14-0629-80
antibody from Invitrogen Antibodies
Targeting: SELL
CD62L, hLHRc, LAM-1, LAM1, Leu-8, LNHR, LSEL, Lyam-1, LYAM1, PLNHR
Antibody data
- Antibody Data
- Antigen structure
- References [15]
- Comments [0]
- Validations
- Flow cytometry [2]
- Other assay [6]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-0629-80 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD62L (L-Selectin) Monoclonal Antibody (DREG-56 (DREG56)), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The DREG-56 monoclonal antibody reacts with human CD62L, a 76 kDa member of the selectin family. CD62L is expressed by neutrophils, monocytes, and subsets of T, B, and NK cells and binds a number of glycosylated, fucosylated, sulfated sialylated glycoproteins including CD34, glycam-1 and MAdCAM-1. These interactions mediate rolling of lymphocytes on activated endothelium at the sites of inflammation and homing of cells to the high endothelial venules (HEV) of peripheral lymphoid tissues. Applications Reported: This DREG-56 (DREG56) antibody has been reported for use in flow cytometric analysis, non-reducing conditions-immunoblotting (WB), and immunohistology staining of frozen tissue sections. DREG-56 has also been reported in inhibition of binding to HEV. (Please use Functional Grade purified DREG-56 (DREG56), Product # 16-0629, in functional assays). Applications Tested: The DREG-56 (DREG56) antibody has been tested by flow cytometric analysis of normal human peripheral blood cells. This can be used at less than or equal to 1 µg per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- DREG-56 (DREG56)
- Vial size
- 25 μg
- Concentration
- 0.5 mg/mL
- Storage
- 4°C
Submitted references Induction of memory-like CD8+ T cells and CD4+ T cells from human naive T cells in culture.
Internalization of HMGB1 (High Mobility Group Box 1) Promotes Angiogenesis in Endothelial Cells.
Inhibition of exosomal miR-24-3p in diabetes restores angiogenesis and facilitates wound repair via targeting PIK3R3.
EZH2 and KDM6B Expressions Are Associated with Specific Epigenetic Signatures during EMT in Non Small Cell Lung Carcinomas.
C reactive protein impairs adaptive immunity in immune cells of patients with melanoma.
Heterogeneity of human bone marrow and blood natural killer cells defined by single-cell transcriptome.
MicroRNA-140-5p inhibits cell proliferation, migration and promotes cell apoptosis in gastric cancer through the negative regulation of THY1-mediated Notch signaling.
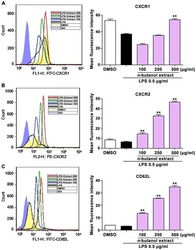
n-butanol extract from Folium isatidis inhibits the lipopolysaccharide-induced downregulation of CXCR1 and CXCR2 on human neutrophils.
Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans.
L-selectin controls trafficking of chronic lymphocytic leukemia cells in lymph node high endothelial venules in vivo.
Cell surface topography is a regulator of molecular interactions during chemokine-induced neutrophil spreading.
Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans.
Systemic hypoxia enhances bactericidal activities of human polymorphonuclear leuocytes.
L-selectin serves as an E-selectin ligand on cultured human T lymphoblasts.
Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule.
Tokumoto Y, Araki Y, Narizuka Y, Mizuno Y, Ohshima S, Mimura T
Clinical and experimental immunology 2022 Jan 28;207(1):95-103
Clinical and experimental immunology 2022 Jan 28;207(1):95-103
Internalization of HMGB1 (High Mobility Group Box 1) Promotes Angiogenesis in Endothelial Cells.
Lan J, Luo H, Wu R, Wang J, Zhou B, Zhang Y, Jiang Y, Xu J
Arteriosclerosis, thrombosis, and vascular biology 2020 Dec;40(12):2922-2940
Arteriosclerosis, thrombosis, and vascular biology 2020 Dec;40(12):2922-2940
Inhibition of exosomal miR-24-3p in diabetes restores angiogenesis and facilitates wound repair via targeting PIK3R3.
Xu Y, Ouyang L, He L, Qu Y, Han Y, Duan D
Journal of cellular and molecular medicine 2020 Dec;24(23):13789-13803
Journal of cellular and molecular medicine 2020 Dec;24(23):13789-13803
EZH2 and KDM6B Expressions Are Associated with Specific Epigenetic Signatures during EMT in Non Small Cell Lung Carcinomas.
Lachat C, Bruyère D, Etcheverry A, Aubry M, Mosser J, Warda W, Herfs M, Hendrick E, Ferrand C, Borg C, Delage-Mourroux R, Feugeas JP, Guittaut M, Hervouet E, Peixoto P
Cancers 2020 Dec 5;12(12)
Cancers 2020 Dec 5;12(12)
C reactive protein impairs adaptive immunity in immune cells of patients with melanoma.
Yoshida T, Ichikawa J, Giuroiu I, Laino AS, Hao Y, Krogsgaard M, Vassallo M, Woods DM, Stephen Hodi F, Weber J
Journal for immunotherapy of cancer 2020 Apr;8(1)
Journal for immunotherapy of cancer 2020 Apr;8(1)
Heterogeneity of human bone marrow and blood natural killer cells defined by single-cell transcriptome.
Yang C, Siebert JR, Burns R, Gerbec ZJ, Bonacci B, Rymaszewski A, Rau M, Riese MJ, Rao S, Carlson KS, Routes JM, Verbsky JW, Thakar MS, Malarkannan S
Nature communications 2019 Sep 2;10(1):3931
Nature communications 2019 Sep 2;10(1):3931
MicroRNA-140-5p inhibits cell proliferation, migration and promotes cell apoptosis in gastric cancer through the negative regulation of THY1-mediated Notch signaling.
Wu K, Zou J, Lin C, Jie ZG
Bioscience reports 2019 Jul 31;39(7)
Bioscience reports 2019 Jul 31;39(7)
n-butanol extract from Folium isatidis inhibits the lipopolysaccharide-induced downregulation of CXCR1 and CXCR2 on human neutrophils.
Wu B, Wang L, Jiang L, Dong L, Xu F, Lu Y, Jin J, Wang Z, Liang G, Shan X
Molecular medicine reports 2018 Jan;17(1):179-185
Molecular medicine reports 2018 Jan;17(1):179-185
Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans.
Rathod KS, Kapil V, Velmurugan S, Khambata RS, Siddique U, Khan S, Van Eijl S, Gee LC, Bansal J, Pitrola K, Shaw C, D'Acquisto F, Colas RA, Marelli-Berg F, Dalli J, Ahluwalia A
The Journal of clinical investigation 2017 Jan 3;127(1):169-182
The Journal of clinical investigation 2017 Jan 3;127(1):169-182
L-selectin controls trafficking of chronic lymphocytic leukemia cells in lymph node high endothelial venules in vivo.
Lafouresse F, Bellard E, Laurent C, Moussion C, Fournié JJ, Ysebaert L, Girard JP
Blood 2015 Sep 10;126(11):1336-45
Blood 2015 Sep 10;126(11):1336-45
Cell surface topography is a regulator of molecular interactions during chemokine-induced neutrophil spreading.
Lomakina EB, Marsh G, Waugh RE
Biophysical journal 2014 Sep 16;107(6):1302-12
Biophysical journal 2014 Sep 16;107(6):1302-12
Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans.
Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK
Journal of immunology (Baltimore, Md. : 1950) 2013 Mar 1;190(5):2001-8
Journal of immunology (Baltimore, Md. : 1950) 2013 Mar 1;190(5):2001-8
Systemic hypoxia enhances bactericidal activities of human polymorphonuclear leuocytes.
Wang JS, Liu HC
Clinical science (London, England : 1979) 2009 May 1;116(11):805-17
Clinical science (London, England : 1979) 2009 May 1;116(11):805-17
L-selectin serves as an E-selectin ligand on cultured human T lymphoblasts.
Jutila MA, Kurk S, Jackiw L, Knibbs RN, Stoolman LM
Journal of immunology (Baltimore, Md. : 1950) 2002 Aug 15;169(4):1768-73
Journal of immunology (Baltimore, Md. : 1950) 2002 Aug 15;169(4):1768-73
Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule.
Kishimoto TK, Jutila MA, Butcher EC
Proceedings of the National Academy of Sciences of the United States of America 1990 Mar;87(6):2244-8
Proceedings of the National Academy of Sciences of the United States of America 1990 Mar;87(6):2244-8
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
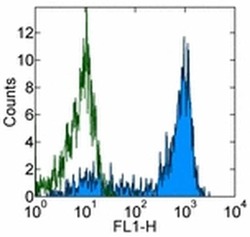
- Experimental details
- Staining of normal human peripheral blood cells with 0.5 µg of Mouse IgG1 kappa Isotype Control Purified (Product # 14-4714-82) (open histogram) or 0.5 µg of Anti-Human CD62P (P-Selectin) Purified (filled histogram) followed by Anti-Mouse IgG FITC (Product # 11-4011-85). Cells in the lymphocyte gate were used for analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
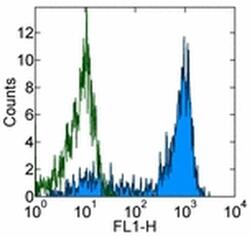
- Experimental details
- Staining of normal human peripheral blood cells with 0.5 µg of Mouse IgG1 kappa Isotype Control Purified (Product # 14-4714-82) (open histogram) or 0.5 µg of Anti-Human CD62P (P-Selectin) Purified (filled histogram) followed by Anti-Mouse IgG FITC (Product # 11-4011-85). Cells in the lymphocyte gate were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
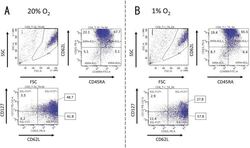
- Fig. 1. Activated CD8 + T cells in either normal or hypoxic culture. Naive CD8 + T cells derived from a healthy donor were cultured in human T-activator CD3/CD28 and IL-2 containing medium for 8 days. ( A ) in 20% O 2 condition and ( B ) in 1% O 2 condition. The 7-AAD-negative cells in the area gated as P1 in the FSC/SSC panel were considered as living cells. The expression pattern of CD45RA, CD62L, and CD127 of living cells were analyzed by FACS. The numbers on the FACS-plot panel mean the frequencies (%) of population of cells. We repeated this experiment four times.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
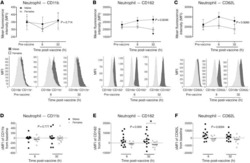
- Figure 5 Reduced inflammatory cell activation state in cantharidin-induced blister exudates in female compared with male healthy volunteers. Mean fluorescence intensity (MFI) of the expression molecules CD162, CD62L, and CD11b on ( A ) neutrophils, ( B ) inflammatory monocytes, and ( C ) CD4 + and CD8 + T cells in healthy male ( n = 16) and female ( n = 16) volunteers. Data are shown as mean +- SEM. Statistical significances determined using 2-way ANOVA, * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001; followed by Sidak's post tests, # P < 0.05, ## P < 0.01, and #### P < 0.0001 comparing the sexes at each time point for all the panels.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
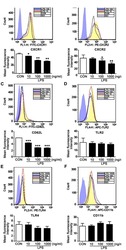
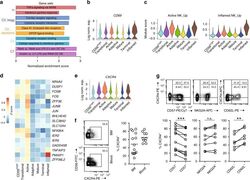
- Fig. 4 Active NK cells with a unique transcriptome profile. a Top two enriched gene sets (ranked by normalized enrichment score) of five different datasets from GSEA of the ""Inflamed NK"" cluster compared to the rest of the cells were plotted. b The expression of CD69 in the BM sample was shown as a violin plot. The y -axis represents log-normalized expression value. c Module score was calculated using up-regulated DEGs of ""Active NK"" (left) or ""Inflamed NK"" (right) cluster from BM sample and plotted via violin plots. d Up-regulated IEGs from ""Active NK"" cluster were plotted using heatmap of the BM sample. e The expression of CXCR4 in the BM sample was shown as a violin plot. The y -axis represents log-normalized expression value. f Percentage of CXCR4 + NK cells (gated on Lin - CD56 + cells) was evaluated via flow cytometry. g The expression of CXCR4 in CD57 +/- , CD62L +/- , or NKG2A +/- CD56 dim NK populations from BM was assessed via flow cytometry (top). Percentage of CXCR4 + cells within each population were quantified (bottom). n >= 6 from two to five independent experiments. Paired Student's t test was used for the statistical analysis. * P < 0.05; ** p < 0.01; *** p < 0.001; n.s. stands for ""not significant."" Source data for f and g are provided as a Source Data file. See also Supplementary Fig. 5
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Flow cytometry
Flow cytometry