrier: Evidence Using a Reconstituted Choroid Plexus Epithelium.
Strazielle N, Creidy R, Malcus C, Boucraut J, Ghersi-Egea JF
PloS one 2016;11(3):e0150945
PloS one 2016;11(3):e0150945
The serine protease-mediated increase in intestinal epithelial barrier function is dependent on occludin and requires an intact tight junction.
Ronaghan NJ, Shang J, Iablokov V, Zaheer R, Colarusso P, Dion S, Désilets A, Leduc R, Turner JR, MacNaughton WK
American journal of physiology. Gastrointestinal and liver physiology 2016 Sep 1;311(3):G466-79
American journal of physiology. Gastrointestinal and liver physiology 2016 Sep 1;311(3):G466-79
Gata4 is critical to maintain gut barrier function and mucosal integrity following epithelial injury.
Lepage D, Bélanger É, Jones C, Tremblay S, Allaire JM, Bruneau J, Asselin C, Perreault N, Menendez A, Gendron FP, Boudreau F
Scientific reports 2016 Nov 9;6:36776
Scientific reports 2016 Nov 9;6:36776
Hypotonic Stress-induced Down-regulation of Claudin-1 and -2 Mediated by Dephosphorylation and Clathrin-dependent Endocytosis in Renal Tubular Epithelial Cells.
Fujii N, Matsuo Y, Matsunaga T, Endo S, Sakai H, Yamaguchi M, Yamazaki Y, Sugatani J, Ikari A
The Journal of biological chemistry 2016 Nov 18;291(47):24787-24799
The Journal of biological chemistry 2016 Nov 18;291(47):24787-24799
Identification of Proteins Bound to Dengue Viral RNA In Vivo Reveals New Host Proteins Important for Virus Replication.
Phillips SL, Soderblom EJ, Bradrick SS, Garcia-Blanco MA
mBio 2016 Jan 5;7(1):e01865-15
mBio 2016 Jan 5;7(1):e01865-15
Modeling immune functions of the mouse blood-cerebrospinal fluid barrier in vitro: primary rather than immortalized mouse choroid plexus epithelial cells are suited to study immune cell migration across this brain barrier.
Lazarevic I, Engelhardt B
Fluids and barriers of the CNS 2016 Jan 29;13:2
Fluids and barriers of the CNS 2016 Jan 29;13:2
Claudin-1, -2, -4, and -5: comparison of expression levels and distribution in equine tissues.
Lee B, Kang HY, Lee DO, Ahn C, Jeung EB
Journal of veterinary science 2016 Dec 30;17(4):445-451
Journal of veterinary science 2016 Dec 30;17(4):445-451
Shear Stress-Induced Alteration of Epithelial Organization in Human Renal Tubular Cells.
Maggiorani D, Dissard R, Belloy M, Saulnier-Blache JS, Casemayou A, Ducasse L, Grès S, Bellière J, Caubet C, Bascands JL, Schanstra JP, Buffin-Meyer B
PloS one 2015;10(7):e0131416
PloS one 2015;10(7):e0131416
Alterations of intercellular junctions in peritoneal mesothelial cells from patients undergoing dialysis: effect of retinoic Acid.
Retana C, Sanchez E, Perez-Lopez A, Cruz A, Lagunas J, Cruz C, Vital S, Reyes JL
Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis 2015 May-Jun;35(3):275-87
Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis 2015 May-Jun;35(3):275-87
Mode of action of claudin peptidomimetics in the transient opening of cellular tight junction barriers.
Staat C, Coisne C, Dabrowski S, Stamatovic SM, Andjelkovic AV, Wolburg H, Engelhardt B, Blasig IE
Biomaterials 2015 Jun;54:9-20
Biomaterials 2015 Jun;54:9-20
A non-tight junction function of claudin-7-Interaction with integrin signaling in suppressing lung cancer cell proliferation and detachment.
Lu Z, Kim DH, Fan J, Lu Q, Verbanac K, Ding L, Renegar R, Chen YH
Molecular cancer 2015 Jun 17;14:120
Molecular cancer 2015 Jun 17;14:120
Tumor necrosis factor-α induces a biphasic change in claudin-2 expression in tubular epithelial cells: role in barrier functions.
Amoozadeh Y, Dan Q, Xiao J, Waheed F, Szászi K
American journal of physiology. Cell physiology 2015 Jul 1;309(1):C38-50
American journal of physiology. Cell physiology 2015 Jul 1;309(1):C38-50
EGF regulates claudin-2 and -4 expression through Src and STAT3 in MDCK cells.
García-Hernández V, Flores-Maldonado C, Rincon-Heredia R, Verdejo-Torres O, Bonilla-Delgado J, Meneses-Morales I, Gariglio P, Contreras RG
Journal of cellular physiology 2015 Jan;230(1):105-15
Journal of cellular physiology 2015 Jan;230(1):105-15
Expression of tight-junction proteins in human proximal small intestinal mucosa before and after Roux-en-Y gastric bypass surgery.
Casselbrant A, Elias E, Fändriks L, Wallenius V
Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2015 Jan-Feb;11(1):45-53
Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2015 Jan-Feb;11(1):45-53
Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease.
Rossi G, Pengo G, Caldin M, Palumbo Piccionello A, Steiner JM, Cohen ND, Jergens AE, Suchodolski JS
PloS one 2014;9(4):e94699
PloS one 2014;9(4):e94699
Loss of EP2 receptor subtype in colonic cells compromise epithelial barrier integrity by altering claudin-4.
Lejeune M, Moreau F, Chadee K
PloS one 2014;9(11):e113270
PloS one 2014;9(11):e113270
Impaired intestinal mucosal barrier upon ischemia-reperfusion: "patching holes in the shield with a simple surgical method".
Rosero O, Ónody P, Kovács T, Molnár D, Lotz G, Tóth S, Turóczi Z, Fülöp A, Garbaisz D, Harsányi L, Szijártó A
BioMed research international 2014;2014:210901
BioMed research international 2014;2014:210901
Diets high in fermentable protein and fibre alter tight junction protein composition with minor effects on barrier function in piglet colon.
Richter JF, Pieper R, Zakrzewski SS, Günzel D, Schulzke JD, Van Kessel AG
The British journal of nutrition 2014 Mar 28;111(6):1040-9
The British journal of nutrition 2014 Mar 28;111(6):1040-9
Ouabain induces endocytosis and degradation of tight junction proteins through ERK1/2-dependent pathways.
Rincon-Heredia R, Flores-Benitez D, Flores-Maldonado C, Bonilla-Delgado J, García-Hernández V, Verdejo-Torres O, Castillo AM, Larré I, Poot-Hernández AC, Franco M, Gariglio P, Reyes JL, Contreras RG
Experimental cell research 2014 Jan 1;320(1):108-18
Experimental cell research 2014 Jan 1;320(1):108-18
Transmigration of neural stem cells across the blood brain barrier induced by glioma cells.
Díaz-Coránguez M, Segovia J, López-Ornelas A, Puerta-Guardo H, Ludert J, Chávez B, Meraz-Cruz N, González-Mariscal L
PloS one 2013;8(4):e60655
PloS one 2013;8(4):e60655
Guanylate cyclase C limits systemic dissemination of a murine enteric pathogen.
Mann EA, Harmel-Laws E, Cohen MB, Steinbrecher KA
BMC gastroenterology 2013 Sep 2;13:135
BMC gastroenterology 2013 Sep 2;13:135
Cholera toxin disrupts barrier function by inhibiting exocyst-mediated trafficking of host proteins to intestinal cell junctions.
Guichard A, Cruz-Moreno B, Aguilar B, van Sorge NM, Kuang J, Kurkciyan AA, Wang Z, Hang S, Pineton de Chambrun GP, McCole DF, Watnick P, Nizet V, Bier E
Cell host & microbe 2013 Sep 11;14(3):294-305
Cell host & microbe 2013 Sep 11;14(3):294-305
H. pylori-encoded CagA disrupts tight junctions and induces invasiveness of AGS gastric carcinoma cells via Cdx2-dependent targeting of Claudin-2.
Song X, Chen HX, Wang XY, Deng XY, Xi YX, He Q, Peng TL, Chen J, Chen W, Wong BC, Chen MH
Cellular immunology 2013 Nov-Dec;286(1-2):22-30
Cellular immunology 2013 Nov-Dec;286(1-2):22-30
Role of tight junction proteins in gastroesophageal reflux disease.
Mönkemüller K, Wex T, Kuester D, Fry LC, Kandulski A, Kropf S, Roessner A, Malfertheiner P
BMC gastroenterology 2012 Sep 20;12:128
BMC gastroenterology 2012 Sep 20;12:128
Phosphorylation of Rab11-FIP2 regulates polarity in MDCK cells.
Lapierre LA, Avant KM, Caldwell CM, Oztan A, Apodaca G, Knowles BC, Roland JT, Ducharme NA, Goldenring JR
Molecular biology of the cell 2012 Jun;23(12):2302-18
Molecular biology of the cell 2012 Jun;23(12):2302-18
Effects of proinflammatory cytokines on the claudin-19 rich tight junctions of human retinal pigment epithelium.
Peng S, Gan G, Rao VS, Adelman RA, Rizzolo LJ
Investigative ophthalmology & visual science 2012 Jul 27;53(8):5016-28
Investigative ophthalmology & visual science 2012 Jul 27;53(8):5016-28
Functional characterization and localization of a gill-specific claudin isoform in Atlantic salmon.
Engelund MB, Yu AS, Li J, Madsen SS, Færgeman NJ, Tipsmark CK
American journal of physiology. Regulatory, integrative and comparative physiology 2012 Jan 15;302(2):R300-11
American journal of physiology. Regulatory, integrative and comparative physiology 2012 Jan 15;302(2):R300-11
Complexity and developmental changes in the expression pattern of claudins at the blood-CSF barrier.
Kratzer I, Vasiljevic A, Rey C, Fevre-Montange M, Saunders N, Strazielle N, Ghersi-Egea JF
Histochemistry and cell biology 2012 Dec;138(6):861-79
Histochemistry and cell biology 2012 Dec;138(6):861-79
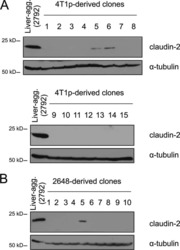
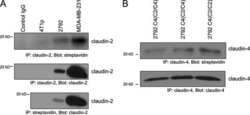
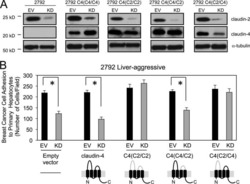
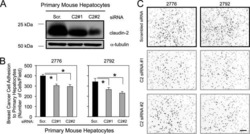
Claudin-2 promotes breast cancer liver metastasis by facilitating tumor cell interactions with hepatocytes.
Tabariès S, Dupuy F, Dong Z, Monast A, Annis MG, Spicer J, Ferri LE, Omeroglu A, Basik M, Amir E, Clemons M, Siegel PM
Molecular and cellular biology 2012 Aug;32(15):2979-91
Molecular and cellular biology 2012 Aug;32(15):2979-91
A novel microfluidics-based method for probing weak protein-protein interactions.
Tan DC, Wijaya IP, Andreasson-Ochsner M, Vasina EN, Nallani M, Hunziker W, Sinner EK
Lab on a chip 2012 Aug 7;12(15):2726-35
Lab on a chip 2012 Aug 7;12(15):2726-35
Dynamics of claudins expression in colitis and colitis-associated cancer in rat.
Arimura Y, Nagaishi K, Hosokawa M
Methods in molecular biology (Clifton, N.J.) 2011;762:409-25
Methods in molecular biology (Clifton, N.J.) 2011;762:409-25
Matrigel improves functional properties of primary human salivary gland cells.
Maria OM, Zeitouni A, Gologan O, Tran SD
Tissue engineering. Part A 2011 May;17(9-10):1229-38
Tissue engineering. Part A 2011 May;17(9-10):1229-38
Ouabain modulates ciliogenesis in epithelial cells.
Larre I, Castillo A, Flores-Maldonado C, Contreras RG, Galvan I, Muñoz-Estrada J, Cereijido M
Proceedings of the National Academy of Sciences of the United States of America 2011 Dec 20;108(51):20591-6
Proceedings of the National Academy of Sciences of the United States of America 2011 Dec 20;108(51):20591-6
Loss of Smad5 leads to the disassembly of the apical junctional complex and increased susceptibility to experimental colitis.
Allaire JM, Darsigny M, Marcoux SS, Roy SA, Schmouth JF, Umans L, Zwijsen A, Boudreau F, Perreault N
American journal of physiology. Gastrointestinal and liver physiology 2011 Apr;300(4):G586-97
American journal of physiology. Gastrointestinal and liver physiology 2011 Apr;300(4):G586-97
Ovarian hormones control the changing expression of claudins and occludin in rat uterine epithelial cells during early pregnancy.
Nicholson MD, Lindsay LA, Murphy CR
Acta histochemica 2010;112(1):42-52
Acta histochemica 2010;112(1):42-52
Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells.
Chang TL, Ito K, Ko TK, Liu Q, Salto-Tellez M, Yeoh KG, Fukamachi H, Ito Y
Gastroenterology 2010 Jan;138(1):255-65.e1-3
Gastroenterology 2010 Jan;138(1):255-65.e1-3
Tight junction proteins in human Schwann cell autotypic junctions.
Alanne MH, Pummi K, Heape AM, Grènman R, Peltonen J, Peltonen S
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2009 Jun;57(6):523-9
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2009 Jun;57(6):523-9
Distribution of tight junction proteins in adult human salivary glands.
Maria OM, Kim JW, Gerstenhaber JA, Baum BJ, Tran SD
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2008 Dec;56(12):1093-8
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2008 Dec;56(12):1093-8
Cadmium causes delayed effects on renal function in the offspring of cadmium-contaminated pregnant female rats.
Jacquillet G, Barbier O, Rubera I, Tauc M, Borderie A, Namorado MC, Martin D, Sierra G, Reyes JL, Poujeol P, Cougnon M
American journal of physiology. Renal physiology 2007 Nov;293(5):F1450-60
American journal of physiology. Renal physiology 2007 Nov;293(5):F1450-60
Differences in claudin synthesis in primary cultures of acinar cells from rat salivary gland are correlated with the specific three-dimensional organization of the cells.
Qi B, Fujita-Yoshigaki J, Michikawa H, Satoh K, Katsumata O, Sugiya H
Cell and tissue research 2007 Jul;329(1):59-70
Cell and tissue research 2007 Jul;329(1):59-70
Tight and adherens junctions in the ovine uterus: differential regulation by pregnancy and progesterone.
Satterfield MC, Dunlap KA, Hayashi K, Burghardt RC, Spencer TE, Bazer FW
Endocrinology 2007 Aug;148(8):3922-31
Endocrinology 2007 Aug;148(8):3922-31
Zinc protects renal function during cadmium intoxication in the rat.
Jacquillet G, Barbier O, Cougnon M, Tauc M, Namorado MC, Martin D, Reyes JL, Poujeol P
American journal of physiology. Renal physiology 2006 Jan;290(1):F127-37
American journal of physiology. Renal physiology 2006 Jan;290(1):F127-37
An optimized in vitro model of the respiratory tract wall to study particle cell interactions.
Blank F, Rothen-Rutishauser BM, Schurch S, Gehr P
Journal of aerosol medicine : the official journal of the International Society for Aerosols in Medicine 2006 Fall;19(3):392-405
Journal of aerosol medicine : the official journal of the International Society for Aerosols in Medicine 2006 Fall;19(3):392-405
Impairment of blood-cerebrospinal fluid barrier properties by retrovirus-activated T lymphocytes: reduction in cerebrospinal fluid-to-blood efflux of prostaglandin E2.
Khuth ST, Strazielle N, Giraudon P, Belin MF, Ghersi-Egea JF
Journal of neurochemistry 2005 Sep;94(6):1580-93
Journal of neurochemistry 2005 Sep;94(6):1580-93
Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells.
Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE
Laboratory investigation; a journal of technical methods and pathology 2005 Sep;85(9):1139-62
Laboratory investigation; a journal of technical methods and pathology 2005 Sep;85(9):1139-62
Inducible expression of Snail selectively increases paracellular ion permeability and differentially modulates tight junction proteins.
Carrozzino F, Soulié P, Huber D, Mensi N, Orci L, Cano A, Féraille E, Montesano R
American journal of physiology. Cell physiology 2005 Oct;289(4):C1002-14
American journal of physiology. Cell physiology 2005 Oct;289(4):C1002-14
Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells.
Guo X, Rao JN, Liu L, Zou T, Keledjian KM, Boneva D, Marasa BS, Wang JY
American journal of physiology. Gastrointestinal and liver physiology 2005 Jun;288(6):G1159-69
American journal of physiology. Gastrointestinal and liver physiology 2005 Jun;288(6):G1159-69
Amphiregulin causes functional downregulation of adherens junctions in psoriasis.
Chung E, Cook PW, Parkos CA, Park YK, Pittelkow MR, Coffey RJ
The Journal of investigative dermatology 2005 Jun;124(6):1134-40
The Journal of investigative dermatology 2005 Jun;124(6):1134-40
Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells.
Lipschutz JH, Li S, Arisco A, Balkovetz DF
The Journal of biological chemistry 2005 Feb 4;280(5):3780-8
The Journal of biological chemistry 2005 Feb 4;280(5):3780-8
A three-dimensional cellular model of the human respiratory tract to study the interaction with particles.
Rothen-Rutishauser BM, Kiama SG, Gehr P
American journal of respiratory cell and molecular biology 2005 Apr;32(4):281-9
American journal of respiratory cell and molecular biology 2005 Apr;32(4):281-9
Cultured monolayers of the dog jejunum with the structural and functional properties resembling the normal epithelium.
Weng XH, Beyenbach KW, Quaroni A
American journal of physiology. Gastrointestinal and liver physiology 2005 Apr;288(4):G705-17
American journal of physiology. Gastrointestinal and liver physiology 2005 Apr;288(4):G705-17
Paracellular Cl- permeability is regulated by WNK4 kinase: insight into normal physiology and hypertension.
Kahle KT, Macgregor GG, Wilson FH, Van Hoek AN, Brown D, Ardito T, Kashgarian M, Giebisch G, Hebert SC, Boulpaep EL, Lifton RP
Proceedings of the National Academy of Sciences of the United States of America 2004 Oct 12;101(41):14877-82
Proceedings of the National Academy of Sciences of the United States of America 2004 Oct 12;101(41):14877-82
Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms.
McLaughlin J, Padfield PJ, Burt JP, O'Neill CA
American journal of physiology. Cell physiology 2004 Nov;287(5):C1412-7
American journal of physiology. Cell physiology 2004 Nov;287(5):C1412-7
Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells.
Singh AB, Harris RC
The Journal of biological chemistry 2004 Jan 30;279(5):3543-52
The Journal of biological chemistry 2004 Jan 30;279(5):3543-52
Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation.
Barmeyer C, Harren M, Schmitz H, Heinzel-Pleines U, Mankertz J, Seidler U, Horak I, Wiedenmann B, Fromm M, Schulzke JD
American journal of physiology. Gastrointestinal and liver physiology 2004 Feb;286(2):G244-52
American journal of physiology. Gastrointestinal and liver physiology 2004 Feb;286(2):G244-52
Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells.
Yeaman C, Grindstaff KK, Nelson WJ
Journal of cell science 2004 Feb 1;117(Pt 4):559-70
Journal of cell science 2004 Feb 1;117(Pt 4):559-70
Tight junction proteins ZO-1, occludin, and claudins in developing and adult human perineurium.
Pummi KP, Heape AM, Grénman RA, Peltonen JT, Peltonen SA
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2004 Aug;52(8):1037-46
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2004 Aug;52(8):1037-46
Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium.
Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G
American journal of physiology. Renal physiology 2004 Aug;287(2):F305-18
American journal of physiology. Renal physiology 2004 Aug;287(2):F305-18
The specific fates of tight junction proteins in apoptotic epithelial cells.
Bojarski C, Weiske J, Schöneberg T, Schröder W, Mankertz J, Schulzke JD, Florian P, Fromm M, Tauber R, Huber O
Journal of cell science 2004 Apr 15;117(Pt 10):2097-107
Journal of cell science 2004 Apr 15;117(Pt 10):2097-107
Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation.
Yu AS, Enck AH, Lencer WI, Schneeberger EE
The Journal of biological chemistry 2003 May 9;278(19):17350-9
The Journal of biological chemistry 2003 May 9;278(19):17350-9
Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins.
Van Itallie CM, Fanning AS, Anderson JM
American journal of physiology. Renal physiology 2003 Dec;285(6):F1078-84
American journal of physiology. Renal physiology 2003 Dec;285(6):F1078-84
Bryostatin-1 enhances barrier function in T84 epithelia through PKC-dependent regulation of tight junction proteins.
Yoo J, Nichols A, Mammen J, Calvo I, Song JC, Worrell RT, Matlin K, Matthews JB
American journal of physiology. Cell physiology 2003 Aug;285(2):C300-9
American journal of physiology. Cell physiology 2003 Aug;285(2):C300-9
Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells.
Poliak S, Matlis S, Ullmer C, Scherer SS, Peles E
The Journal of cell biology 2002 Oct 28;159(2):361-72
The Journal of cell biology 2002 Oct 28;159(2):361-72
E-Cadherin and tight junctions between epithelial cells of different animal species.
Contreras RG, Shoshani L, Flores-Maldonado C, Lázaro A, Monroy AO, Roldán ML, Fiorentino R, Cereijido M
Pflugers Archiv : European journal of physiology 2002 Jul;444(4):467-75
Pflugers Archiv : European journal of physiology 2002 Jul;444(4):467-75
Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells.
Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M
Journal of cell science 2002 Dec 15;115(Pt 24):4969-76
Journal of cell science 2002 Dec 15;115(Pt 24):4969-76
Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells.
Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M
Journal of cell science 2002 Dec 15;115(Pt 24):4969-76
Journal of cell science 2002 Dec 15;115(Pt 24):4969-76
The renal segmental distribution of claudins changes with development.
Reyes JL, Lamas M, Martin D, del Carmen Namorado M, Islas S, Luna J, Tauc M, González-Mariscal L
Kidney international 2002 Aug;62(2):476-87
Kidney international 2002 Aug;62(2):476-87
Mechanisms of diarrhea in collagenous colitis.
Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD
Gastroenterology 2002 Aug;123(2):433-43
Gastroenterology 2002 Aug;123(2):433-43
Mechanisms of diarrhea in collagenous colitis.
Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD
Gastroenterology 2002 Aug;123(2):433-43
Gastroenterology 2002 Aug;123(2):433-43
Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions.
Nishiyama R, Sakaguchi T, Kinugasa T, Gu X, MacDermott RP, Podolsky DK, Reinecker HC
The Journal of biological chemistry 2001 Sep 21;276(38):35571-80
The Journal of biological chemistry 2001 Sep 21;276(38):35571-80
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
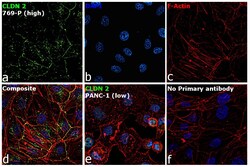
- Immunofluorescence analysis of Claudin-2 was performed using 70% confluent log phase 769-P cells. The cells were fixed with 4% paraformaldehyde for 5 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with Claudin 2 Polyclonal Antibody (MH44) (Product # 51-6100) at 1:100 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32790), (1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cell junction and cytoplasm localization. Panel e represents low expressing PANC-1 cells. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
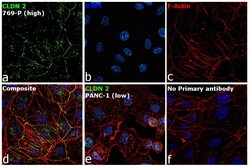
- Immunofluorescence analysis of Claudin-2 was performed using 70% confluent log phase 769-P cells. The cells were fixed with 4% paraformaldehyde for 5 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with Claudin 2 Polyclonal Antibody (MH44) (Product # 51-6100) at 1:100 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32790), (1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cell junction and cytoplasm localization. Panel e represents low expressing PANC-1 cells. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
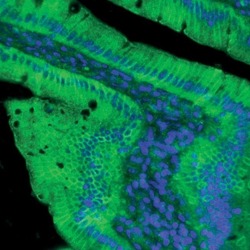
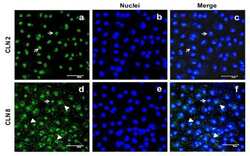
- Indirect immunofluorescent analysis of rat small intestine using Zymed Rb anti-Claudin-2 (Product # 51-6100).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
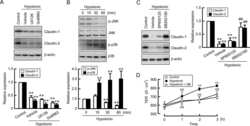
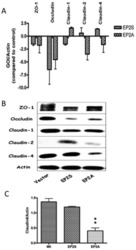
- FIGURE 10: Overexpression of either GFP-Rab11-FIP2-WT or GFP-Rab11-FIP2 (S227E) altered the claudin content of tight junctions. GFP-Rab11-FIP2-WT-on and GFP-Rab11-FIP2(S227E)-on and -off were grown for 5 d postconfluence on Transwell filters. The cells were fixed then stained for claudin-1 (A), claudin-2 (B), or claudin-4 (C) and ZO-1. In the merged image, GFP-Rab11-FIP2s are pseudocolored green, the claudins are red, and ZO-1 is blue. (D) Cells were grown as described, then lysed, run on Western blots, and probed for the different claudins and VDAC. Three separate blots were scanned and quantified using ImageJ and normalized to control (VDAC). *p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
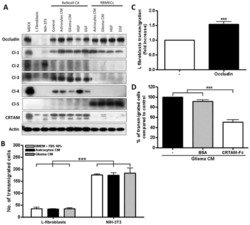
- Figure 6 Expression of TJ proteins in transmigrating cells favors their passage across RBMECs. A) Analysis by Western blots of the expression of occludin, claudins 1 to 5 and CRTAM in NIH-3T3and L-fibroblasts, ReNcells CX and RBMECs. B) The transmigration of NIH-3T3 and L-fibroblasts acrossRBMECs is independent of the CM present in the basal compartment, but is significantly higher inNIH-3T3 fibroblasts that express claudin-2 than in L-fibroblast that do not express TJ proteins.N = 3, F(1,6) = 232.22, ***P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
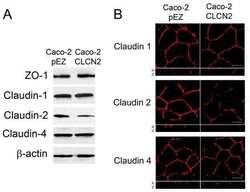
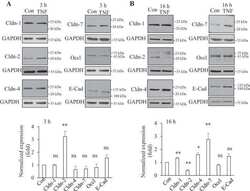
- Figure 3 The expression of tight junction mRNA and proteins in Caco-2 EP2 receptor transfectants. (A) The mRNA expression for tight junction proteins (ZO-1, occludin, claudin-1, claudin-2 and claudin-4) in Caco-2 EP2S and EP2A cells was measured by Q-PCR. The expression of gene of interest (GOI) was normalized with the internal control (Actin) and expressed as fold changes with that of the vector control. (B) A representative immunoblot showing constitutive expression of the important tight junction proteins (ZO-1, occludin, claudin-1, claudin-2 and claudin-4) in EP2S, EP2A and vector control cells. Actin used as internal control. (C) Densitometry analysis for claudin-4 expression in EP2S, EP2A and vector control cells. EP2A compared statistically with the vector control. p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
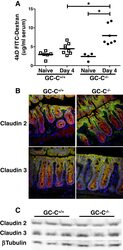
- Figure 5 GC-C is essential to maintain intestinal barrier function during C. rodentium infection. A : GC-C +/+ and GC-C -/- mice were given a FITC-dextran tracer by oral gavage on day 4 p.i. and serum fluorescence was measured four hours later. Relative to naive mice as well as infected GC-C +/+ animals, GC-C -/- mice have a substantial and significant increase in tracer movement from the gut lumen into the blood indicating a loss of intestinal barrier function. (* P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
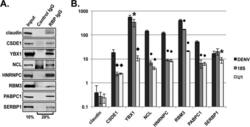
- FIG 2 Validation of RNA-protein interactions by RNA immunoprecipitation. Huh7 cells were infected with DENV2 NGC at an MOI of 1. Infected cell lysates were incubated with an isotype control antibody or an antibody directed against the indicated cellular proteins. Bound material was captured on Dynabeads protein G for analysis. (A) Western blot analysis of immunoprecipitated material. Input represents 10% of total input sample. IgG lanes represent 20% of immunoprecipitated protein. (B) RT-qPCR analysis of immunoprecipitated RNA. Statistical significance relative to DENV measured by Student's t test: *, P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
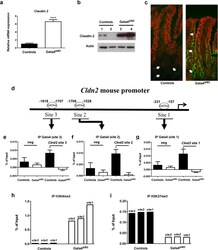
- Figure 2 Claudin-2 expression is derepressed in the jejunum of Gata4 mutant mice. ( a ) Total RNA was isolated from the jejunum of control and Gata4 DeltaIEC mice (n = 3-4 per group) and RT-qPCR was performed to quantify claudin-2 gene transcripts. **** P < 0.0001 (Student-t test). ( b ) Western blot analysis was performed using a claudin-2 polyclonal antibody on total lysates prepared from control and Gata4 DeltaIEC mice. An actin polyclonal antibody was used as a loading control to monitor protein integrity. Cropped blots are displayed and full-length blots are included in the supplementary information. ( c ) Immunofluorescence detection of claudin-2 on jejunum sections prepared from control and Gata4 DeltaIEC mice. Arrows display typical labeling in the tight junctions. Original magnification: 10X. ( d ) Schematic representation of the Cldn2 gene with its predicted binding sites for GATA. ( e - g ) ChIP analysis of three paired biological samples (n = 3) obtained from the jejunum of control and Gata4 DeltaIEC mice. Data were obtained by qPCR and are expressed as the percent of total DNA input used for precipitation with an antibody against Gata4 relative to the DNA precipitated with normal goat-IgG. * P < 0.05 (ANOVA test). ( h , i ) ChIP analysis from the jejunum of control and Gata4 DeltaIEC mice. Data were obtained by qPCR and are expressed as the percent of total DNA input used for precipitation with an antibody against H3K4me3 ( h ) or H3K27me3 ( i ) relative to the D
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
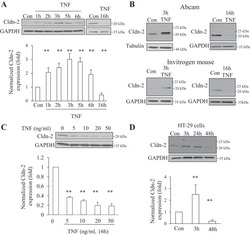
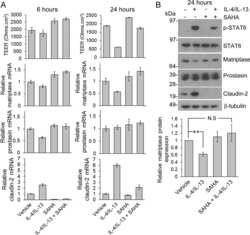
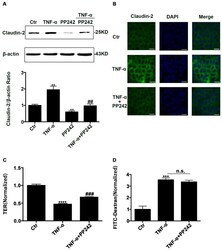
- Figure 5 PP242 alleviated the change of claudin-2 expression and intestinal epithelial tight junction function in TNF-alpha treated Caco-2 cells. ( A ) Western blot analysis of claudin-2 after TNF-alpha (10 ng/mL, 48 h) and/or PP242 (1 mum, 24 h) treatment. ( B ) Immunofluorescence staining of claudin-2 after TNF-alpha and/or PP242 treatment in Caco-2 cell monolayers. The green and blue fluorescence were represented the claudin-2 protein and nucleus, respectively. Scale bar: 20 mum. ( C ) The effect of PP242 (1 mum, 24 h) on TER of Caco-2 cell monolayers treated with TNF-alpha (10 ng/mL, 48 h). ( D ) The effect of PP242 on the permeability of FITC-dextran in TNF-alpha treated Caco-2 cell monolayers. Data were shown as mean +- SD and replicated three times. ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus control group. ## p < 0.01, ### p < 0.001 versus TNF-alpha group. n.s., no significant. Ctr, control group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
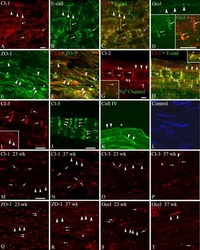
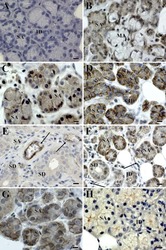
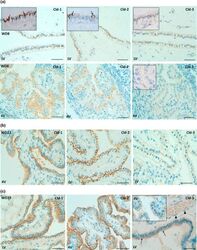
- Fig. 8 Immunohistochemical detection of claudins in choroid plexuses of developing human brain. Immunoreactivity of Cld-1, -2, and -3 is presented on the left, central, and right panels , respectively, with hematoxylin counterstaining. a Staining of a WD8 fetal brain. Cld-1 labeling, observed in both LVCP and 4VCP, was continuous and localized apically at intercellular junctions of the epithelium ( arrows in inserted high magnification micrograph ). Cld-2 immunoreactivity displayed a similar apical, but patchy pattern in both LVCP and 4VCP. The higher magnification ( insert in LVCP) illustrates the discontinuous labeling ( arrows ). Cld-3 was also detected in the epithelium of both CP ( arrows ). Inserts are higher magnifications highlighting the continuous staining pattern. b Staining of a WD22 fetal brain. Cld-1 immunoreactivity was present at all epithelial junctions (shown in 4VCP). A continuous apical labeling of the epithelium was observed for Cld-2 (LVCP is shown). Cld-3 labeling was faint but continuous in LVCP epithelial cells. c Staining of a WD39 fetal brain. A distinct and continuous labeling of the choroidal epithelium was observed for all Cld-1 (shown for LVCP), -2 (shown for 4VCP), and -3. The continuous signal for Cld-3 was only associated with the epithelium ( arrows ) and not with stromal vessels ( arrowheads in LVCP). The insert shows a similar Cld-3 staining for 4VCP. Abbreviations as in Fig. 2 . Scale bar 50 mum
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
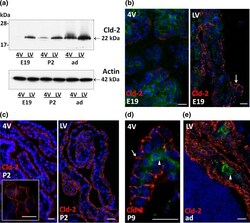
- Fig. 4 Differential expression of claudin-2 in choroid plexus during rat brain development. a Representative Western blot of LVCP and 4VCP dissected from E19, P2, and adult animals (10 mug per lane). Cld-2 expression increased in CPs during development, and was distinctively higher in LVCP than in 4VCP of E19 and P2 animals ( upper panel band at 22 kDa). Actin used as a loading indicator is shown in the lower panel (42 kDa band). b - e Cld-2 immunoreactivity ( red ) in the developing and adult brain. LVCP and 4VCP sections have been labeled simultaneously and pictures generated with identical camera settings. Cld-2 staining was associated with the epithelium throughout the LVCP of E19 animals ( arrow ), while the staining in 4VCP was faint and patchy, with large portions of the epithelium remaining unlabeled ( b ). In P2 animals ( c ), the staining was more homogeneous in 4VCP and became continuous between epithelial cells ( insert ), as in the LVCP ( right panel ). The epithelial staining was strong in all CPs at P9 ( d arrow ) and in adult animals ( e ). In b , d , e double immunolabeling with anti-RECA-1 Ab allowed visualizing the choroidal vessels unlabeled by the anti-Cld-2 Ab ( arrowheads ). DAPI was used for nuclei staining. Scale bars 20 mum. Abbreviations as in Fig. 2
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
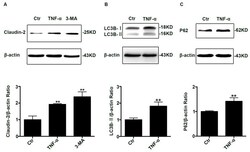
- Figure 1 Change of claudin-2, LC3B-II, and P62 protein in TNF-alpha treated Caco-2 cell monolayers. ( A ) Western blotting analysis of caludin-2 in Caco-2 cell monolayer after TNF-alpha (10 ng/mL) administration or 3-MA (5 mM) treatment for 48 h. ( B , C ) Western blotting analysis of LC3B-II and P62 in Caco-2 cells after TNF-alpha (10 ng/mL) administration for 48 h. beta-actin was used as an internal control. Data were shown as mean +- SD and replicated three times. ** p < 0.01 versus control group. Ctr, control group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
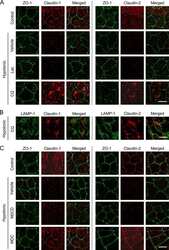
- Figure 3 Representative photographs of claudin-2, claudin-3, claudin-4, and zonula occludens-1 immunostained in longitudinal jejunal sections of the three experimental groups. Claudin-2 immunoreaction in the sham-operated group (a) was low and was mainly found in the cytoplasm of the intestinal epithelial cells. Claudin-2 expression in the groups exposed to ischemia-reperfusion was substantially higher and was principally found in the lateral membrane of the epithelial cells (b, c). The sham-operated group presented strong membrane claudin-3 immunostaining (d), which was remarkably decreased in the ischemia-reperfusion- (IR-) group (e), but not in the postconditioned- (PC-) group (f). Minimal claudin-4 immunostaining was observed in the jejunal samples of the sham-operated animals (g). The two groups challenged with 60 minutes of mesenteric ischemia and 6 hours of reperfusion (h, i) showed high membrane claudin-4 expression, which was limited to the tips of the villi. Zonula occludens-1 was strongly immunostained in the jejunal samples of the sham-operated animals (j). Following small intestinal ischemia-reperfusion, intestinal epithelial cell with zonula occludens-1 membrane immunostaining notably decreased in the IR-group (k); in the PC-group minimal loss of zonula occludens-1 membrane immunostaining was detected (l). Each photograph was taken with the same magnification. Scale bar: 20 mu m.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
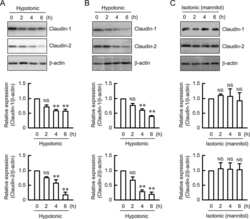
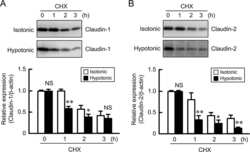
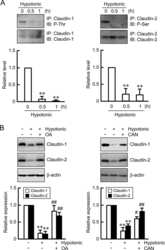
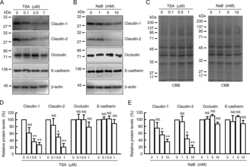
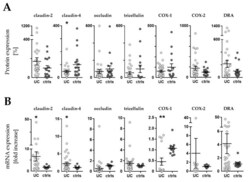
- Figure 3 Western blot and RT-qPCR. ( A ) Expression levels of barrier related proteins by densitometric analysis of Western blot (WB). Values are expressed as % of a mean of all controls (ctrls). Bars indicate mean +- standard error of the mean. Asterisk indicates statistically significant difference between groups (* p < 0.05). ( B ) mRNA levels by RT-qPCR. Values are expressed relative to a mean of all controls. Three controls (18%, 3/17) presented a different baseline of housekeeping genes, while two controls (12%, 2/17) presented outliers, which were removed, leaving the final number of controls for mRNA analysis at 12 (70% 12/17). One outlier (3%, 1/33) was removed from the UC group. n = number of UC observations: COX-1 ( n = 16), COX-2 ( n = 11), claudin-2 ( n = 23), claudin-4 ( n = 33), occludin ( n = 31), tricellulin ( n = 29), and DRA ( n = 33). Bars indicate mean +- standard error of the mean. Asterisks indicate statistically significant difference between two groups (* p < 0.05, ** p < 0.01).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
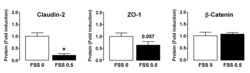
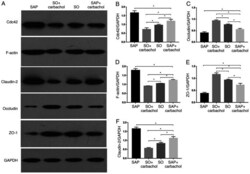
- Figure 3 Western blotting analysis. Representative western blotting images of (A) claudin-2, occludin, ZO-1, F-actin and Cdc42 in the intestinal epithelium. Relative expression of (B) Cdc42, (C) occludin, (D) F-actin, (E) ZO-1 and (F) claudin-2 determined by optical densitometry. Results are expressed as the mean +- standard deviation (n=6 randomly selected mice in each group). * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
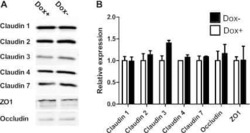
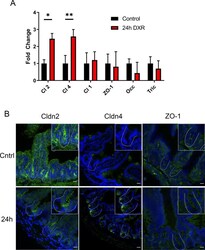
- Figure 6 The murine jejunal tight junctional network is affected by DXR exposure. ( A ) RT-qPCR of tight junctional genes in jejunal crypt epithelium isolated from control tissues and tissues collected 24 h after DXR from conventionally raised mice ( n = 3/group). All data is presented as fold change normalized to Actb . Student's t test; *p < 0.05, **p < 0.01. ( B ) Representative images of immunofluorescent staining of tight junction associated proteins Claudin-2 (Cldn2), Claudin-4 (Cldn4), and Zona Occludens-1 (ZO-1) in control tissues (Cntrl) and tissues collected 24 h after DXR (24 h) from conventionally raised mice. Images are representative of n = 3 per time point. Scale bar 20 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
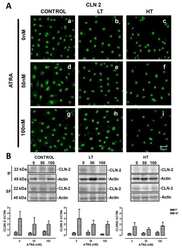
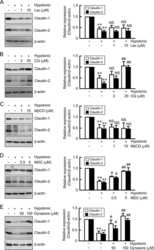
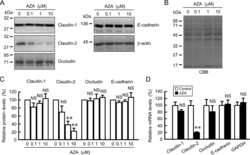
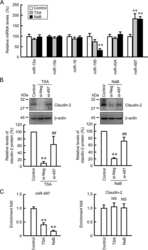
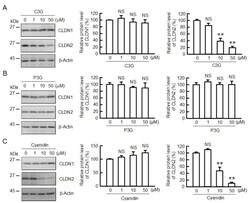
- Figure 1 Effects of anthocyanins on the protein levels of CLDN1 and CLDN2 in A549 cells. Cells were incubated with 0, 1, 10, or 50 uM C3G ( A ), P3G ( B ), or cyanidin ( C ) for 24 h. Cell lysates were immunoblotted with anti-CLDN1, anti-CLDN2, and anti-beta-actin antibodies. The protein levels of CLDN1 and CLDN2 are represented as a percentage relative to 0 uM. n = 3-4. ** p < 0.01 and NS p > 0.05 compared with 0 uM.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
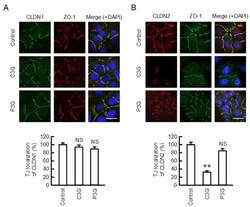
- Figure 2 Effects of anthocyanins on the cellular localization of CLDN1 and CLDN2. Cells cultured on cover glasses were incubated in the absence (control) and presence of 50 uM C3G or P3G for 24 h. ( A ) The cells were stained with anti-CLDN1 (green), anti-ZO-1 (red), and nuclear marker DAPI (blue). ( B ) The cells were stained with anti-CLDN2 (red), anti-ZO-1 (green), and DAPI (blue). Merged images are shown in the right panel. The scale bar represents 10 um. The TJ localizations of CLDN1 and CLDN2 are shown as percentage of control ( n = 132-220 cells). ** p < 0.01 and NS p > 0.05 compared with control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
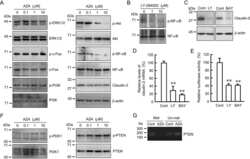
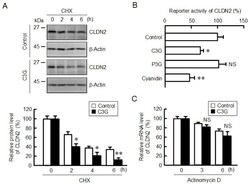
- Figure 5 Decrease in the protein stability and reporter activity of CLDN2 by C3G. ( A ) Cells were incubated with 10 uM CHX in the absence and presence of 50 uM C3G for 0, 2, 4, and 6 h. Cell lysates were immunoblotted with anti-CLDN2 and anti-beta-actin antibodies. The protein levels of CLDN2 are represented as a percentage relative to control. ( B ) Cells were incubated in the absence (control) and presence of 50 uM anthocyanins for 6 h, and then the reporter activity was measured by the Dual-Luciferase Reporter Assay System. The reporter activity is represented as a percentage relative to control. ( C ) Cells were incubated with 1 µg/mL actinomycin D in the absence and presence of 50 uM C3G for 0, 3, and 6 h. The mRNA level of CLDN2 was measured by quantitative real-time PCR and represented as a percentage relative to 0 h. n = 3-5. ** p < 0.01, * p < 0.05 and NS p > 0.05 compared with control or 0 h.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
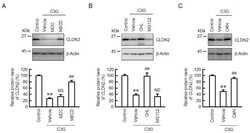
- Figure 6 Effect of C3G on the degradation and endocytosis of the CLDN2 protein. ( A ) Cells were incubated in the absence (control) and presence of 50 uM C3G, 5 uM MDC, or 10 uM MbetaCD for 24 h. ( B ) Cells were incubated in the absence (control) and presence of 50 uM C3G, 20 uM CHL, or 5 uM MG132 for 24 h. ( C ) Cells were incubated in the absence (control) and presence of 50 uM C3G for 24 h. CAN (0.5 uM) was added into the medium 9 h before collection. Cell lysates were immunoblotted with anti-CLDN2 and anti-beta-actin antibodies. The protein levels of CLDN2 are represented as a percentage relative to control. n = 3-5. ** p < 0.01 compared with control. ## p < 0.01, and NS p > 0.05 compared with vehicle.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
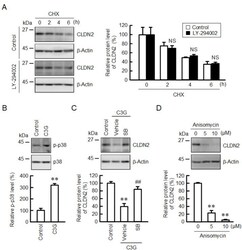
- Figure 7 Effect of C3G on the levels of p-p38 and CLDN2. ( A ) Cells were incubated with 10 uM CHX in the absence (control) and presence of 10 uM LY-294002 for 0, 2, 4, and 6 h. Cell lysates were immunoblotted with anti-CLDN2 and anti-beta-actin antibodies. The protein levels of CLDN2 are represented as a percentage relative to 0 h. ( B ) Cells were incubated in the absence and presence of 50 uM C3G for 1 h. Cell lysates were immunoblotted with anti-p-p38 and anti-p38 antibodies. The p-p38 levels are represented as a percentage relative to control. ( C ) Cells were incubated in the absence and presence of 50 uM C3G and 10 uM SB203580 (SB) for 24 h. Cell lysates were immunoblotted with anti-CLDN2 and anti-beta-actin antibodies. The protein levels are represented as a percentage relative to control. ( D ) Cells were incubated with 0, 5, and 10 uM anisomycin for 24 h. Cell lysates were immunoblotted with anti-CLDN2 and anti-beta-actin antibodies. The protein levels are represented as a percentage relative to 0 uM. n = 3-4. ** p < 0.01 and NS p > 0.05 compared with control. ## p < 0.01 compared with vehicle.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
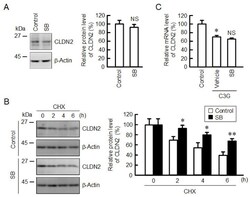
- Figure 8 Effects of anisomycin and SB203580 on CLDN2 expression. ( A ) Cells were incubated in the absence (vehicle) and presence of 10 uM SB203580 (SB) for 24 h. Cell lysates were immunoblotted with anti-CLDN2 and anti-beta-actin antibodies. The protein levels of CLDN2 are represented as a percentage relative to vehicle. ( B ) Cells were incubated with 10 uM CHX in the absence (control) and presence of 10 uM SB203580 (SB) for 0, 2, 4, and 6 h. The protein levels are represented relative to 0 h. ( C ) Cells were incubated in the absence (control) and presence of 10 uM C3G and 10 uM SB203580 for 6 h. The mRNA levels are represented as a percentage relative to control. n = 3-4. ** p < 0.01 and * p < 0.05 compared with 0 uM or control. NS p > 0.05 compared with vehicle.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
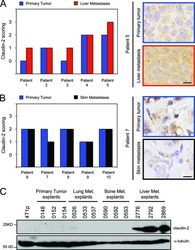
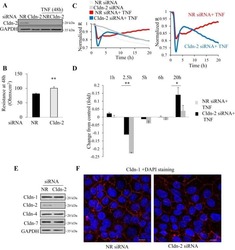
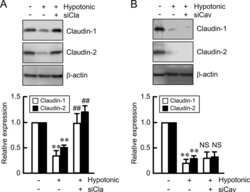
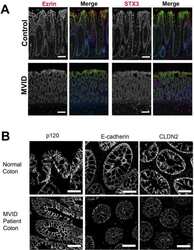
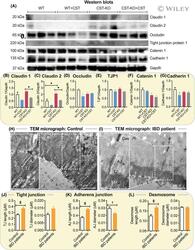
- 3 FIGURE Elevated levels of Claudin-2 in colon of CST-KO mice and elongated epithelial junctions in IBD patients. (A) Western blots and (B) densitometric analyses of Claudin-1 (B), Claudin-2 (C), Occludin (D), Tjp1 (E), Catenin 1 (F) and Cadherin 1 (G). H and I, TEM micrographs (n = 3) showing tight junction (TJ), adherens junction (AJ) and desmosomes (Des) in healthy control and IBD patients. Microvillus (MV). J-L, Morphometrical analyses (n = 20-54) showing increased lengths and diameter of TJ (J), AJ (K) and Des (L) in IBD patients. * P < .05; + P < .01; ++ P < .001
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
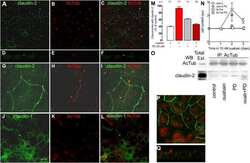
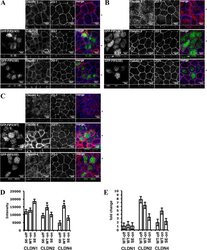
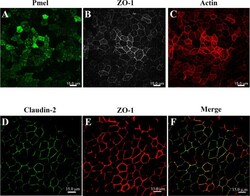
- Figure 2 Confocal micrographs of ARPE-19 cells cultured for 4 months. The ARPE-19 cells were plated on laminin-coated Transwell (r) filter membrane at a density of 3 x 10 5 cells/cm 2 and grown for 4 months as outlined in the Methods section. Rabbit anti- zonula occludens-1 (ZO-1), mouse anti-premelanosome protein (PMEL), mouse anti-claudin-2 primary antibodies, and Alexa Fluor secondary antibodies were used to immunostain the ARPE-19 cells at 4 months post-confluency; rhodamine-phalloidin was used to stain actin. A : PMEL immunostaining correlates with the presence of melanosome pigmentation. B : ZO-1 immunostaining shows good junctional development and localization at the cell borders containing a mixture of elongated and polygonal cells. C : Actin immunostaining reveals circumferential actin distribution throughout the height of the cells. D : Claudin-2 immunostaining shows tight junction localization uniformly across the monolayer. E : ZO-1 immunostaining shows good junctional development and localization at the cell borders regardless of the secondary antibody used. F : Claudin-2 immunofluorescent labeling shows colocalization with ZO-1 in the tight junction complexes (merged image of D and E ). Images were taken using an Andor Revolution XD spinning disk confocal microscope using the 40X Plan Fluor oil immersion objective. Scale bar = 15 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
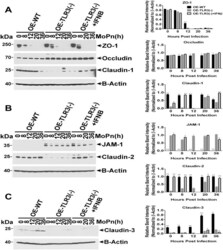
- Fig 6 TLR3 deficiency leads to altered TJ protein expression in OE cell monolayers during Chlamydia infection. WT, TLR3-deficient, and IFN-beta pre-treated TLR3-deficient OE cells were infected with 10 IFU/ cell C . muridarum for up to 36 hours before cell lysates were harvested at the various time-points shown. Protein expression levels were measured by western blot analyses for: (A) ZO-1, occludin, and claudin-1; (B) JAM1 and claudin-2; and (C) claudin-3. OE-129WT = wild-type and OE-TLR3(-) = TLR3-deficient OE cells. Blots shown are representative data . Right-side plots show densitometry of tight-junction proteins normalized to the beta-actin control for the representative data. The relative band intensity is the ratio number of respective band intensity for each TJ protein (minus background) at the indicated time-point, divided by the band intensity for beta-actin (minus background) at that specific time-point shown in the representative western blot data . Significance was determined by comparing the ratio number of the representative data for WT and IFN-beta pre-treated TLR3-deficient OE cells, to the ratio number of OE-TLR3(-) cells divided by beta-actin for three independent experiments. P-values were calculated using a two-tailed unpaired t -test, error bars represent mean (SD) * = p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
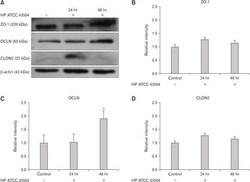
- Figure 4 Expression of zonula occludens-1 (ZO-1), occludin (OCLN), and claudin-2 (CLDN2) in HFE 145 cell lines after cultured with Helicobacter pylori 43504 strain (HP ATCC 43504; O4 strain). (A) Western blot analysis of ZO-1, OCLN, and CLDN2. (B, D) There were no significant differences in the expression of ZO-1 and CLDN2 between control and H. pylori infection group. (C) The expression of OCLN was significantly elevated 48 hours after the culture with H. pylori strain 43504. Data are expressed as means +- SE for 3 experiments. * P < 0.05 significantly different from the control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
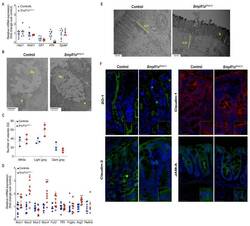
- Figure 3 BmpR1a DeltaFoxL1+ mice displayed abnormalities in goblet cell vesicles and presented dysregulated mucus maturation and structural gene expression. ( A ) Relative mRNA levels of the colonocyte/secretory lineage specification Hes1 and Atoh1 , along with committed-secretory cell toward the goblet fate or maturation Gfi1 , Spdef and Klf4 . A significant decrease was observed for Klf4 mRNA levels in mutant mice while other mRNAs were not modulated between mutant and control mice ( N = 7-9). ( B ) Ultrastructural analysis of the goblet cells revealed vesicles without mucin diversity, disturbed morphology in BmpR1a DeltaFoxL1+ mice ( N = 4). ( C ) Vesicles pattern quantification revealed a significant increase in light grey vesicles in mutant goblet cells when compared to controls ( N = 3). ( D ) BmpR1a DeltaFoxL1+ mice showed a significant increase in Fut2 , Muc2 , Muc4 and Agr2 mRNA levels in mutant mice. ( E ) Ultrastructural analysis of the glycocalyx and the apical junctional complex (yellow bracket) revealed that the latter was not modified between both groups and showed loss of glycocalyx in BmpR1a DeltaFoxL1+ mice ( N = 4). ( F ) Immunofluorescence against ZO-1, claudin-1 and 2 as well as JAM-A revealed no modulation in the tight junction complex proteins between both groups ( N = 3). ( C ) 2-way ANOVA test. Data are presented as the mean +- SD. ( D ) Mann-Whitney test. N: Nucleus; Mu: Mucin vesicles; G: Glycocalyx; AJC: Apical junctional complex. Fold-change was n
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. 1. Cldn2 and Cldn12 confer independent Ca 2+ permeability to the proximal colon. P Ca measured ex vivo in Ussing's chambers across the proximal colon compared to WT of Cldn2 KO ( A ) ( n = 8 per group and P = 0.016); Cldn12 KO ( B ) ( n = 8 per group and P = 0.012); and Cldn2/12 DKO ( C ) ( n = 8 per group and P = 0.019). Full results of bionic dilution potential experiments on small intestine and colon of DKO mice are in SI Appendix , Fig. S2 and Tables S1-S4 . Means were compared by Student's t test. ( D ) Data from A-C expressed as the percentage change in P Ca relative to WT for each genotype. Data are presented as mean +- SD. One-way ANOVA with Dunnett correction for multiple comparisons to compare mean from DKO mice to Cldn2 KO ( P = 0.017) and Cldn12 KO ( P = 0.010) was performed. The Cldn12 coding exon was replaced with a LacZ cassette. ( E and F ) We used this to localize Cldn12 expression by X-gal staining of colon from Cldn12 WT ( E ) and Cldn12 heterozygous mice ( F ). Staining is present in colonic crypt epithelial cells in Cldn12 heterozygous mouse (cyan). (Scale bars, 100 um [ E ] and 25 um [ F ]). ( G ) X-gal staining of colon for Cldn12 (cyan) and immunohistochemical staining for claudin-2 (brown) from a Cldn12 heterozygous mouse. Cr = crypts, SM = smooth muscle, and L = colonic lumen. (Scale bar, 25 um.) * P < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
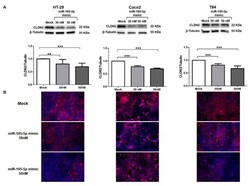
- Experimental details
- Regulation of CLDN2 protein expression by miR-195-5p in colonic epithelial cell lines. ( A ) Western blot analysis of CLDN2 protein expression in HT-29, Caco2, and T84 cell lines after miR-195-5p mimic transfection. A significant reduction of CLDN2 expression was detected in all cell lines. Raw data of the independent experiments of Western blot were reported in Appendix A . ( B ) Immunofluorescence staining of CLDN2 in HT-29, Caco2 and T84 cell cultures after miR-195-5p mimic transfection. In accordance with Western blot results, after transfection, the expression of CLDN2 decreased compared with mock-control. Single channel images of DAPI and CLDN2 staining before merge were reported in Appendix B . Data of WB were obtained by dividing the normalized transfected sample values by the normalized control sample values. beta-Tubulin was used as housekeeping protein to normalize the data. Data are representative of four independent experiments. The histograms correspond to mean +- SEM. Scale bar = 50 um ** p < 0.01; *** p < 0.001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
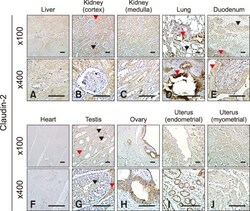
- Experimental details
- Fig. 3 Localization of claudin-2 in equine tissues via immunohistochemistry. Scale bar = 100 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 3 Analysis of Cldn2 expression and localization in MDCK II cells following quercetin treatment. ( A ) Western blot analysis of Cldn2 expression in cell lysates from control and 400 uM quercetin-treated MDCK II cells. Actin was used as a loading control. ( B ) Densitometry measurements of Cldn2 and actin intensity on western blot were normalized to control at 1 h. Normalized Cldn2 expression relative to normalized actin is plotted (P int = 0.052; P time = 0.096; P treat = 0.0021). ( C ) Immunofluorescence of control and 400 uM of quercetin-treated MDCK II cells at 1, 6, 24, and 48 h. Cldn2 is shown in green and ZO1 is shown in red. ( D ) Localization of claudin at the tight junction was assessed by determining the amount of ZO1 that co-localized with claudin expression (P int = 0.15; P time = 0.46; P treat = 0.0055). For all graphs, each point corresponds to an independent experiment; mean and SEM are shown. C = Control and Q = Quercetin. Scale bar, 20 um.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry