Antibody data
- Antibody Data
- Antigen structure
- References [25]
- Comments [0]
- Validations
- Flow cytometry [1]
- Other assay [24]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 32-2400 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Cullin 1 Monoclonal Antibody (2H4C9)
- Antibody type
- Monoclonal
- Antigen
- Synthetic peptide
- Description
- CUL-1 Antibody (2H4C9), Prod# 32-2400 specifically detects neddylated and unmodified Cullin 1 in human cells.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 2H4C9
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- -20°C
Submitted references Cullin neddylation inhibitor attenuates hyperglycemia by enhancing hepatic insulin signaling through insulin receptor substrate stabilization.
Cyclin B/CDK1 and Cyclin A/CDK2 phosphorylate DENR to promote mitotic protein translation and faithful cell division.
The IMiD target CRBN determines HSP90 activity toward transmembrane proteins essential in multiple myeloma.
Antagonistic activities of CDC14B and CDK1 on USP9X regulate WT1-dependent mitotic transcription and survival.
The Balance between Mono- and NEDD8-Chains Controlled by NEDP1 upon DNA Damage Is a Regulatory Module of the HSP70 ATPase Activity.
NS5A Promotes Constitutive Degradation of IP3R3 to Counteract Apoptosis Induced by Hepatitis C Virus.
Cand1-Mediated Adaptive Exchange Mechanism Enables Variation in F-Box Protein Expression.
Multiple UBXN family members inhibit retrovirus and lentivirus production and canonical NFκΒ signaling by stabilizing IκBα.
Geminin is an indispensable inhibitor of Cdt1 in mouse embryonic stem cells.
Immunomodulatory drugs disrupt the cereblon-CD147-MCT1 axis to exert antitumor activity and teratogenicity.
Isolation of ubiquitinated substrates by tandem affinity purification of E3 ligase-polyubiquitin-binding domain fusions (ligase traps).
USP9X stabilizes XIAP to regulate mitotic cell death and chemoresistance in aggressive B-cell lymphoma.
Ubiquitin-conjugating enzyme Cdc34 and ubiquitin ligase Skp1-cullin-F-box ligase (SCF) interact through multiple conformations.
Ubiquitin-conjugating enzyme Cdc34 and ubiquitin ligase Skp1-cullin-F-box ligase (SCF) interact through multiple conformations.
Peptidic degron in EID1 is recognized by an SCF E3 ligase complex containing the orphan F-box protein FBXO21.
F-box protein FBXL16 binds PP2A-B55α and regulates differentiation of embryonic stem cells along the FLK1+ lineage.
Early insights into the function of KIAA1199, a markedly overexpressed protein in human colorectal tumors.
Small molecule-induced mitochondrial disruption directs prostate cancer inhibition via UPR signaling.
Designer reagents for mass spectrometry-based proteomics: clickable cross-linkers for elucidation of protein structures and interactions.
Cullin-3 regulates late endosome maturation.
Structure of a glomulin-RBX1-CUL1 complex: inhibition of a RING E3 ligase through masking of its E2-binding surface.
UBXN7 docks on neddylated cullin complexes using its UIM motif and causes HIF1α accumulation.
The glomuvenous malformation protein Glomulin binds Rbx1 and regulates cullin RING ligase-mediated turnover of Fbw7.
SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction.
Control of chromosome stability by the beta-TrCP-REST-Mad2 axis.
Chen C, Gu L, Matye DJ, Clayton YD, Hasan MN, Wang Y, Friedman JE, Li T
Proceedings of the National Academy of Sciences of the United States of America 2022 Feb 8;119(6)
Proceedings of the National Academy of Sciences of the United States of America 2022 Feb 8;119(6)
Cyclin B/CDK1 and Cyclin A/CDK2 phosphorylate DENR to promote mitotic protein translation and faithful cell division.
Clemm von Hohenberg K, Müller S, Schleich S, Meister M, Bohlen J, Hofmann TG, Teleman AA
Nature communications 2022 Feb 3;13(1):668
Nature communications 2022 Feb 3;13(1):668
The IMiD target CRBN determines HSP90 activity toward transmembrane proteins essential in multiple myeloma.
Heider M, Eichner R, Stroh J, Morath V, Kuisl A, Zecha J, Lawatscheck J, Baek K, Garz AK, Rudelius M, Deuschle FC, Keller U, Lemeer S, Verbeek M, Götze KS, Skerra A, Weber WA, Buchner J, Schulman BA, Kuster B, Fernández-Sáiz V, Bassermann F
Molecular cell 2021 Mar 18;81(6):1170-1186.e10
Molecular cell 2021 Mar 18;81(6):1170-1186.e10
Antagonistic activities of CDC14B and CDK1 on USP9X regulate WT1-dependent mitotic transcription and survival.
Dietachmayr M, Rathakrishnan A, Karpiuk O, von Zweydorf F, Engleitner T, Fernández-Sáiz V, Schenk P, Ueffing M, Rad R, Eilers M, Gloeckner CJ, Clemm von Hohenberg K, Bassermann F
Nature communications 2020 Mar 9;11(1):1268
Nature communications 2020 Mar 9;11(1):1268
The Balance between Mono- and NEDD8-Chains Controlled by NEDP1 upon DNA Damage Is a Regulatory Module of the HSP70 ATPase Activity.
Bailly AP, Perrin A, Serrano-Macia M, Maghames C, Leidecker O, Trauchessec H, Martinez-Chantar ML, Gartner A, Xirodimas DP
Cell reports 2019 Oct 1;29(1):212-224.e8
Cell reports 2019 Oct 1;29(1):212-224.e8
NS5A Promotes Constitutive Degradation of IP3R3 to Counteract Apoptosis Induced by Hepatitis C Virus.
Kuchay S, Saeed M, Giorgi C, Li J, Hoffmann HH, Pinton P, Rice CM, Pagano M
Cell reports 2018 Oct 23;25(4):833-840.e3
Cell reports 2018 Oct 23;25(4):833-840.e3
Cand1-Mediated Adaptive Exchange Mechanism Enables Variation in F-Box Protein Expression.
Liu X, Reitsma JM, Mamrosh JL, Zhang Y, Straube R, Deshaies RJ
Molecular cell 2018 Mar 1;69(5):773-786.e6
Molecular cell 2018 Mar 1;69(5):773-786.e6
Multiple UBXN family members inhibit retrovirus and lentivirus production and canonical NFκΒ signaling by stabilizing IκBα.
Hu Y, O'Boyle K, Auer J, Raju S, You F, Wang P, Fikrig E, Sutton RE
PLoS pathogens 2017 Feb;13(2):e1006187
PLoS pathogens 2017 Feb;13(2):e1006187
Geminin is an indispensable inhibitor of Cdt1 in mouse embryonic stem cells.
Hosogane M, Bosu L, Fukumoto E, Yamada H, Sato S, Nakayama K
Genes to cells : devoted to molecular & cellular mechanisms 2017 Apr;22(4):360-375
Genes to cells : devoted to molecular & cellular mechanisms 2017 Apr;22(4):360-375
Immunomodulatory drugs disrupt the cereblon-CD147-MCT1 axis to exert antitumor activity and teratogenicity.
Eichner R, Heider M, Fernández-Sáiz V, van Bebber F, Garz AK, Lemeer S, Rudelius M, Targosz BS, Jacobs L, Knorn AM, Slawska J, Platzbecker U, Germing U, Langer C, Knop S, Einsele H, Peschel C, Haass C, Keller U, Schmid B, Götze KS, Kuster B, Bassermann F
Nature medicine 2016 Jul;22(7):735-43
Nature medicine 2016 Jul;22(7):735-43
Isolation of ubiquitinated substrates by tandem affinity purification of E3 ligase-polyubiquitin-binding domain fusions (ligase traps).
Mark KG, Loveless TB, Toczyski DP
Nature protocols 2016 Feb;11(2):291-301
Nature protocols 2016 Feb;11(2):291-301
USP9X stabilizes XIAP to regulate mitotic cell death and chemoresistance in aggressive B-cell lymphoma.
Engel K, Rudelius M, Slawska J, Jacobs L, Ahangarian Abhari B, Altmann B, Kurutz J, Rathakrishnan A, Fernández-Sáiz V, Brunner A, Targosz BS, Loewecke F, Gloeckner CJ, Ueffing M, Fulda S, Pfreundschuh M, Trümper L, Klapper W, Keller U, Jost PJ, Rosenwald A, Peschel C, Bassermann F
EMBO molecular medicine 2016 Aug;8(8):851-62
EMBO molecular medicine 2016 Aug;8(8):851-62
Ubiquitin-conjugating enzyme Cdc34 and ubiquitin ligase Skp1-cullin-F-box ligase (SCF) interact through multiple conformations.
Sandoval D, Hill S, Ziemba A, Lewis S, Kuhlman B, Kleiger G
The Journal of biological chemistry 2015 Jan 9;290(2):1106-18
The Journal of biological chemistry 2015 Jan 9;290(2):1106-18
Ubiquitin-conjugating enzyme Cdc34 and ubiquitin ligase Skp1-cullin-F-box ligase (SCF) interact through multiple conformations.
Sandoval D, Hill S, Ziemba A, Lewis S, Kuhlman B, Kleiger G
The Journal of biological chemistry 2015 Jan 9;290(2):1106-18
The Journal of biological chemistry 2015 Jan 9;290(2):1106-18
Peptidic degron in EID1 is recognized by an SCF E3 ligase complex containing the orphan F-box protein FBXO21.
Zhang C, Li X, Adelmant G, Dobbins J, Geisen C, Oser MG, Wucherpfenning KW, Marto JA, Kaelin WG Jr
Proceedings of the National Academy of Sciences of the United States of America 2015 Dec 15;112(50):15372-7
Proceedings of the National Academy of Sciences of the United States of America 2015 Dec 15;112(50):15372-7
F-box protein FBXL16 binds PP2A-B55α and regulates differentiation of embryonic stem cells along the FLK1+ lineage.
Honarpour N, Rose CM, Brumbaugh J, Anderson J, Graham RL, Sweredoski MJ, Hess S, Coon JJ, Deshaies RJ
Molecular & cellular proteomics : MCP 2014 Mar;13(3):780-91
Molecular & cellular proteomics : MCP 2014 Mar;13(3):780-91
Early insights into the function of KIAA1199, a markedly overexpressed protein in human colorectal tumors.
Tiwari A, Schneider M, Fiorino A, Haider R, Okoniewski MJ, Roschitzki B, Uzozie A, Menigatti M, Jiricny J, Marra G
PloS one 2013;8(7):e69473
PloS one 2013;8(7):e69473
Small molecule-induced mitochondrial disruption directs prostate cancer inhibition via UPR signaling.
Rico-Bautista E, Zhu W, Kitada S, Ganapathy S, Lau E, Krajewski S, Ramirez J, Bush JA, Yuan Z, Wolf DA
Oncotarget 2013 Aug;4(8):1212-29
Oncotarget 2013 Aug;4(8):1212-29
Designer reagents for mass spectrometry-based proteomics: clickable cross-linkers for elucidation of protein structures and interactions.
Sohn CH, Agnew HD, Lee JE, Sweredoski MJ, Graham RL, Smith GT, Hess S, Czerwieniec G, Loo JA, Heath JR, Deshaies RJ, Beauchamp JL
Analytical chemistry 2012 Mar 20;84(6):2662-9
Analytical chemistry 2012 Mar 20;84(6):2662-9
Cullin-3 regulates late endosome maturation.
Huotari J, Meyer-Schaller N, Hubner M, Stauffer S, Katheder N, Horvath P, Mancini R, Helenius A, Peter M
Proceedings of the National Academy of Sciences of the United States of America 2012 Jan 17;109(3):823-8
Proceedings of the National Academy of Sciences of the United States of America 2012 Jan 17;109(3):823-8
Structure of a glomulin-RBX1-CUL1 complex: inhibition of a RING E3 ligase through masking of its E2-binding surface.
Duda DM, Olszewski JL, Tron AE, Hammel M, Lambert LJ, Waddell MB, Mittag T, DeCaprio JA, Schulman BA
Molecular cell 2012 Aug 10;47(3):371-82
Molecular cell 2012 Aug 10;47(3):371-82
UBXN7 docks on neddylated cullin complexes using its UIM motif and causes HIF1α accumulation.
Bandau S, Knebel A, Gage ZO, Wood NT, Alexandru G
BMC biology 2012 Apr 26;10:36
BMC biology 2012 Apr 26;10:36
The glomuvenous malformation protein Glomulin binds Rbx1 and regulates cullin RING ligase-mediated turnover of Fbw7.
Tron AE, Arai T, Duda DM, Kuwabara H, Olszewski JL, Fujiwara Y, Bahamon BN, Signoretti S, Schulman BA, DeCaprio JA
Molecular cell 2012 Apr 13;46(1):67-78
Molecular cell 2012 Apr 13;46(1):67-78
SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction.
Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, Xiao Y, Christie AL, Aster J, Settleman J, Gygi SP, Kung AL, Look T, Nakayama KI, DePinho RA, Wei W
Nature 2011 Mar 3;471(7336):104-9
Nature 2011 Mar 3;471(7336):104-9
Control of chromosome stability by the beta-TrCP-REST-Mad2 axis.
Guardavaccaro D, Frescas D, Dorrello NV, Peschiaroli A, Multani AS, Cardozo T, Lasorella A, Iavarone A, Chang S, Hernando E, Pagano M
Nature 2008 Mar 20;452(7185):365-9
Nature 2008 Mar 20;452(7185):365-9
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
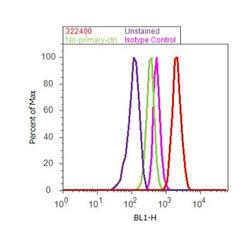
- Flow cytometry analysis of CUL-1 was done on MCF7 cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with CUL-1 Mouse Monoclonal Antibody (322400, red histogram) or with mouse isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Rabbit Anti-Mouse Secondary Antibody (A11059) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
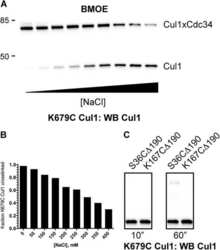
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
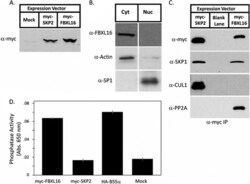
- Experimental details
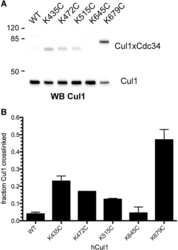
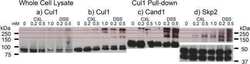
- Fig. 2. FBXL16 is a cytoplasmic protein that binds SKP1 and PP2A but does not bind CUL1. A , expression of myc-FBXL16. Total cell lysate was prepared from 3T3 cells that were either mock-transfected or stably transfected with plasmids that contained the coding sequences for either myc-SKP2 or myc-FBXL16. Lysates were evaluated via SDS-PAGE followed by immunoblot with anti-myc. B , myc-FBXL16 is a cytosolic protein. Cytosolic (Cyt) and nuclear (Nuc) fractions prepared from 3T3 cells stably expressing myc-FBXL16 were fractionated by means of SDS-PAGE and immunoblotted for myc-FBXL16, alpha-actin (a cytosolic marker), and SP1 (a nuclear marker). C , myc-FBXL16 binds SKP1 and PP2A but not CUL1. Total cell lysates from 293 cells that were either mock-transfected or transfected with plasmids that contained the coding sequences for either myc-SKP2 or myc-FBXL16 were subjected to immunoprecipitation with anti-myc. Immunoprecipitates were fractionated via SDS-PAGE and immunoblotted with antibodies against SKP1, CUL1, and PP2A subunit A. D , FBXL16, but not SKP2, co-immunoprecipitates serine/threonine phosphatase activity. Total cell lysates from 293 cells that were either mock-transfected or transfected with plasmids that contained the coding sequences for myc-SKP2, myc-FBXL16, or HA-B55alpha (included as a positive control) were subjected to immunoprecipitation with either anti-myc (SKP2 and FBXL16) or anti-HA (B55alpha). Immunoprecipitates were assayed for their content of serine/th
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
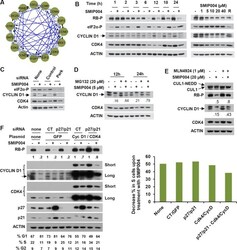
- Experimental details
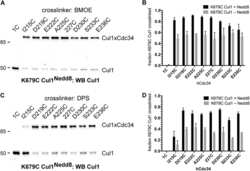
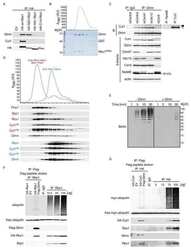
- Figure 3 Effect of SMIP004 on cell cycle pathways (A) RB subnetwork components downregulated by SMIP004 as revealed by Reactome network analysis (B) Kinetics and dose response of SMIP004 effects on RB pathway components as revealed by immunoblotting. LNCaP-S14 cells were treated with 40 muM SMIP004 for the indicated times (left panel) or were treated for 24 h with the indicated doses of the compound (right panel). (C) LNCaP-S14 cells were transfected with siRNA for PERK, followed by treatment with DMSO or SMIP004 (40 muM). The levels of cyclin D1, CDK4 and eIF2alpha-P were determined by immunoblotting. (D) Cells were pre-incubated with MG132 for 1h followed by treatment with SMIP004 for the indicated times. Cyclin D1 and CDK4 levels were analyzed by immunoblotting. (E) Cells were pre-treated with the neddylation inhibitor MLN4924 (1 muM) for 1h followed by treatment with SMIP004 (20 muM) for 6h. Levels of cyclin D1, CDK4, RB-P and cullin 1 (neddylated and unneddylated forms) were evaluated by immunoblotting. (F) LNCaP-S14 cells were transfected with p27 and p21 siRNAs for 24 h followed by transient transfection with cyclin D1 and CDK4 expression plasmids for another 24 h. Cells were then incubated for an additional 24 h in the presence or absence of SMIP004 (40 muM). Cells were collected for flow cytometry and immunoblotting analysis. Quantifications of phospho-RB levels are relative to the loading control actin. Short and long exposures are shown for cyclin D1 and CDK4 blots
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
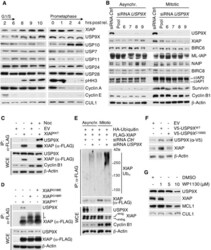
- Experimental details
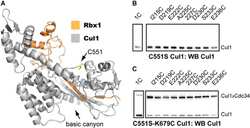
- Figure 1 USP9X binds, deubiquitylates, and stabilizes XIAP in mitosis Immunoblot analysis of HeLa cells using the indicated antibodies that were synchronized in G1/S phase using a double thymidine block or in mitosis using sequential thymidine and nocodazole treatment and collected at the given time points. Immunoblot analysis of HeLa cells that were transfected with siRNA oligonucleotides as indicated and left untreated or synchronized in mitosis using sequential thymidine and nocodazole treatment. Co-immunoprecipitation of XIAP and endogenous USP9X in HEK 293T cells that were transfected with FLAG-tagged XIAP or empty vector (EV) and synchronized in mitosis using nocodazole or left untreated. Co-immunoprecipitation of XIAP and endogenous USP9X in HEK 293T cells that were treated as in (C), but with additional transfection of the FLAG-tagged XIAP single amino acid mutants G188R and G188E. Only mitotic samples are shown. The asterisk denotes ubiquitylated forms of XIAP. In vivo ubiquitylation of XIAP in HeLa cells that were infected with the indicated expression constructs carrying FLAG-tagged XIAP and transfected with siRNA oligonucleotides as specified. Cells were synchronized in mitosis using sequential thymidine/nocodazole treatment, as indicated. Subsequent to treatment with MG132, whole-cell extracts (WCE) were prepared and ubiquitylated XIAP was isolated by anti-FLAG immunoprecipitation (IP) under denaturing conditions. Immunoblot analysis of NIH 3T3 cells that were tr
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
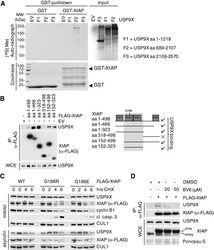
- Experimental details
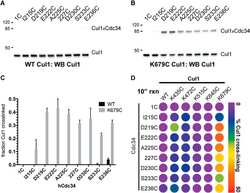
- Figure EV1 USP 9X interacts with XIAP in a direct manner and its active site binds to the BIR 2 domain of XIAP via glycine 188 In vitro co-immunoprecipitation of GST-purified XIAP with in vitro translated fragments of human USP9X with F2 containing the active site (aa 1556-1902). Co-immunoprecipitation of either full-length or different fragments of FLAG-tagged XIAP with endogenous USP9X from HEK 293T cells that were transfected with the indicated expression constructs and synchronized in mitosis using nocodazole. Immunoblot analyses of HeLa cells that were transfected with the indicated WT and mutant XIAP expression constructs and treated with cycloheximide (CHX) for the times specified. Co-immunoprecipitation of FLAG-tagged XIAP with endogenous USP9X from HEK 293T cells that were treated with BV6 as specified and nocodazole for 12 h. Source data are available online for this figure.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
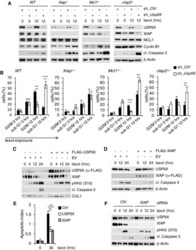
- Experimental details
- Figure 2 USP9X stabilizes XIAP to antagonize mitotic cell death independent of MCL1 Immunoblot analysis of WT, Xiap -/- , Mcl1 -/- , or cIap2 -/- mouse embryonic fibroblasts that were lentivirally infected with shRNA constructs directed against a non-relevant mRNA (Ctrl) or against Usp9X mRNA and treated with taxol as specified. Two-dimensional cell cycle analysis (BrdU/PI) of cells described in (A). Sub-G1 and G2/M fractions of cells were quantified and averaged with two additional, independent experiments ( n = 3, +- SD; WT: ** P = 0.0016; *** P = 0.0004; Mcl1 -/- : *** P = 0.0003; ** P = 0.0017; cIap2 -/- : ** P = 0.0043, Student's t -test). Black bars exemplify shRNA Ctrl and white bars shRNA Usp9X samples. Immunoblot analysis of HeLa cells transfected with a FLAG-tagged USP9X expression construct or empty vector (EV) and treated with taxol for the indicated times. Immunoblot analysis of HeLa cells transfected with a FLAG-tagged XIAP expression construct or empty vector and treated as in (C). Two-dimensional cell cycle analysis (BrdU/PI) of cells described in (C) and (D). Apoptotic indices represent ratios of sub-G1 to G1/S cells and are shown for analyses at the indicated time points ( n = 3, +- SD). ** P = 0.0020; *** P = 0.0004; Student's t -test. Immunoblot analysis of HeLa cells that were transfected with siRNA oligonucleotides directed against a non-relevant mRNA (Ctrl) or against Xiap mRNA and treated with taxol as specified. Source data are available onl
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
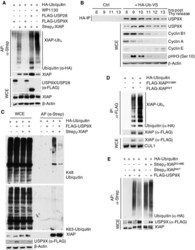
- Experimental details
- Figure EV2 USP 9X deubiquitylates XIAP - WT , but not XIAP -G188R or XIAP -G188E, in mitosis In vivo ubiquitylation of XIAP in HEK 293T cells that were co-transfected with the indicated expression constructs, synchronized in mitosis using nocodazole, and treated with MG132 prior to harvesting. The USP9X inhibitor WP1130 was added for 2 h as specified. XIAP was isolated by streptavidin affinity purification (AP) using denaturing conditions. HeLa cells were arrested in S phase with double thymidine block, released, and collected at the indicated time points. Deubiquitination activity was assessed by addition of HA-tagged dominant negative diubiquitin and following HA-IP under denaturing conditions. Immunoblot analysis of in vivo ubiquitylated XIAP (prepared as in A) using K48- or K63-specific ubiquitin antibodies. In vivo ubiquitylation of XIAP WT or XIAP G188R in HEK 293T cells that were co-transfected with the indicated expression constructs, synchronized in mitosis, and treated with MG132 as in (A). XIAP WT or XIAP G188R were isolated by anti-FLAG immunoprecipitation under denaturing conditions. In vivo ubiquitylation of XIAP WT or XIAP G188E in HEK 293T cells that were co-transfected with the indicated expression constructs and treated as in (A). XIAP WT or XIAP G188E were isolated by streptavidin affinity purification under denaturing conditions. Source data are available online for this figure.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
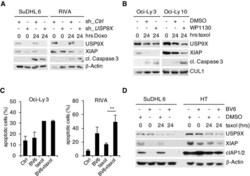
- Experimental details
- Figure EV3 Mitotic stabilization of XIAP by USP 9X mediates resistance to spindle poisons Immunoblot analyses of the indicated DLBCL cell lines that were lentivirally transduced with IRES-GFP shRNA constructs against USP9X or a non-relevant mRNA, FACS sorted for GFP + PI - cells and exposed to doxorubicin for the indicated periods of time. Immunoblot analyses of the indicated DLBCL cell lines that were exposed to taxol for the indicated periods of time. Two hours before collecting, WP1130 at a concentration of 5 muM or DMSO was added as specified. FACS analysis (propidium iodide (PI) uptake) of DLBCL cell lines treated with taxol and/or the SMAC mimetic BV6 as indicated. Results displayed are from three independent experiments each ( n = 3, +- SD). ** P = 0.00643; Student's t -test. Immunoblot analyses of the indicated DLBCL cell lines that were treated with taxol or the SMAC mimetic BV6 as indicated. Source data are available online for this figure.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
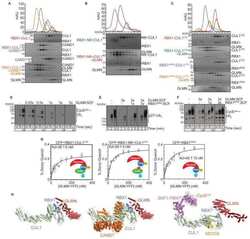
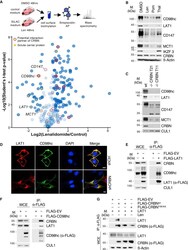
- Figure 1 Cell surface proteomics reveal a broad effect of IMiDs on transmembrane protein abundance and specify CD98hc/LAT1 as novel CRBN clients (A) Quantitative cell surface proteome of MM1s cells treated with lenalidomide versus solvent. MM1S cells were cultured in SILAC and control medium and subjected to treatment with 10 muM lenalidomide or DMSO for 48 h, followed by cell surface biotinylation, streptavidin affinity purification, and mass spectrometry (MS) analysis. Plasma membrane proteins (according to Gene Ontology cellular component [GOCC]) are depicted by blue circles. Solute carrier protein family members are marked by a yellow dot. Potential interactors of CRBN identified by tandem-affinity purification and MS are circled in red. (B) Representative immunoblot analysis of MM1S cells that were treated with DMSO, 10 muM lenalidomide (Len), 1 muM pomalidomide (Pom), or 100 muM thalidomide (Thal) for 72 h. (C) Representative immunoblot analysis of MM1S WT or MM1S with CRISPR-Cas9-mediated homozygous deletion of CRBN (clones T11 and T21). (D) Representative immunofluorescence images of HeLa cells that were transfected with constructs encoding both HA-CD98hc and FLAG-LAT1 and expressing the indicated short hairpin RNAs (shRNAs). Cells were stained with antibodies to HA (green) and FLAG (red), and DNA was stained with DAPI (blue). Scale bars, 10 mum. Quantification and confirmation of knockdown are presented in Figure S1 E. (E) Immunoprecipitation (IP) of FLAG-tagged LAT1
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
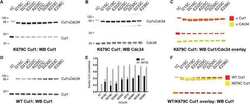
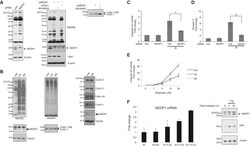
- Figure 3 Conserved Role for NEDP1 in the DNA Damage-Induced Apoptosis (A) Western blotting of extracts from U2OS cells transfected with siRNAs and treated with the NEDD8 E1 inhibitor MLN4924 (1 muM, 15 h) as indicated. Extracts were re-analyzed with cullin-1 antibodies (right panel). (B) Western blot analysis of extracts from parental U2OS or U2OS NEDP1 knockout clones (C6 and H6). (C and D) Similar experiment as in (A), except cells were treated with IR (7 Gy) and analyzed either for caspase 3/7 activity (C) or AnnexinV staining (D) 9 h later. (E) Caspase 3/7 activity in parental or NEDP1 knockout U2OS cells exposed to etoposide for 15 h. Average values (n = 3) +- SEM. (F) MCF7 cells were irradiated with 2 or 10 Gy for the indicated times. Quantitative real-time PCR for NEDP1 was carried out as described in the STAR Methods . The experiments were performed in triplicates; average values +- SEM (left panel). Western blot analysis in extracts of MCF7 cells treated as indicated (right panel).
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Flow cytometry
Flow cytometry