PA3-013
antibody from Invitrogen Antibodies
Targeting: HSP90AA1
FLJ31884, Hsp89, Hsp90, HSP90N, HSPC1, HSPCA
Antibody data
- Antibody Data
- Antigen structure
- References [53]
- Comments [0]
- Validations
- Immunocytochemistry [4]
- Immunoprecipitation [1]
- Immunohistochemistry [4]
- Other assay [32]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA3-013 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- HSP90 alpha Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA3-013 detects heat shock protein 90 (HSP90) from human, monkey, mouse, sheep (adrenal) and rat tissues and cells. This antibody does not detect HSP84. PA3-013 has been successfully used in Western blot, immunofluorescence, immunohistochemistry (paraffin) and immunoprecipitation procedures. By Western blot, this antibody detects an ~86 kDa protein representing HSP86 from HeLa cell lysate. Immunofluorescence staining of HSP86 in HeLa cells with PA3-013 yields a pattern consistent with cytoplasmic staining. Immunoprecipitation experiments with this antibody suggest that HSP86 exists primarily as homodimers in HeLa cells. Furthermore, the antibody is capable of precipitating HSP86 that is complexed with other proteins such as the aryl hydrocarbon (Ah) receptor. The PA3-013 immunogen is a synthetic peptide corresponding to residues P(2) E E T Q T Q D Q P M(12) of mouse HSP86. The N-terminal regions of HSP84 and HSP86 show the largest difference in amino acid sequence.
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Tissue differences in the exosomal/small extracellular vesicle proteome and their potential as indicators of altered tissue metabolism.
Brain region-specific susceptibility of Lewy body pathology in synucleinopathies is governed by α-synuclein conformations.
HSP90 promotes radioresistance of cervical cancer cells via reducing FBXO6-mediated CD147 polyubiquitination.
Loss of ARID1A Promotes Epithelial-Mesenchymal Transition and Sensitizes Pancreatic Tumors to Proteotoxic Stress.
RINT1 Loss Impairs Retinogenesis Through TRP53-Mediated Apoptosis.
Adipose tissue-liver crosstalk during pathologic changes caused by vinyl chloride metabolites in mice.
HSP90A inhibition promotes anti-tumor immunity by reversing multi-modal resistance and stem-like property of immune-refractory tumors.
Human coronavirus dependency on host heat shock protein 90 reveals an antiviral target.
Heat Shock Protein 90 as a Prognostic Marker and Therapeutic Target for Adrenocortical Carcinoma.
Augmentation of vaccine-induced humoral and cellular immunity by a physical radiofrequency adjuvant.
Molecular and functional crosstalk between extracellular Hsp90 and ephrin A1 signaling.
LSD1 engages a corepressor complex for the activation of the estrogen receptor α by estrogen and cAMP.
Acetylation of androgen receptor by ARD1 promotes dissociation from HSP90 complex and prostate tumorigenesis.
Novobiocin Analogues That Inhibit the MAPK Pathway.
A Remodeled Hsp90 Molecular Chaperone Ensemble with the Novel Cochaperone Aarsd1 Is Required for Muscle Differentiation.
A C-terminal HSP90 inhibitor restores glucocorticoid sensitivity and relieves a mouse allograft model of Cushing disease.
Integration-independent Transgenic Huntington Disease Fragment Mouse Models Reveal Distinct Phenotypes and Life Span in Vivo.
c-Myc regulates cell proliferation during lens development.
HSP90α plays an important role in piRNA biogenesis and retrotransposon repression in mouse.
Cruentaren A binds F1F0 ATP synthase to modulate the Hsp90 protein folding machinery.
Vacuolar H+ ATPase expression and activity is required for Rab27B-dependent invasive growth and metastasis of breast cancer.
Selective inhibition of HER2-positive breast cancer cells by the HIV protease inhibitor nelfinavir.
An interaction network predicted from public data as a discovery tool: application to the Hsp90 molecular chaperone machine.
Subcellular heterogeneity of ryanodine receptor properties in ventricular myocytes with low T-tubule density.
A systematic protocol for the characterization of Hsp90 modulators.
Transactivation of the epidermal growth factor receptor by heat shock protein 90 via Toll-like receptor 4 contributes to the migration of glioblastoma cells.
Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis.
The molecular chaperone Hsp90α is required for meiotic progression of spermatocytes beyond pachytene in the mouse.
(-)-Epigallocatechin-3-gallate is a novel Hsp90 inhibitor.
KU135, a novel novobiocin-derived C-terminal inhibitor of the 90-kDa heat shock protein, exerts potent antiproliferative effects in human leukemic cells.
Tubulin acetylation favors Hsp90 recruitment to microtubules and stimulates the signaling function of the Hsp90 clients Akt/PKB and p53.
Importance of the C-terminal domain of Harc for binding to Hsp70 and Hop as well as its response to heat shock.
Pregnancy-enhanced endothelial nitric oxide synthase (eNOS) activation in uterine artery endothelial cells shows altered sensitivity to Ca2+, U0126, and wortmannin but not LY294002--evidence that pregnancy adaptation of eNOS activation occurs at multiple levels of cell signaling.
Domain-mediated dimerization of the Hsp90 cochaperones Harc and Cdc37.
A central role for the Hsp90.Cdc37 molecular chaperone module in interleukin-1 receptor-associated-kinase-dependent signaling by toll-like receptors.
Functional characterization of HUVEC-CS: Ca2+ signaling, ERK 1/2 activation, mitogenesis and vasodilator production.
Novobiocin induces a distinct conformation of Hsp90 and alters Hsp90-cochaperone-client interactions.
Tyrosine phosphorylation of HSP-90 during mammalian sperm capacitation.
Characterization of the phosphorylation status of the hepatitis B virus X-associated protein 2.
Characterization of the phosphorylation status of the hepatitis B virus X-associated protein 2.
Purification of polyglutamine aggregates and identification of elongation factor-1alpha and heat shock protein 84 as aggregate-interacting proteins.
Purification of polyglutamine aggregates and identification of elongation factor-1alpha and heat shock protein 84 as aggregate-interacting proteins.
Zonal expression of endothelial nitric oxide synthase in sheep and rhesus adrenal cortex.
Identification and characterization of Harc, a novel Hsp90-associating relative of Cdc37.
p50(cdc37) is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules.
p50(cdc37) is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules.
Hsp and chaperone distribution during endochondral bone development in mouse embryo.
Hsp and chaperone distribution during endochondral bone development in mouse embryo.
Modular folding and evidence for phosphorylation-induced stabilization of an hsp90-dependent kinase.
Function and regulation of heat shock factor 2 during mouse embryogenesis.
Function and regulation of heat shock factor 2 during mouse embryogenesis.
Subunit composition of the heteromeric cytosolic aryl hydrocarbon receptor complex.
Localization and characterization of the 86- and 84-kDa heat shock proteins in Hepa 1c1c7 cells.
Garcia-Martin R, Brandao BB, Thomou T, Altindis E, Kahn CR
Cell reports 2022 Jan 18;38(3):110277
Cell reports 2022 Jan 18;38(3):110277
Brain region-specific susceptibility of Lewy body pathology in synucleinopathies is governed by α-synuclein conformations.
de Boni L, Watson AH, Zaccagnini L, Wallis A, Zhelcheska K, Kim N, Sanderson J, Jiang H, Martin E, Cantlon A, Rovere M, Liu L, Sylvester M, Lashley T, Dettmer U, Jaunmuktane Z, Bartels T
Acta neuropathologica 2022 Apr;143(4):453-469
Acta neuropathologica 2022 Apr;143(4):453-469
HSP90 promotes radioresistance of cervical cancer cells via reducing FBXO6-mediated CD147 polyubiquitination.
Song Q, Wen J, Li W, Xue J, Zhang Y, Liu H, Han J, Ning T, Lu Z
Cancer science 2022 Apr;113(4):1463-1474
Cancer science 2022 Apr;113(4):1463-1474
Loss of ARID1A Promotes Epithelial-Mesenchymal Transition and Sensitizes Pancreatic Tumors to Proteotoxic Stress.
Tomihara H, Carbone F, Perelli L, Huang JK, Soeung M, Rose JL, Robinson FS, Lissanu Deribe Y, Feng N, Takeda M, Inoue A, Poggetto ED, Deem AK, Maitra A, Msaouel P, Tannir NM, Draetta GF, Viale A, Heffernan TP, Bristow CA, Carugo A, Genovese G
Cancer research 2021 Jan 15;81(2):332-343
Cancer research 2021 Jan 15;81(2):332-343
RINT1 Loss Impairs Retinogenesis Through TRP53-Mediated Apoptosis.
Gomes AL, Matos-Rodrigues GE, Frappart PO, Martins RAP
Frontiers in cell and developmental biology 2020;8:711
Frontiers in cell and developmental biology 2020;8:711
Adipose tissue-liver crosstalk during pathologic changes caused by vinyl chloride metabolites in mice.
Kaelin BR, McKenzie CM, Hempel KW, Lang AL, Arteel GE, Beier JI
Toxicology and applied pharmacology 2020 Jul 15;399:115068
Toxicology and applied pharmacology 2020 Jul 15;399:115068
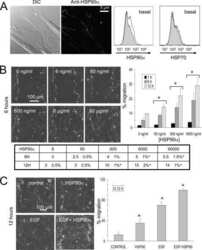
HSP90A inhibition promotes anti-tumor immunity by reversing multi-modal resistance and stem-like property of immune-refractory tumors.
Song KH, Oh SJ, Kim S, Cho H, Lee HJ, Song JS, Chung JY, Cho E, Lee J, Jeon S, Yee C, Lee KM, Hewitt SM, Kim JH, Woo SR, Kim TW
Nature communications 2020 Jan 28;11(1):562
Nature communications 2020 Jan 28;11(1):562
Human coronavirus dependency on host heat shock protein 90 reveals an antiviral target.
Li C, Chu H, Liu X, Chiu MC, Zhao X, Wang D, Wei Y, Hou Y, Shuai H, Cai J, Chan JF, Zhou J, Yuen KY
Emerging microbes & infections 2020 Dec;9(1):2663-2672
Emerging microbes & infections 2020 Dec;9(1):2663-2672
Heat Shock Protein 90 as a Prognostic Marker and Therapeutic Target for Adrenocortical Carcinoma.
Siebert C, Ciato D, Murakami M, Frei-Stuber L, Perez-Rivas LG, Monteserin-Garcia JL, Nölting S, Maurer J, Feuchtinger A, Walch AK, Haak HR, Bertherat J, Mannelli M, Fassnacht M, Korpershoek E, Reincke M, Stalla GK, Hantel C, Beuschlein F
Frontiers in endocrinology 2019;10:487
Frontiers in endocrinology 2019;10:487
Augmentation of vaccine-induced humoral and cellular immunity by a physical radiofrequency adjuvant.
Cao Y, Zhu X, Hossen MN, Kakar P, Zhao Y, Chen X
Nature communications 2018 Sep 12;9(1):3695
Nature communications 2018 Sep 12;9(1):3695
Molecular and functional crosstalk between extracellular Hsp90 and ephrin A1 signaling.
Daoud A, Gopal U, Kaur J, Isaacs JS
Oncotarget 2017 Dec 5;8(63):106807-106819
Oncotarget 2017 Dec 5;8(63):106807-106819
LSD1 engages a corepressor complex for the activation of the estrogen receptor α by estrogen and cAMP.
Bennesch MA, Segala G, Wider D, Picard D
Nucleic acids research 2016 Oct 14;44(18):8655-8670
Nucleic acids research 2016 Oct 14;44(18):8655-8670
Acetylation of androgen receptor by ARD1 promotes dissociation from HSP90 complex and prostate tumorigenesis.
DePaolo JS, Wang Z, Guo J, Zhang G, Qian C, Zhang H, Zabaleta J, Liu W
Oncotarget 2016 Nov 1;7(44):71417-71428
Oncotarget 2016 Nov 1;7(44):71417-71428
Novobiocin Analogues That Inhibit the MAPK Pathway.
Hall JA, Seedarala S, Zhao H, Garg G, Ghosh S, Blagg BS
Journal of medicinal chemistry 2016 Feb 11;59(3):925-33
Journal of medicinal chemistry 2016 Feb 11;59(3):925-33
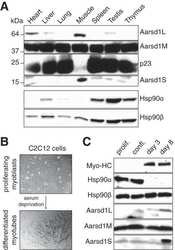
A Remodeled Hsp90 Molecular Chaperone Ensemble with the Novel Cochaperone Aarsd1 Is Required for Muscle Differentiation.
Echeverría PC, Briand PA, Picard D
Molecular and cellular biology 2016 Apr;36(8):1310-21
Molecular and cellular biology 2016 Apr;36(8):1310-21
A C-terminal HSP90 inhibitor restores glucocorticoid sensitivity and relieves a mouse allograft model of Cushing disease.
Riebold M, Kozany C, Freiburger L, Sattler M, Buchfelder M, Hausch F, Stalla GK, Paez-Pereda M
Nature medicine 2015 Mar;21(3):276-80
Nature medicine 2015 Mar;21(3):276-80
Integration-independent Transgenic Huntington Disease Fragment Mouse Models Reveal Distinct Phenotypes and Life Span in Vivo.
O'Brien R, DeGiacomo F, Holcomb J, Bonner A, Ring KL, Zhang N, Zafar K, Weiss A, Lager B, Schilling B, Gibson BW, Chen S, Kwak S, Ellerby LM
The Journal of biological chemistry 2015 Jul 31;290(31):19287-306
The Journal of biological chemistry 2015 Jul 31;290(31):19287-306
c-Myc regulates cell proliferation during lens development.
Cavalheiro GR, Matos-Rodrigues GE, Gomes AL, Rodrigues PM, Martins RA
PloS one 2014;9(2):e87182
PloS one 2014;9(2):e87182
HSP90α plays an important role in piRNA biogenesis and retrotransposon repression in mouse.
Ichiyanagi T, Ichiyanagi K, Ogawa A, Kuramochi-Miyagawa S, Nakano T, Chuma S, Sasaki H, Udono H
Nucleic acids research 2014 Oct 29;42(19):11903-11
Nucleic acids research 2014 Oct 29;42(19):11903-11
Cruentaren A binds F1F0 ATP synthase to modulate the Hsp90 protein folding machinery.
Hall JA, Kusuma BR, Brandt GE, Blagg BS
ACS chemical biology 2014 Apr 18;9(4):976-85
ACS chemical biology 2014 Apr 18;9(4):976-85
Vacuolar H+ ATPase expression and activity is required for Rab27B-dependent invasive growth and metastasis of breast cancer.
Hendrix A, Sormunen R, Westbroek W, Lambein K, Denys H, Sys G, Braems G, Van den Broecke R, Cocquyt V, Gespach C, Bracke M, De Wever O
International journal of cancer 2013 Aug 15;133(4):843-54
International journal of cancer 2013 Aug 15;133(4):843-54
Selective inhibition of HER2-positive breast cancer cells by the HIV protease inhibitor nelfinavir.
Shim JS, Rao R, Beebe K, Neckers L, Han I, Nahta R, Liu JO
Journal of the National Cancer Institute 2012 Oct 17;104(20):1576-90
Journal of the National Cancer Institute 2012 Oct 17;104(20):1576-90
An interaction network predicted from public data as a discovery tool: application to the Hsp90 molecular chaperone machine.
Echeverría PC, Bernthaler A, Dupuis P, Mayer B, Picard D
PloS one 2011;6(10):e26044
PloS one 2011;6(10):e26044
Subcellular heterogeneity of ryanodine receptor properties in ventricular myocytes with low T-tubule density.
Biesmans L, Macquaide N, Heinzel FR, Bito V, Smith GL, Sipido KR
PloS one 2011;6(10):e25100
PloS one 2011;6(10):e25100
A systematic protocol for the characterization of Hsp90 modulators.
Matts RL, Brandt GE, Lu Y, Dixit A, Mollapour M, Wang S, Donnelly AC, Neckers L, Verkhivker G, Blagg BS
Bioorganic & medicinal chemistry 2011 Jan 1;19(1):684-92
Bioorganic & medicinal chemistry 2011 Jan 1;19(1):684-92
Transactivation of the epidermal growth factor receptor by heat shock protein 90 via Toll-like receptor 4 contributes to the migration of glioblastoma cells.
Thuringer D, Hammann A, Benikhlef N, Fourmaux E, Bouchot A, Wettstein G, Solary E, Garrido C
The Journal of biological chemistry 2011 Feb 4;286(5):3418-28
The Journal of biological chemistry 2011 Feb 4;286(5):3418-28
Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis.
Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den Broecke R, Lambert J, Van Belle S, Cocquyt V, Gespach C, Bracke M, Seabra MC, Gahl WA, De Wever O, Westbroek W
Journal of the National Cancer Institute 2010 Jun 16;102(12):866-80
Journal of the National Cancer Institute 2010 Jun 16;102(12):866-80
The molecular chaperone Hsp90α is required for meiotic progression of spermatocytes beyond pachytene in the mouse.
Grad I, Cederroth CR, Walicki J, Grey C, Barluenga S, Winssinger N, De Massy B, Nef S, Picard D
PloS one 2010 Dec 31;5(12):e15770
PloS one 2010 Dec 31;5(12):e15770
(-)-Epigallocatechin-3-gallate is a novel Hsp90 inhibitor.
Yin Z, Henry EC, Gasiewicz TA
Biochemistry 2009 Jan 20;48(2):336-45
Biochemistry 2009 Jan 20;48(2):336-45
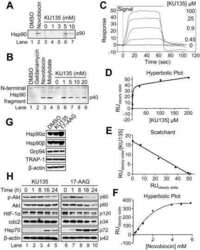
KU135, a novel novobiocin-derived C-terminal inhibitor of the 90-kDa heat shock protein, exerts potent antiproliferative effects in human leukemic cells.
Shelton SN, Shawgo ME, Matthews SB, Lu Y, Donnelly AC, Szabla K, Tanol M, Vielhauer GA, Rajewski RA, Matts RL, Blagg BS, Robertson JD
Molecular pharmacology 2009 Dec;76(6):1314-22
Molecular pharmacology 2009 Dec;76(6):1314-22
Tubulin acetylation favors Hsp90 recruitment to microtubules and stimulates the signaling function of the Hsp90 clients Akt/PKB and p53.
Giustiniani J, Daire V, Cantaloube I, Durand G, Poüs C, Perdiz D, Baillet A
Cellular signalling 2009 Apr;21(4):529-39
Cellular signalling 2009 Apr;21(4):529-39
Importance of the C-terminal domain of Harc for binding to Hsp70 and Hop as well as its response to heat shock.
Cartledge K, Elsegood C, Roiniotis J, Hamilton JA, Scholz GM
Biochemistry 2007 Dec 25;46(51):15144-52
Biochemistry 2007 Dec 25;46(51):15144-52
Pregnancy-enhanced endothelial nitric oxide synthase (eNOS) activation in uterine artery endothelial cells shows altered sensitivity to Ca2+, U0126, and wortmannin but not LY294002--evidence that pregnancy adaptation of eNOS activation occurs at multiple levels of cell signaling.
Sullivan JA, Grummer MA, Yi FX, Bird IM
Endocrinology 2006 May;147(5):2442-57
Endocrinology 2006 May;147(5):2442-57
Domain-mediated dimerization of the Hsp90 cochaperones Harc and Cdc37.
Roiniotis J, Masendycz P, Ho S, Scholz GM
Biochemistry 2005 May 3;44(17):6662-9
Biochemistry 2005 May 3;44(17):6662-9
A central role for the Hsp90.Cdc37 molecular chaperone module in interleukin-1 receptor-associated-kinase-dependent signaling by toll-like receptors.
De Nardo D, Masendycz P, Ho S, Cross M, Fleetwood AJ, Reynolds EC, Hamilton JA, Scholz GM
The Journal of biological chemistry 2005 Mar 18;280(11):9813-22
The Journal of biological chemistry 2005 Mar 18;280(11):9813-22
Functional characterization of HUVEC-CS: Ca2+ signaling, ERK 1/2 activation, mitogenesis and vasodilator production.
Gifford SM, Grummer MA, Pierre SA, Austin JL, Zheng J, Bird IM
The Journal of endocrinology 2004 Sep;182(3):485-99
The Journal of endocrinology 2004 Sep;182(3):485-99
Novobiocin induces a distinct conformation of Hsp90 and alters Hsp90-cochaperone-client interactions.
Yun BG, Huang W, Leach N, Hartson SD, Matts RL
Biochemistry 2004 Jun 29;43(25):8217-29
Biochemistry 2004 Jun 29;43(25):8217-29
Tyrosine phosphorylation of HSP-90 during mammalian sperm capacitation.
Ecroyd H, Jones RC, Aitken RJ
Biology of reproduction 2003 Dec;69(6):1801-7
Biology of reproduction 2003 Dec;69(6):1801-7
Characterization of the phosphorylation status of the hepatitis B virus X-associated protein 2.
Dull AB, Carlson DB, Petrulis JR, Perdew GH
Archives of biochemistry and biophysics 2002 Oct 15;406(2):209-21
Archives of biochemistry and biophysics 2002 Oct 15;406(2):209-21
Characterization of the phosphorylation status of the hepatitis B virus X-associated protein 2.
Dull AB, Carlson DB, Petrulis JR, Perdew GH
Archives of biochemistry and biophysics 2002 Oct 15;406(2):209-21
Archives of biochemistry and biophysics 2002 Oct 15;406(2):209-21
Purification of polyglutamine aggregates and identification of elongation factor-1alpha and heat shock protein 84 as aggregate-interacting proteins.
Mitsui K, Nakayama H, Akagi T, Nekooki M, Ohtawa K, Takio K, Hashikawa T, Nukina N
The Journal of neuroscience : the official journal of the Society for Neuroscience 2002 Nov 1;22(21):9267-77
The Journal of neuroscience : the official journal of the Society for Neuroscience 2002 Nov 1;22(21):9267-77
Purification of polyglutamine aggregates and identification of elongation factor-1alpha and heat shock protein 84 as aggregate-interacting proteins.
Mitsui K, Nakayama H, Akagi T, Nekooki M, Ohtawa K, Takio K, Hashikawa T, Nukina N
The Journal of neuroscience : the official journal of the Society for Neuroscience 2002 Nov 1;22(21):9267-77
The Journal of neuroscience : the official journal of the Society for Neuroscience 2002 Nov 1;22(21):9267-77
Zonal expression of endothelial nitric oxide synthase in sheep and rhesus adrenal cortex.
Peterson JK, Moran F, Conley AJ, Bird IM
Endocrinology 2001 Dec;142(12):5351-63
Endocrinology 2001 Dec;142(12):5351-63
Identification and characterization of Harc, a novel Hsp90-associating relative of Cdc37.
Scholz GM, Cartledge K, Hall NE
The Journal of biological chemistry 2001 Aug 17;276(33):30971-9
The Journal of biological chemistry 2001 Aug 17;276(33):30971-9
p50(cdc37) is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules.
Hartson SD, Irwin AD, Shao J, Scroggins BT, Volk L, Huang W, Matts RL
Biochemistry 2000 Jun 27;39(25):7631-44
Biochemistry 2000 Jun 27;39(25):7631-44
p50(cdc37) is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules.
Hartson SD, Irwin AD, Shao J, Scroggins BT, Volk L, Huang W, Matts RL
Biochemistry 2000 Jun 27;39(25):7631-44
Biochemistry 2000 Jun 27;39(25):7631-44
Hsp and chaperone distribution during endochondral bone development in mouse embryo.
Loones MT, Morange M
Cell stress & chaperones 1998 Dec;3(4):237-44
Cell stress & chaperones 1998 Dec;3(4):237-44
Hsp and chaperone distribution during endochondral bone development in mouse embryo.
Loones MT, Morange M
Cell stress & chaperones 1998 Dec;3(4):237-44
Cell stress & chaperones 1998 Dec;3(4):237-44
Modular folding and evidence for phosphorylation-induced stabilization of an hsp90-dependent kinase.
Hartson SD, Ottinger EA, Huang W, Barany G, Burn P, Matts RL
The Journal of biological chemistry 1998 Apr 3;273(14):8475-82
The Journal of biological chemistry 1998 Apr 3;273(14):8475-82
Function and regulation of heat shock factor 2 during mouse embryogenesis.
Rallu M, Loones M, Lallemand Y, Morimoto R, Morange M, Mezger V
Proceedings of the National Academy of Sciences of the United States of America 1997 Mar 18;94(6):2392-7
Proceedings of the National Academy of Sciences of the United States of America 1997 Mar 18;94(6):2392-7
Function and regulation of heat shock factor 2 during mouse embryogenesis.
Rallu M, Loones M, Lallemand Y, Morimoto R, Morange M, Mezger V
Proceedings of the National Academy of Sciences of the United States of America 1997 Mar 18;94(6):2392-7
Proceedings of the National Academy of Sciences of the United States of America 1997 Mar 18;94(6):2392-7
Subunit composition of the heteromeric cytosolic aryl hydrocarbon receptor complex.
Chen HS, Perdew GH
The Journal of biological chemistry 1994 Nov 4;269(44):27554-8
The Journal of biological chemistry 1994 Nov 4;269(44):27554-8
Localization and characterization of the 86- and 84-kDa heat shock proteins in Hepa 1c1c7 cells.
Perdew GH, Hord N, Hollenback CE, Welsh MJ
Experimental cell research 1993 Dec;209(2):350-6
Experimental cell research 1993 Dec;209(2):350-6
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
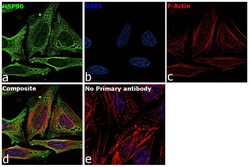
- Experimental details
- Immunofluorescence analysis of HSP90 alpha was performed using 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with HSP90 alpha Polyclonal Antibody (Product # PA3-013) at 5 µg/mL in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32731), (1:2000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasm and weak Nucleus localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
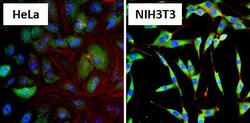
- Experimental details
- Immunofluorescent analysis of Heat Shock Protein 86 (HSP86, green) in HeLa cells and NIH3T3 cells. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature and blocked with 1% Blocker BSA (Product # 37525) for 15 minutes at room temperature. Cells were probed with a HSP86 polyclonal antibody (Product # PA3-013), at a dilution of 1:100 for at least 1 hour at room temperature, washed with PBS, and incubated with DyLight 488 goat-anti-rabbit IgG secondary antibody (Product # 35552) at a dilution of 1:400 for 30 minutes at room temperature. Nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ArrayScan at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
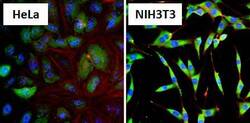
- Experimental details
- Immunofluorescent analysis of Heat Shock Protein 86 (HSP86, green) in HeLa cells and NIH3T3 cells. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature and blocked with 1% Blocker BSA (Product # 37525) for 15 minutes at room temperature. Cells were probed with a HSP86 polyclonal antibody (Product # PA3-013), at a dilution of 1:100 for at least 1 hour at room temperature, washed with PBS, and incubated with DyLight 488 goat-anti-rabbit IgG secondary antibody (Product # 35552) at a dilution of 1:400 for 30 minutes at room temperature. Nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ArrayScan at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
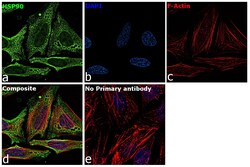
- Experimental details
- Immunofluorescence analysis of HSP90 alpha was performed using 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with HSP90 alpha Polyclonal Antibody (Product # PA3-013) at 5 µg/mL in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32731), (1:2000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasm and weak Nucleus localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
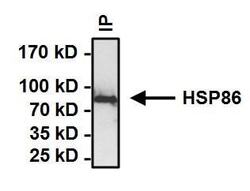
- Experimental details
- Immunoprecipitation of Heat Shock Protein (HSP86) was performed on HeLa cells. Antigen-antibody complexes formed by incubating 500 µg whole cell lysate with 2 µg of HSP86 polyclonal antibody (Product # PA3-013) overnight on a rocking platform at 4°C. The immune complexes were captured on 50 µL Protein A/G Plus Agarose (Product # 20421), washed extensively, and eluted with Lane Marker Reducing Sample Buffer (Product # 39000). Samples were then resolved on a 4-20% Tris-HCl polyacrylamide gel, transferred to a PVDF membrane and blocked with 5% BSA/TBST for at least 1 hour. The membrane was probed with a HSP86 polyclonal antibody (Product # PA3-013) at a dilution of 1:1000 overnight rotating at 4C. The membrane was washed in TBST, and probed with Clean-Blot IP detection Reagent (Product # 21230) at a dilution of 1:1000 for at least 1 hour. Chemiluminescent detection was performed using Pierce SuperSignal West Dura (Product # 34075).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
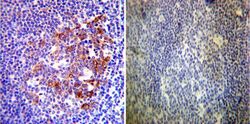
- Experimental details
- Immunohistochemistry was performed on normal deparaffinized Human tonsil tissue tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a rabbit polyclonal antibody recognizing Heat Shock Protein 90 (86) (Product # PA3-013) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
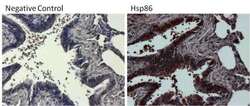
- Experimental details
- Immunohistochemistry was performed on differentiated colon adenocarcinoma from paraffin-embedded human colon tissue sections. To expose target proteins, heat-induced epitope retrieval was performed using 10mM sodium citrate (pH 6.0) buffer for 20 minutes at 95ºC. Following antigen retrieval, tissues were blocked in 3% BSA (Product # 37525) in PBST for 30 minutes at room temperature and then probed with a Heat Shock Protein 86 (Hsp86) polyclonal antibody (Product # PA3-013) at a dilution of 1:100 for 1 hour in a humidified chamber (right panel). As a negative control, the primary antibody was eliminated from the staining procedure (left panel). Tissues were washed extensively with PBS/0.025% Tween-20 (Product # 28314) and endogenous peroxidase activity quenched with Peroxidase Suppressor (Product # 35000) for 30 minutes at room temperature. Detection was performed using an HRP-conjugated goat anti-rabbit IgG-HRP secondary antibody (Product # 31466) at a dilution of 1:250 followed by colorimetric detection using Metal Enhanced DAB Substrate Kit (Product # 34065). Tissues were counterstained with hematoxylin and prepped for mouting. Images were taken on a Zeiss Axiovision microscope at 40X magnification (x1.6 Optovar).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
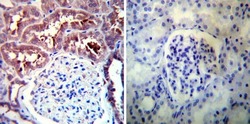
- Experimental details
- Immunohistochemistry was performed on normal deparaffinized Human kidney tissue tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a rabbit polyclonal antibody recognizing Heat Shock Protein 90 (86) (Product # PA3-013) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
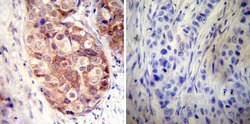
- Immunohistochemistry was performed on cancer biopsies of deparaffinized Human breast carcinoma tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:50 with a rabbit polyclonal antibody recognizing Heat Shock Protein 90 (86) (Product # PA3-013) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
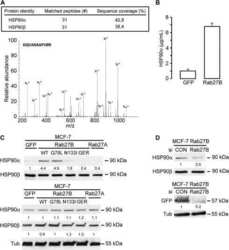
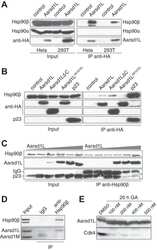
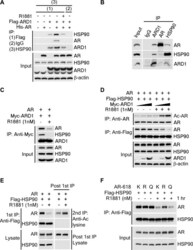
- Figure 5 ARD1-mediated AR acetylation drives ligand-induced AR-HSP90 dissociation ( A ) His-AR and Flag-ARD1 were co-transfected in 293T cells. In vitro interaction of AR, ARD1, and HSP90 were analyzed by sequential immunoprecipitation and immunoblotting with antibodies as indicated. ( B ) In vivo interaction of endogenous ARD1, AR, and HSP90 in LNCaP cells by immunoprecipitation and immunoblotting using antibodies as indicated. ( C ) Immunoblot of interaction between AR-WT and Myc-ARD1-WT transfected in Cos-7 cells in the presence or absence of R1881, immunoprecipitated with anti-Myc antibody. ( D ) AR and Flag-HSP90 were co-transfected with increased amounts of Myc-ARD1 in Cos-7 cells. Ligand-induced and ARD1 dose dependent AR acetylation were measured by immunoprecipitation with an anti-AR antibody and immunoblotted with an antibody specifically against acetylated lysine, while AR-HSP90 dissociation was measured by immunoprecipitation with an anti-Flag antibody and HSP90 co-immunoprecipitated AR was observed by Western blot. ( E ) AR and Flag-HSP90 co-transfected in Cos-7 cells in the presence or absence of R1881 were immunoprecipitated (first IP) with an anti-Flag antibody and immunoblotted using an antibody against AR (left two lanes). A secondary immunoprecipitation was performed on the supernatant after first IP using antibody specifically against acetylated lysine and immunoblotted with an anti-AR antibody (right two lanes). ( F ) Flag-HSP90 co-transfected with AR-WT,
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
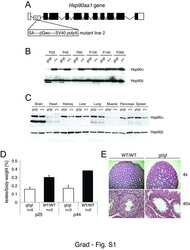
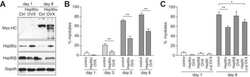
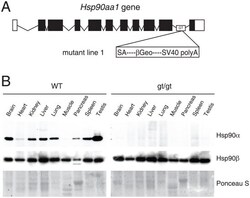
- Figure 1 Functional disruption of the Hsp90alpha gene in the mouse. (A) Schematic representation of the disruption of the mouse Hsp90aa1 gene by insertion of a gene trap (GT) in intron 10 (in mutant mouse line 1). Open and black boxes indicate non-coding and coding exons, respectively. The gene trap consists of a splice acceptor (SA), a beta-galactosidase-neomycin resistance fusion (betaGeo) and an SV40 polyadenylation site. (B) Immunoblots showing the expression of Hsp90alpha and Hsp90beta in a panel of tissues in wild-type (WT) and mutant (gt/gt) mice (of 136 days postnatal). The Ponceau S staining of the immunoblot filter gives an indication of protein loading.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
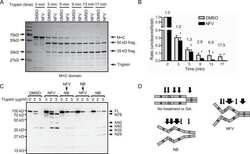
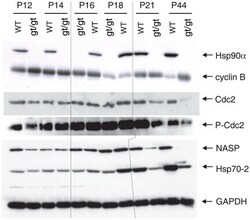
- Figure 6 Immunoblot analysis of a panel of Hsp90- and Hsp70-related proteins. The results shown are from a representative analysis of testis extracts from wild-type and mutant animals from P12 until completion of the first wave of spermatogenesis at P44. GAPDH serves as loading control. Note that the order of the sample pairs is reversed for the P16 and P18 samples at the center of the blots (area highlighted by hairlines).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
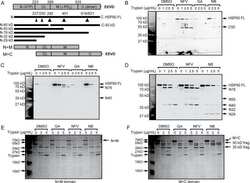
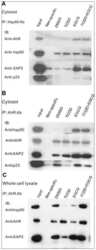
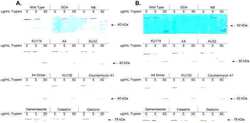
- Figure 6 Experimental validation of the involvement of Aha1 in nucleocytoplasmic transport. (A) PPI map of the proteins (red nodes) in the GO module ""nucleocytoplasmic transport"" of the Aha1-Hsp90 PPI subset of Figure 5 . It contains potential and known (dashed and full line edges, respectively) Aha1 interactions. The Hsp90 client protein GR was also integrated into the predicted PPI (upper panel). The co-immunoprecipitation assays of panel B allowed the experimental confirmation and new demonstration of PPIs as indicated by red and green edges, respectively (lower panel). (B) Co-immunoprecipitation experiment demonstrating interactions between Aha1 or exportin-1 (XPO1) and components of the GO module ""nucleocytoplasmic transport"" shown in panel A. Flag-tagged Aha1, exportin-1, and GPR30 as an unrelated control protein were exogenously expressed in 293T cells. IPO4, importin-4; KPNA5, importin-alpha6. (C) and (D) Nuclear localization of GR in mouse fibroblasts with and without Aha1. Panel C shows representative micrographs of the localization of Tom.GR with our without treatment with dexamethasone (Dex) for 40 min, and an immunoblot on the right verifying the absence of Aha1 in the Aha1- fibroblasts. Panel D shows the nuclear accumulation of Tom.GR over time in wild-type (^) and Aha1- (V) cells. Nuclear localization of GR was initiated at time zero with the addition of 10 nM dexamethasone. Data points are the means with standard errors of three independent experim
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
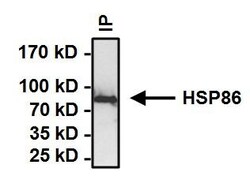
- Immunoprecipitation of Heat Shock Protein (HSP86) was performed on HeLa cells. Antigen-antibody complexes formed by incubating 500 µg whole cell lysate with 2 µg of HSP86 polyclonal antibody (Product # PA3-013) overnight on a rocking platform at 4°C. The immune complexes were captured on 50 µL Protein A/G Plus Agarose (Product # 20421), washed extensively, and eluted with Lane Marker Reducing Sample Buffer (Product # 39000). Samples were then resolved on a 4-20% Tris-HCl polyacrylamide gel, transferred to a PVDF membrane and blocked with 5% BSA/TBST for at least 1 hour. The membrane was probed with a HSP86 polyclonal antibody (Product # PA3-013) at a dilution of 1:1000 overnight rotating at 4C. The membrane was washed in TBST, and probed with Clean-Blot IP detection Reagent (Product # 21230) at a dilution of 1:1000 for at least 1 hour. Chemiluminescent detection was performed using Pierce SuperSignal West Dura (Product # 34075).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
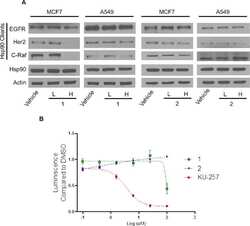
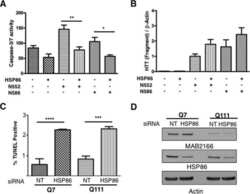
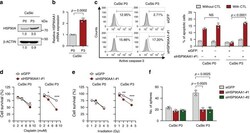
- Fig. 1 HSP90A is required for multi-aggressive properties of immune-edited tumor cells. a HSP90A protein level in CaSki P0 and P3 cells was determined by Western blot. beta-ACTIN was included as an internal loading control. Numbers below blot images indicate the expression as measured by fold change. b HSP90AA1 mRNA level in CaSki P0 and P3 cells was determined by qRT-PCR. c-e CaSki P0 and P3 cells were transfected with siGFP or siHSP90AA1-#1. c The frequency of apoptotic (active caspase-3 + ) cells in the MART-1 peptide pulsed cells after incubation with or without MART-1-specific CTLs at a 1:1 ratio for 4 h was estimated by flow cytometry analysis. d and e Cells were treated with indicated concentrations of cisplatin d and irradiation e . The percentage of viable cells was determined by trypan blue exclusion assay at 24 h after either cisplatin treatment or irradiation. * p < 0.01, ** p < 0.001, and *** p < 0.0001. f CaSki P0 and P3 cells were transfected with siGFP, siHSP90AA1-#1, or siHSP90AA1-#2. Sphere-forming capacity of the cells in low-density suspension culture. All experiments were performed in triplicate. The p -values by two-tailed Student's t test b or two-way ANOVA c-f are indicated. NS, not significant. Data represent the mean +- SD. Source data are provided as a Source Data file.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
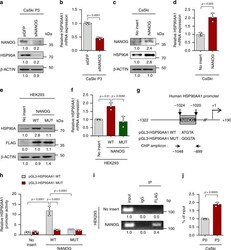
- Fig. 2 HSP90AA1 expression is directly regulated by NANOG. a and b CaSki P3 cells were transfected with siRNA-targeting GFP or NANOG. a Levels of NANOG and HSP90A protein were analyzed by Western blot. b HSP90AA1 mRNA expression was analyzed by qRT-PCR. c and d CaSki P0 cells were stably transfected with empty vector (no insert) or NANOG. c Levels of NANOG and HSP90A protein were analyzed by Western blot. d HSP90AA1 mRNA expression was analyzed by qRT-PCR. e and f HEK293 cells were transfected with empty vector (no insert), FLAG-NANOG wild type (NANOG WT) or FLAG-NANOG mutant (NANOG MUT). e Levels of HSP90A and FLAG-NANOG proteins were proved by Western blot. f HSP90AA1 mRNA expression was analyzed by qRT-PCR. g Diagram of HSP90AA1 promoter region (-1322 to +190) containing NANOG binding element. The arrows indicate ChIP amplicon corresponding to -1048 to -899. h Luciferase assay in HEK293 cells transfected with the pGL3-HSP90AA1 WT or MUT plasmid, together no insert, NANOG WT or NANOG MUT plasmids. i Chromatin immunoprecipitation assay was carried out using HEK293 cells transfected with FLAG-NANOG. Cross-linked chromatin was immunoprecipitated with anti-FLAG antibodies. Immunoprecipitated DNAs were amplified with PCR primers specific for the HSP90AA1 promoter region indicated above. Mouse IgG was used as a negative control. The input represents 2% of the total chromatin. j ChIP assay was carried out using CaSki P0 and P3 cells. Cross-linked chromatin was immunoprecipitated w
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
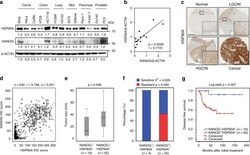
- Fig. 3 NANOG-HSP90A axis is conserved across various human cancer types. a Protein levels of NANOG and HSP90A in various human cancer cells were determined by immunoblotting. This experiment was performed in triplicate. b Correlation between NANOG and HSP90A level normalized by beta-ACTIN level in various human cancer cells (Spearnan r = 0.7741, p = 0.0028). c Representative images of IHC staining of HSP90A in cervical tissue from normal ( n = 328), LGCIN ( n = 65), HGCIN ( n = 160), and cervical carcinoma ( n = 151) patients. Scale bar shown is 250 um. LGCIN low-grade CIN; HGCIN high-grade CIN. d Correlation between NANOG and HSP90A in patients with cervical cancer (Spearnan r = 0.746, p < 0.001). e and f Combined level of NANOG + /HSP90A + was significantly associated with large-sized tumors e and chemoradiation resistance f in patients with cervical cancer. Chemoradiation resistance was calculated only cases with available information of chemoradiation response. g Patients with NANOG + /HSP90A + level displayed worse disease-free survival ( p = 0.007) than patients with NANOG - /HSP90A - level. Cut-off value of NANOG + and HSP90A + are 160 and 127, respectively. The p -values were determined by Mann-Whitney U test e and f and Log-rank (Mantel-Cox) test g or spearman correlation ( r ) b and d . In the box plots, the top and bottom edges of boxes indicate the first and third quartiles, respectively; the center lines indicate the medians; and the ends of whiskers indicate the
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
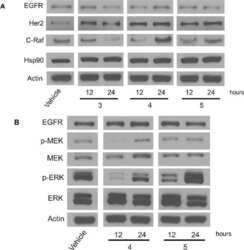
- Experimental details
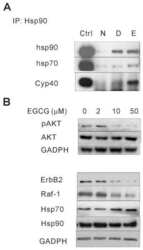
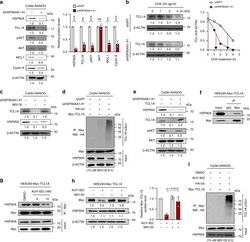
- Fig. 4 HSP90A contributes to NANOG-induced AKT activation through TCL1A-stabilization. a-e CaSki-NANOG cells were transfected with siGFP or siHSP90AA1-#1. a Levels of HSP90A, TCL1A, pAKT, AKT, MCL1, and Cyclin A were analyzed by immunoblotting. Graph depicts the experimental quantitation based on at least three independent experiments. ** p < 0.001, *** p < 0.0001 by two-tailed Student's t test. NS not significant. b The cells were treated with cycloheximide (CHX) for the indicated times. Cell lysates were subjected to immunoblotting with anti-TCL1A antibodies. Graph represents the means +- SD of three quantified data, after normalization to the corresponding beta-ACTIN level. c The cells were treated with or without MG132 (10 muM) for 8 h. Cell lysates were subjected to immunoblotting with anti-HSP90A and anti-TCL1A antibodies. d The cells, transfecte d with indicated plasmids, were treated with MG132. Cell lysates were immunoprecipitated with anti-Myc antibody, and immunoblotted with the anti-HA antibody. The input represents 5% of total proteins. e siHSP90AA1-transfect e d CaSki-NANOG cells were transfected with TCL1A-Myc constructs. Levels of HSP90A, TCL1A, pAKT, and AKT were analyzed by immunoblotting. f HEK293 cells were transfected with TCL1A-Myc. g TCL1A-Myc-transfected HEK293 cells were treated with DMSO or AUY-922 as indicated. f and g Cell lysates were immunoprecipitated with anti-Myc antibody, followed by western blotting using anti-Myc and anti-HSP90A antibodies.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
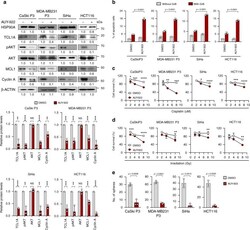
- Fig. 5 HSP90A inhibition reduces multi-aggressive properties of NANOG high tumor cells. a-e CaSki P3, MDA-MB231 P3, SiHa, and HCT116 cells were treated with DMSO or AUY-922. a Levels of HSP90A, TCL1A, pAKT, AKT, MCL1, Cyclin A, and beta-ACTIN were proved by Western blot. beta-ACTIN was included as an internal loading control. Numbers below blot images indicate the expression as measured by fold change. Graph depicts the experimental quantitation based on at least three independent experiments. b Flow cytometry analysis of the frequency of apoptotic (active caspase-3 + ) cells in the cells after intracellular delivery of granzyme B for 4 h. c and d Cells were treated with indicated concentrations of cisplatin c and irradiation d . The percentage of viable cells was determined by trypan blue exclusion assay at 24 h after either drug challenge or irradiation. e Sphere-forming capacity of the cells in low-density suspension culture. All experiments were performed in triplicate. The p -value by two-tailed Student's t test a , one-way ANOVA b and e or two-way ANOVA c and d are indicated. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Data represent the mean +- SD. Source data are provided as a Source Data file.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
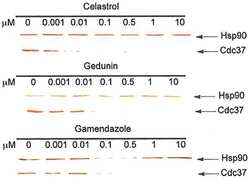
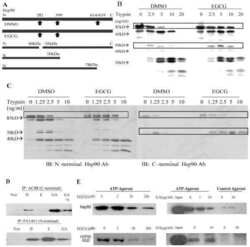
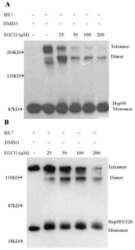
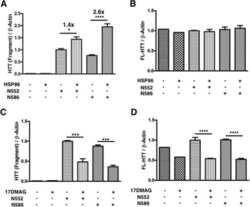
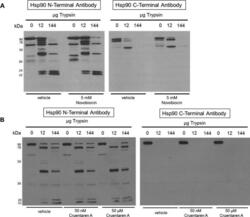
- Figure 3 Proteolysis of Hsp90from TnT reticulocyte lysate incubated underconditions of protein synthesis with (A) vehicle (1% DMSO) and 5 mMnovobiocin and (B) vehicle (1% DMSO) or 50 nM or 50 muM cruentarenA. Antibodies specific to either the N- or C-terminus of Hsp90 wereused to identify the Hsp90 fragments produced in the presence of increasingamount of trypsin.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry