Antibody data
- Antibody Data
- Antigen structure
- References [5]
- Comments [0]
- Validations
- Immunocytochemistry [1]
- Other assay [4]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA1-10837 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- MBP Monoclonal Antibody (MBP101)
- Antibody type
- Monoclonal
- Antigen
- Purifed from natural sources
- Description
- MA1-10837 detects Myelin Basic Protein from human, non-human primate, rabbit, sheep, goat, rat, and mouse samples. MA1-10837 has been successfully used in ELISA, Western blot, and immunohistochemistry applications. The MA1-10837 immunogen is purified human myelin basic protein.
- Reactivity
- Human, Mouse, Rat, Goat, Rabbit
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- MBP101
- Vial size
- 500 μg
- Concentration
- 1.7 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Neuronal and Glial Distribution of Tau Protein in the Adult Rat and Monkey.
BCAS1-positive immature oligodendrocytes are affected by the α-synuclein-induced pathology of multiple system atrophy.
KUS121, a valosin-containing protein modulator, attenuates ischemic stroke via preventing ATP depletion.
Peroxisome proliferator-activated receptor-γ is expressed in eosinophils in nasal polyps.
Tumor-specific diagnostic marker for transmissible facial tumors of Tasmanian devils: immunohistochemistry studies.
Kanaan NM, Grabinski T
Frontiers in molecular neuroscience 2021;14:607303
Frontiers in molecular neuroscience 2021;14:607303
BCAS1-positive immature oligodendrocytes are affected by the α-synuclein-induced pathology of multiple system atrophy.
Kaji S, Maki T, Ueda J, Ishimoto T, Inoue Y, Yasuda K, Sawamura M, Hikawa R, Ayaki T, Yamakado H, Takahashi R
Acta neuropathologica communications 2020 Jul 29;8(1):120
Acta neuropathologica communications 2020 Jul 29;8(1):120
KUS121, a valosin-containing protein modulator, attenuates ischemic stroke via preventing ATP depletion.
Kinoshita H, Maki T, Yasuda K, Kishida N, Sasaoka N, Takagi Y, Kakizuka A, Takahashi R
Scientific reports 2019 Aug 8;9(1):11519
Scientific reports 2019 Aug 8;9(1):11519
Peroxisome proliferator-activated receptor-γ is expressed in eosinophils in nasal polyps.
Asaka C, Honda K, Ito E, Fukui N, Chihara J, Ishikawa K
International archives of allergy and immunology 2011;155 Suppl 1:57-63
International archives of allergy and immunology 2011;155 Suppl 1:57-63
Tumor-specific diagnostic marker for transmissible facial tumors of Tasmanian devils: immunohistochemistry studies.
Tovar C, Obendorf D, Murchison EP, Papenfuss AT, Kreiss A, Woods GM
Veterinary pathology 2011 Nov;48(6):1195-203
Veterinary pathology 2011 Nov;48(6):1195-203
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
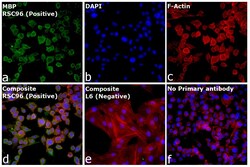
- Experimental details
- Immunofluorescence analysis of MBP was performed using 70% confluent log phase RSC96 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with MBP Monoclonal Antibody (Product # MA1-10837, 1.6 µg/mL) in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor™ Plus 488 (Product # A32766, 1:2,000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic side of myelin membrane localization. Panel e represents L6 cells with no expression. Panel f represents control cells with no primary antibody to assess background. The images were captured at 40x magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
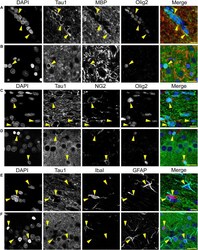
- Experimental details
- FIGURE 8 Multi-label immunofluorescence reveals tau positive interfascicular glia are mature oligodendrocytes, not oligodendrocyte precursor cells, astrocytes or microglia, in adult rat brains. (A,B) To determine whether interfascicular and perineuronal glial cells containing tau are mature oligodendrocytes, tissue sections were dephosphorylated and stained with multi-label immunofluorescence for DAPI (nuclear counterstain, blue), Tau1 (mid-tau antibody, green), myelin basic protein (MBP, mature oligodendrocyte marker, red), and oligodendrocyte lineage transcription factor 2 (Olig2, an oligodendrocyte marker, cyan). In the corpus callosum (A) and hippocampal pyramidal cell layers (B) , the Tau1-positive glial cells were also stained with MBP and Olig2 indicating they are mature oligodendrocytes. MBP labeling in tau/Olig2-positive cells was evident, but not all tau/Olig2-positive cells were clearly labeled with MBP suggesting either levels of cytoplasmic MBP were below detection or a subset of unidentified Olig2-positive cells express tau. (C,D) To further explore if the tau-positive glial cells were oligodendrocyte precursor cells, tissue sections were stained with multi-label immunofluorescence for DAPI (blue), Tau1 (green), neural/glial antigen 2 (NG2, an oligodendrocyte precursor marker, red), and Olig2 (cyan). In the corpus callosum (C) and hippocampal pyramidal cell layers (D) , the Olig2-positive cells that were NG2-positive showed little to no tau reactivity indicating
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
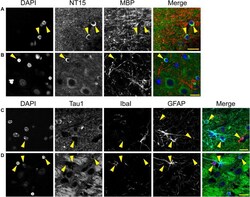
- Experimental details
- FIGURE 12 Multi-label immunofluorescence reveals tau positive interfascicular glia are mature oligodendrocytes, not astrocytes or microglia, in adult rhesus macaque brains. (A,B) To determine whether interfascicular and perineuronal glial cells containing tau are mature oligodendrocytes, tissue sections were dephosphorylated and stained with multi-label immunofluorescence for DAPI (nuclear counterstain, blue), NT15 (N-terminal tau antibody, green), and myelin basic protein (MBP, mature oligodendrocyte marker, red). In the alveus (A) and hippocampal pyramidal cell layers (B) , the Tau1-positive glial cells were also stained with MBP indicating they are mature oligodendrocytes. (C,D) To establish whether tau-positive glial cells were astrocytes or microglia, tissue sections were stained with multi-label immunofluorescence for DAPI (blue), Tau1 (mid-tau antibody, green), ionized calcium-binding adapter molecule 1 (IbaI, a microglial marker, red), and glial fibrillary acidic protein (GFAP, an astrocyte marker, cyan). In the alveus (C) and hippocampal pyramidal cell layers (D) , the tau-positive cells showed little to no co-labeling with GFAP-positive cells or IbaI-positive cells indicating that the tau-positive glial cells are not astrocytes or microglia. Representative cells of each phenotype are indicated with yellow arrows in images. Scale bars in merged images are 10 mum in panels (A,C) and 20 mum in panel (B,D) .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
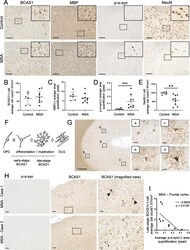
- Experimental details
- Fig. 1 The differentiation of BCAS1(+) cells was affected by p-alpha-syn-immunoreactive inclusions in the frontal cortices of MSA brains. a Serial sections of brain tissues from the frontal cortices of an aged non-neurodegenerative disease control (upper) and an MSA patient (lower) which were immunostained with antibodies against BCAS1, MBP, p-alpha-syn, and NeuN. Scale bar = 100 mum. The regions marked by dotted squares are magnified in the right upper corners. The magnified images of BCAS1 staining show altered morphology of BCAS1(+) cells in the MSA case. Images of control and MSA cases are provided from case 21 and case 2 (Additional file 1 : Table S1), respectively. b , e Quantification of BCAS1(+) cell counts ( b ), MBP(+) cell counts ( c ), p-alpha-syn(+) area ( d ), and NeuN(+) cell counts ( e ) in control and MSA brains. Mean +- SEM; control, N = 6, MSA, N = 9; Mann-Whitney, p ** < 0.01, p *** < 0.001. f Illustration of oligodendroglial differentiation showing the morphological classification of early-stage and late-stage BCAS1(+) cells. g Immunostaining of an aged non-neurodegenerative disease control (case 21, Additional file 1 : Table S1) with the anti-BCAS1 antibody showing representative images of early-stage (a, b) and late-stage (c, d) BCAS1(+) cells in the frontal cortex. Scale bar = 500 mum (low-power field, left) and 20 mum (high-power field, right). (H) Immunostaining of the serial sections from the frontal cortices of two different MSA cases (cases 1 and
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
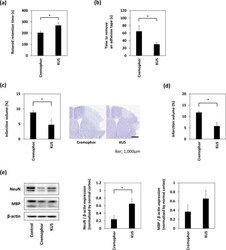
- Figure 3 Treatment with KUS121 improves functional deficits and reduces brain infarction volume. ( a ) Rotarod retention time and ( b ) the time to remove an adhesive tape in C57BL/6 mice subjected to transient occlusion of the distal portion of left middle cerebral artery (MCA). Neurological function was measured by the accelerating rotarod apparatus and adhesive removal test 24 h after occlusion of the distal MCA (n = 16; *p < 0.05). ( c ) Quantification of the infarction volume was calculated by Nissl staining and representative Nissl stained brain sections of C57BL/6 mice (Scale bar: 1,000 mum; vehicle, n = 5; KUS121, n = 4; *p < 0.05). ( d ) Quantification of the infarction volume in B-17 mice measured 24 h after transient occlusion of the distal MCA and calculated by Nissl staining (vehicle, n = 4; KUS121, n = 5; *p < 0.05). ( e ) Western blot analyses of cerebral cortex lysates for Neuronal Nuclei (NeuN) and myelin basic protein (MBP). The cerebral cortex of B-17 mice was collected 24 h after transient occlusion of the distal MCA. The ratio of NeuN or MBP to beta-actin expression levels was calculated (n = 5; *p < 0.05).
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry