Antibody data
- Antibody Data
- Antigen structure
- References [16]
- Comments [0]
- Validations
- Western blot [1]
- Immunocytochemistry [2]
- Immunohistochemistry [4]
- Other assay [10]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA3-032A - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- nNOS Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA3-032A detects brain nitric oxide synthase (bNOS) from bovine, mouse, rabbit and rat tissues. This antibody does not detect endothelial NOS (eNOS) or inducible NOS (iNOS). PA3-032A has been successfully used in Western blot, immunoprecipitation and immunohistochemical procedures. By Western blot, this antibody detects an ~155 kDa protein representing bNOS from rat brain extract. Immunohistochemical staining of bNOS in rat brain with PA3-032A results in staining of the anterior pituitary. The PA3-032A immunogen is a synthetic peptide corresponding to residues T(724) K R R A I G F K K L A E A V K(739) C of rat nNOS.
- Reactivity
- Human, Mouse, Rat, Bovine, Rabbit
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- Conc. Not Determined
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references The neuroprotective effects of AMN082 on neuronal apoptosis in rats after traumatic brain injury.
An autophagic deficit in the uterine vessel microenvironment provokes hyperpermeability through deregulated VEGFA, NOS1, and CTNNB1.
Tau induces PSD95-neuronal NOS uncoupling and neurovascular dysfunction independent of neurodegeneration.
Therapeutic effects of syringaldehyde on spinal cord ischemia in rabbits.
Bubble CPAP Support after Discontinuation of Mechanical Ventilation Protects Rat Lungs with Ventilator-Induced Lung Injury.
Nitric oxide synthase in diabetic rat testicular tissue and the effects of pentoxifylline therapy.
Ameliorative effects of pentoxifylline on NOS induced by diabetes in rat kidney.
Therapeutic effects of pentoxifylline on diabetic heart tissue via NOS.
A role for nitric oxide in the control of breathing in zebrafish (Danio rerio).
Smooth muscle NOS, colocalized with caveolin-1, modulates contraction in mouse small intestine.
Regulation of vascular tone in animals overexpressing the sarcolemmal calcium pump.
The plasmamembrane calmodulin-dependent calcium pump: a major regulator of nitric oxide synthase I.
Granulated metrial gland cells contain nitric oxide synthases during pregnancy in the rat.
Physiological dilation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast.
Physiological dilation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast.
Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase.
Lu CC, Nyam TE, Kuo JR, Lee YL, Chio CC, Wang CC
BMC neuroscience 2021 Jun 25;22(1):44
BMC neuroscience 2021 Jun 25;22(1):44
An autophagic deficit in the uterine vessel microenvironment provokes hyperpermeability through deregulated VEGFA, NOS1, and CTNNB1.
Lee B, Shin H, Oh JE, Park J, Park M, Yang SC, Jun JH, Hong SH, Song H, Lim HJ
Autophagy 2021 Jul;17(7):1649-1666
Autophagy 2021 Jul;17(7):1649-1666
Tau induces PSD95-neuronal NOS uncoupling and neurovascular dysfunction independent of neurodegeneration.
Park L, Hochrainer K, Hattori Y, Ahn SJ, Anfray A, Wang G, Uekawa K, Seo J, Palfini V, Blanco I, Acosta D, Eliezer D, Zhou P, Anrather J, Iadecola C
Nature neuroscience 2020 Sep;23(9):1079-1089
Nature neuroscience 2020 Sep;23(9):1079-1089
Therapeutic effects of syringaldehyde on spinal cord ischemia in rabbits.
Malçok ÜA, Aras AB, Şehitoğlu MH, Akman T, Yüksel Y
Saudi medical journal 2020 Apr;41(4):341-350
Saudi medical journal 2020 Apr;41(4):341-350
Bubble CPAP Support after Discontinuation of Mechanical Ventilation Protects Rat Lungs with Ventilator-Induced Lung Injury.
Wu CS, Chou HC, Huang LT, Lin YK, Chen CM
Respiration; international review of thoracic diseases 2016;91(2):171-9
Respiration; international review of thoracic diseases 2016;91(2):171-9
Nitric oxide synthase in diabetic rat testicular tissue and the effects of pentoxifylline therapy.
Sönmez MF, Kılıç E, Karabulut D, Çilenk K, Deligönül E, Dündar M
Systems biology in reproductive medicine 2016;62(1):22-30
Systems biology in reproductive medicine 2016;62(1):22-30
Ameliorative effects of pentoxifylline on NOS induced by diabetes in rat kidney.
Sönmez MF, Dündar M
Renal failure 2016;38(4):605-13
Renal failure 2016;38(4):605-13
Therapeutic effects of pentoxifylline on diabetic heart tissue via NOS.
Karabulut D, Ulusoy HB, Kaymak E, Sönmez MF
Anatolian journal of cardiology 2016 May;16(5):310-5
Anatolian journal of cardiology 2016 May;16(5):310-5
A role for nitric oxide in the control of breathing in zebrafish (Danio rerio).
Porteus CS, Pollack J, Tzaneva V, Kwong RW, Kumai Y, Abdallah SJ, Zaccone G, Lauriano ER, Milsom WK, Perry SF
The Journal of experimental biology 2015 Dec;218(Pt 23):3746-53
The Journal of experimental biology 2015 Dec;218(Pt 23):3746-53
Smooth muscle NOS, colocalized with caveolin-1, modulates contraction in mouse small intestine.
El-Yazbi AF, Cho WJ, Cena J, Schulz R, Daniel EE
Journal of cellular and molecular medicine 2008 Aug;12(4):1404-15
Journal of cellular and molecular medicine 2008 Aug;12(4):1404-15
Regulation of vascular tone in animals overexpressing the sarcolemmal calcium pump.
Schuh K, Quaschning T, Knauer S, Hu K, Kocak S, Roethlein N, Neyses L
The Journal of biological chemistry 2003 Oct 17;278(42):41246-52
The Journal of biological chemistry 2003 Oct 17;278(42):41246-52
The plasmamembrane calmodulin-dependent calcium pump: a major regulator of nitric oxide synthase I.
Schuh K, Uldrijan S, Telkamp M, Rothlein N, Neyses L
The Journal of cell biology 2001 Oct 15;155(2):201-5
The Journal of cell biology 2001 Oct 15;155(2):201-5
Granulated metrial gland cells contain nitric oxide synthases during pregnancy in the rat.
Sladek SM, Kanbour-Shakir A, Watkins S, Berghorn KA, Hoffman GE, Roberts JM
Placenta 1998 Jan;19(1):55-65
Placenta 1998 Jan;19(1):55-65
Physiological dilation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast.
Nanaev A, Chwalisz K, Frank HG, Kohnen G, Hegele-Hartung C, Kaufmann P
Cell and tissue research 1995 Dec;282(3):407-21
Cell and tissue research 1995 Dec;282(3):407-21
Physiological dilation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast.
Nanaev A, Chwalisz K, Frank HG, Kohnen G, Hegele-Hartung C, Kaufmann P
Cell and tissue research 1995 Dec;282(3):407-21
Cell and tissue research 1995 Dec;282(3):407-21
Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase.
Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH
Nature 1991 Jun 27;351(6329):714-8
Nature 1991 Jun 27;351(6329):714-8
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
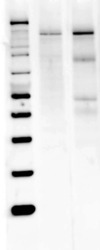
- Experimental details
- Western blot analysis of nNOS was performed by loading 40 µg of Mouse (Lane 1) and Rat Brain (Lane 2) tissue lysate onto a 4-12% Bis-Tris polyacrylamide gel. Proteins were transferred to a Nitrocellulose membrane. Membranes were probed with a rabbit polyclonal antibody (Product # PA3-032A) recognizing nNOS at a dilution of 1:1000. The western blot was performed using the Fast Western Kit, SuperSignal West Dura, Rabbit (Product # 35071).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
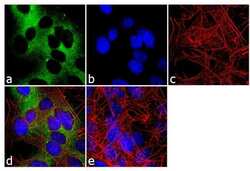
- Experimental details
- Immunofluorescence analysis of nNOS was done on 70% confluent log phase SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with nNOS Rabbit Polyclonal Antibody (Product # PA3-032A) at 1:250 dilution in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
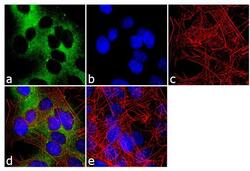
- Experimental details
- Immunofluorescence analysis of nNOS was done on 70% confluent log phase SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with nNOS Rabbit Polyclonal Antibody (Product # PA3-032A) at 1:250 dilution in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
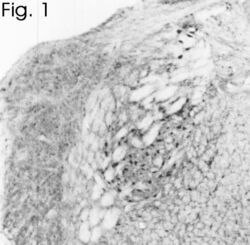
- Experimental details
- Immunohistochemical staining of bNOS in rat brain using Product # PA3-032A.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
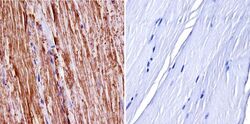
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized mouse skeletal muscle tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:400 with a Rabbit Polyclonal Antibody recognizing nNOS (Product # PA3-032A) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
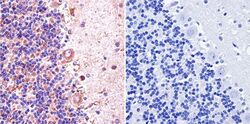
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized Rat cerebellum tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a Rabbit Polyclonal Antibody recognizing nNOS (Product # PA3-032A) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
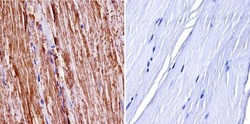
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized mouse skeletal muscle tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:400 with a Rabbit Polyclonal Antibody recognizing nNOS (Product # PA3-032A) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
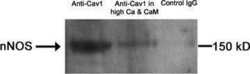
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
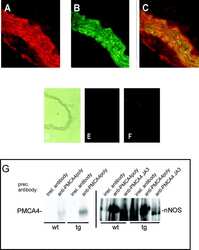
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
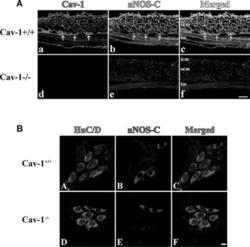
- Experimental details
- Fig. 1 Immunostained cryosections ( A ) and whole mount preparations ( B ) of Cav1 +/+ and Cav1 -/- mice small intestine. In section ( A ), panels (a-c) show caveolin-1 (a, red) and nNOS (b, green) immunoreactivity and their colocalization (c, yellow) in the smooth muscle cell membrane of Cav1 +/+ mice small intestinal tissue. Panels (d-f) show the absence of both caveolin-1 (d) and nNOS (e) immunoreactivities in Cav1 -/- mice small intestine. Panel f is a merged image. The scale bar is 10 mum. icm, inner circular muscle layer; ocm, outer circular muscle layer and lm, longitudinal muscle layer. Arrows point to interstitial cells of Cajal in the myenteric plexus layer. Section ( B ) shows immunostained myenteric ganglia in Cav1 +/+ (a-d) and Cav1 -/- (e-h) mice. Myenteric neuron cell bodies were localized by staining with HuC/D antibodies that stains the cytoplasm of neuron cell bodies (a and e, red). Panels b and e show neuron cells in myenteric ganglia stained with anti-nNOS COOH terminal epitope (blue). Panels c and f are merged images. Scale bars are 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
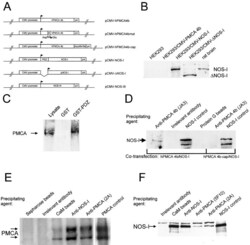
- Experimental details
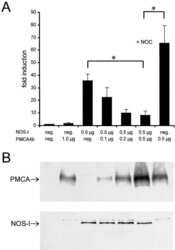
- Figure 1. Schematic overview of constructs used, expression of NOS-I constructs in HEK293 cells, and physical interaction of PMCA with NOS-I. (A) Plasmid constructs used in transfection assays. pCMV-PMCA 4b contains the wild-type human PMCA 4b cDNA under the control of the CMV promoter. pCMV-PMCA 4bmut carries a mutation Asp 672- Glu, thereby reducing the ATPase activity to ~10% ( Adamo et al., 1995 ). In pCMV-hPMCA 4b cap the stop codon is mutated and the myc/6xHis tag is added in frame, resulting in capping of the PDZ-binding COOH- terminal amino acids. NOS-I and NOS-III are also under the control of the CMV promoter, in pCMV-DeltaNOS-I the interacting PDZ domain is deleted. (B) NOS-I was expressed in pCMV-NOS-I-transfected HEK293 cells, rat brain homogenate served as control. DeltaNOS-I was expressed at comparable levels, the size difference is due to deletion of the PDZ domain. In untransfected HEK cells, NOS-I was undetectable. (C) GST pull-down assay ( Brenman et al., 1995 ) demonstrating specific interaction of PMCA 4b with PDZ domain of NOS-I. Lysate of pCMV-hPMCA4b-transfected HEK293 cells was used as expression control (first lane), GST alone did not bind PMCA (second lane), whereas GST-PDZ fusion protein was able to bind PMCA 4b (last lane). (D) NOS-I-PMCA 4b interaction in cotransfected HEK293 cells. In the first lane NOS-I coprecipitation by a hPMCA 4b-specific antibody (JA3) from lysates of cotransfected cells is shown. In the third lane, lysates of NOS-I/PMCA-t
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
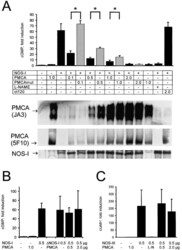
- Figure 2. Functional interaction of PMCA 4b and NOS-I. (A) Dose-dependent inhibition of NOS activity by PMCA 4b. Background cGMP production in untransfected HEK293 cells was set as one (first column), induced cGMP production is shown as fold increase of cGMP levels in relation to protein content of cell lysates. PMCA overexpression showed no effect on cGMP production (second column), whereas NOS-I expression resulted in an ~60-fold increase in cGMP levels (third column). Increasing amounts of PMCA led to a dose-dependent reduction of NOS-I activity, insertion of the point mutation Asp 672 -Glu in the PMCA reduced inhibition dramatically (PMCAmut, grey columns). The ~10-fold higher amounts of mutant PMCA necessary to inhibit NOS-I are in line with the fact that the mutation abolishes ~90% of Ca 2+ transport activity ( Adamo et al., 1995 ). Transfection with pPMCAmut alone had no effect on cGMP production (column 12), treatment of NOS-I transfected cells with L-NAME (Lreceptor-nitro-L-arginine methyl ester; 1 mM) resulted in the expected complete inhibition of cGMP production (penultimate column). Coexpression of the constitutively active mutant of PMCA 4b (""ct120"", last column) did not result in inhibition of NOS-I ( n = 16, mean +-SEM, asterisk indicates P < 0.05 PMCA vs. PMCAmut). Representative Western blots demonstrated expression of relevant proteins: antibody JA3 showed expression of hPMCA4b and hPMCA4bmut, antibody 5F10, specific for a more NH 2 -terminal epitope of P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
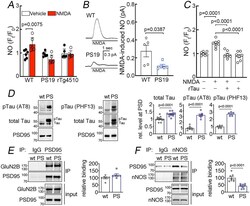
- Figure 7: Suppression of NMDAR-induced neuronal NO production and dissociation of nNOS from PSD95 in tau mice. A. NMDA (40 muM) increases NO production in neurons dissociated from 2-3-month-old WT mice, but not in neurons from age-matched PS19 or rTg4510 mice. N=6/group; two-tailed paired t-test. B . NMDA (100 muM)-induced NO production, detected amperometrically, is attenuated in PS19 mice compared to WT. N=5/group; two-tailed unpaired t-test. C . Recombinant tau (rTau; P301L mutant; 400 nM) attenuates NO production induced by NMDA in WT neurons. N=7/group; one-way ANOVA and Tukey's test. D . Total tau and phosphorylated tau (pTau) are elevated in post-synaptic density (PSD) preparations from 2-3-month-old PS19 (PS) compared to age-matched WT mice. N=12 for WT total Tau, pTau (AT8), and pTau (PHF13); N=13 for PS total tau; N=7 for PS pTau (AT8); N=5 for PS pTau (PHF13); two-tailed unpaired t-test. Data are presented as mean+-SEM. E . The association of GluN2B to PSD95, detected by co-immunoprecipitation (IP), is comparable in PS19 and WT mice. N=6/group. Data are presented as mean+-SEM. F . Association of PSD95 with nNOS, assessed by IP, is markedly reduced in PS19 mice compared to WT. Data are presented as mean+-SEM. N=8/group; two-tailed unpaired t-test. Immunoblots in D, E, and F are cropped; full gel pictures are shown in Source Data 5
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
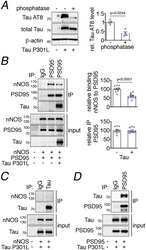
- Figure 8: Tau disrupts PSD95-nNOS association by binding to PSD95. A. Exogenously expressed Tau P301L in HEK293T cells is phosphorylated, as shown by sensitivity to phosphatase treatment. N=4/group; two-tailed paired t-test. Data are presented as mean+-SEM. B . Co-expression of Tau P301L reduces PSD95 association with nNOS. PSD95 was immunoprecipitated (IP) and associated nNOS was detected. N=10/group; two-tailed unpaired t-test. Data are presented as mean+-SEM. C. nNOS does not bind to immunoprecipitated Tau P301L. A representative blot is shown from N=9/group. D . Tau P301L associates with PSD95, which was immunoprecipitated (IP) from HEK293T cell lysates. Data are presented as mean+-SEM. A representative image is shown from N=9/group. Immunoblots in are cropped; full gel pictures are shown in Source Data 6
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
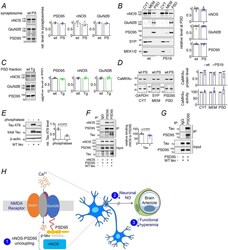
- Extended Data Fig. 10 NMDAR-related proteins in PS19 and rTg4510 mice, effects of WT tau on nNOS-PSD95 coupling, and putative mechanisms of the effect of tau on neurovascular function A-D . Levels and kinase activity of NMDAR-related proteins are not altered in 2-3 month-old PS19 and rTg4510 mice. A . Protein levels of nNOS, GluN2B and PSD95 are unaltered in synaptosomal preparations of PS19 (PS) compared to WT mice. N=3/group. Data are presented as mean+-SEM. B . Protein levels of nNOS, GluN2B and PSD95 in PS19 (PS) and WT mice are comparable. The presynaptic marker synaptophysin (SYP) and MEK1/2 were used as membrane (MEM) and cytosolic (CYT) markers, respectively. N=7/group. Data are presented as mean+-SEM. C . As in PS19 mice, nNOS, GluN2B and PSD95 protein levels are unchanged in PSD preparations of rTg4510 (Tg) mice compared to WT mice. N=4/group. Data are presented as mean+-SEM. D . CaMKIIalpha level and activity quantified with reference to GAPDH, synaptophysin (SYP), or PSD95 associated with cytoplasm (CYT), membrane (MEM), and/or PSD are not altered in PS19 mice, compared to WT. N=3/group. Data are presented as mean+-SEM. Immunoblots in A-D are cropped; full gel pictures are shown in Source Data 28 . E-G . WT tau impairs binding of nNOS to PSD95 through association with PSD95. E. WT tau over-expressed in HEK293T cells is susceptible to phosphatase treatment, and thus hyperphosphorylated. N=3/group; unpaired t-test. F. WT tau co-expression disrupts binding of nNOS to
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
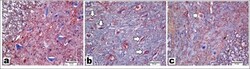
- Figure 5 Representative images of sections immunostained with neuronal nitric oxide synthase (nNOS) primary antibody (x200, scale bar = 50 um). A) Control group showed a few nNOS-positive-stained motor neurons. B) Spinal cord ischemia/reperfusion (SCI/R) group showed strong nNOS positivity in degenerated motor neurons. C) Syringaldehyde-treated ischemia group showed decrease in nNOS expression compared with SCI/R group. Arrows refer to nNOS-positive-stained motor neurons.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunoprecipitation
Immunoprecipitation