Antibody data
- Antibody Data
- Antigen structure
- References [22]
- Comments [0]
- Validations
- Flow cytometry [1]
- Other assay [6]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 11-9994-41 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Perforin Monoclonal Antibody (dG9 (delta G9)), FITC, eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The dG9 antibody clone reacts with human perforin (pore-forming protein, pfp). Perforin is one of the cytolytic mediators present in the cytoplasmic granules of cytotoxic T lymphocytes (CTL) and natural killer cells (NK). Perforin is involved in the killing function by CTLs and NKs and has an important role in the immune response against tumors and virus infections. Applications Reported: This dG9 (delta G9) antibody has been reported for use in intracellular staining followed by flow cytometric analysis, and immunohistochemical staining. Applications Tested: This dG9 (delta G9) antibody has been tested by intracellular flow cytometric analysis of normal human peripheral blood cells. This can be used at 5 µL (0.125 µg) per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. Excitation: 488 nm; Emission: 520 nm; Laser: Blue Laser. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human, Porcine
- Host
- Mouse
- Conjugate
- Green dye
- Isotype
- IgG
- Antibody clone number
- dG9 (delta G9)
- Vial size
- 25 Tests
- Concentration
- 5 μL/Test
- Storage
- 4°C, store in dark, DO NOT FREEZE!
Submitted references Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial.
CD8+ T cell-Dependent Remodeling of the Tumor Microenvironment Overcomes Chemoresistance.
Rapamycin improves Graves' orbitopathy by suppressing CD4+ cytotoxic T lymphocytes.
Single-cell transcriptomics links malignant T cells to the tumor immune landscape in cutaneous T cell lymphoma.
LINC01123 promotes immune escape by sponging miR-214-3p to regulate B7-H3 in head and neck squamous-cell carcinoma.
PD-1-Expressing SARS-CoV-2-Specific CD8(+) T Cells Are Not Exhausted, but Functional in Patients with COVID-19.
Human Thymic CD10(+) PD-1(+) Intraepithelial Lymphocyte Precursors Acquire Interleukin-15 Responsiveness at the CD1a(-) CD95(+) CD28(-) CCR7(-) Developmental Stage.
Neutrophil expansion defines an immunoinhibitory peripheral and intratumoral inflammatory milieu in resected non-small cell lung cancer: a descriptive analysis of a prospectively immunoprofiled cohort.
Enhanced Anti-lymphoma Activity of CAR19-iNKT Cells Underpinned by Dual CD19 and CD1d Targeting.
The Transcription Factor Hobit Identifies Human Cytotoxic CD4(+) T Cells.
Eomesodermin and T-bet mark developmentally distinct human natural killer cells.
Human Asymptomatic Epitopes Identified from the Herpes Simplex Virus Tegument Protein VP13/14 (UL47) Preferentially Recall Polyfunctional Effector Memory CD44high CD62Llow CD8+ TEM Cells and Protect Humanized HLA-A*02:01 Transgenic Mice against Ocular Herpesvirus Infection.
Blocking the recruitment of naive CD4(+) T cells reverses immunosuppression in breast cancer.
Peritoneal carcinomatosis of colorectal cancer is characterized by structural and functional reorganization of the tumor microenvironment inducing senescence and proliferation arrest in cancer cells.
Regulatory T Cell Modulation by CBP/EP300 Bromodomain Inhibition.
IL12-mediated sensitizing of T-cell receptor-dependent and -independent tumor cell killing.
Cutting edge: identification and characterization of human intrahepatic CD49a+ NK cells.
Differentiation and functional regulation of human fetal NK cells.
Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma.
Common gamma chain cytokines promote rapid in vitro expansion of allo-specific human CD8+ suppressor T cells.
Transfer of regulatory properties from tolerogenic to proinflammatory dendritic cells via induced autoreactive regulatory T cells.
The lytic potential of human liver NK cells is restricted by their limited expression of inhibitory killer Ig-like receptors.
Cascone T, Leung CH, Weissferdt A, Pataer A, Carter BW, Godoy MCB, Feldman H, William WN Jr, Xi Y, Basu S, Sun JJ, Yadav SS, Rojas Alvarez FR, Lee Y, Mishra AK, Chen L, Pradhan M, Guo H, Sinjab A, Zhou N, Negrao MV, Le X, Gay CM, Tsao AS, Byers LA, Altan M, Glisson BS, Fossella FV, Elamin YY, Blumenschein G Jr, Zhang J, Skoulidis F, Wu J, Mehran RJ, Rice DC, Walsh GL, Hofstetter WL, Rajaram R, Antonoff MB, Fujimoto J, Solis LM, Parra ER, Haymaker C, Wistuba II, Swisher SG, Vaporciyan AA, Lin HY, Wang J, Gibbons DL, Jack Lee J, Ajami NJ, Wargo JA, Allison JP, Sharma P, Kadara H, Heymach JV, Sepesi B
Nature medicine 2023 Mar;29(3):593-604
Nature medicine 2023 Mar;29(3):593-604
CD8+ T cell-Dependent Remodeling of the Tumor Microenvironment Overcomes Chemoresistance.
Lao L, Zeng W, Huang P, Chen H, Jia Z, Wang P, Huang D, Chen J, Nie Y, Yang L, Wu W, Liu J
Cancer immunology research 2023 Mar 1;11(3):320-338
Cancer immunology research 2023 Mar 1;11(3):320-338
Rapamycin improves Graves' orbitopathy by suppressing CD4+ cytotoxic T lymphocytes.
Zhang M, Chong KK, Chen ZY, Guo H, Liu YF, Kang YY, Li YJ, Shi TT, Lai KK, He MQ, Ye K, Kahaly GJ, Shi BY, Wang Y
JCI insight 2023 Feb 8;8(3)
JCI insight 2023 Feb 8;8(3)
Single-cell transcriptomics links malignant T cells to the tumor immune landscape in cutaneous T cell lymphoma.
Liu X, Jin S, Hu S, Li R, Pan H, Liu Y, Lai P, Xu D, Sun J, Liu Z, Gao Y, Zhao Y, Liu F, Xiao Y, Li Y, Wen Y, Chen Z, Xu B, Lin Y, Ran M, Li Q, Yang S, Li H, Tu P, Haniffa M, Teichmann SA, Bai F, Wang Y
Nature communications 2022 Mar 3;13(1):1158
Nature communications 2022 Mar 3;13(1):1158
LINC01123 promotes immune escape by sponging miR-214-3p to regulate B7-H3 in head and neck squamous-cell carcinoma.
Li H, Yang Z, Yang X, Zhang F, Wang J, Wu Z, Wanyan C, Meng Q, Gao W, Yang X, Wei J
Cell death & disease 2022 Feb 3;13(2):109
Cell death & disease 2022 Feb 3;13(2):109
PD-1-Expressing SARS-CoV-2-Specific CD8(+) T Cells Are Not Exhausted, but Functional in Patients with COVID-19.
Rha MS, Jeong HW, Ko JH, Choi SJ, Seo IH, Lee JS, Sa M, Kim AR, Joo EJ, Ahn JY, Kim JH, Song KH, Kim ES, Oh DH, Ahn MY, Choi HK, Jeon JH, Choi JP, Kim HB, Kim YK, Park SH, Choi WS, Choi JY, Peck KR, Shin EC
Immunity 2021 Jan 12;54(1):44-52.e3
Immunity 2021 Jan 12;54(1):44-52.e3
Human Thymic CD10(+) PD-1(+) Intraepithelial Lymphocyte Precursors Acquire Interleukin-15 Responsiveness at the CD1a(-) CD95(+) CD28(-) CCR7(-) Developmental Stage.
Billiet L, Goetgeluk G, Bonte S, De Munter S, De Cock L, Pille M, Ingels J, Jansen H, Weening K, Van Nieuwerburgh F, Kerre T, Taghon T, Leclercq G, Vandekerckhove B
International journal of molecular sciences 2020 Nov 20;21(22)
International journal of molecular sciences 2020 Nov 20;21(22)
Neutrophil expansion defines an immunoinhibitory peripheral and intratumoral inflammatory milieu in resected non-small cell lung cancer: a descriptive analysis of a prospectively immunoprofiled cohort.
Mitchell KG, Diao L, Karpinets T, Negrao MV, Tran HT, Parra ER, Corsini EM, Reuben A, Federico L, Bernatchez C, Dejima H, Francisco-Cruz A, Wang J, Antonoff MB, Vaporciyan AA, Swisher SG, Cascone T, Wistuba II, Heymach JV, Gibbons DL, Zhang J, Haymaker CL, Sepesi B
Journal for immunotherapy of cancer 2020 Apr;8(1)
Journal for immunotherapy of cancer 2020 Apr;8(1)
Enhanced Anti-lymphoma Activity of CAR19-iNKT Cells Underpinned by Dual CD19 and CD1d Targeting.
Rotolo A, Caputo VS, Holubova M, Baxan N, Dubois O, Chaudhry MS, Xiao X, Goudevenou K, Pitcher DS, Petevi K, Kachramanoglou C, Iles S, Naresh K, Maher J, Karadimitris A
Cancer cell 2018 Oct 8;34(4):596-610.e11
Cancer cell 2018 Oct 8;34(4):596-610.e11
The Transcription Factor Hobit Identifies Human Cytotoxic CD4(+) T Cells.
Oja AE, Vieira Braga FA, Remmerswaal EB, Kragten NA, Hertoghs KM, Zuo J, Moss PA, van Lier RA, van Gisbergen KP, Hombrink P
Frontiers in immunology 2017;8:325
Frontiers in immunology 2017;8:325
Eomesodermin and T-bet mark developmentally distinct human natural killer cells.
Collins A, Rothman N, Liu K, Reiner SL
JCI insight 2017 Mar 9;2(5):e90063
JCI insight 2017 Mar 9;2(5):e90063
Human Asymptomatic Epitopes Identified from the Herpes Simplex Virus Tegument Protein VP13/14 (UL47) Preferentially Recall Polyfunctional Effector Memory CD44high CD62Llow CD8+ TEM Cells and Protect Humanized HLA-A*02:01 Transgenic Mice against Ocular Herpesvirus Infection.
Srivastava R, Khan AA, Garg S, Syed SA, Furness JN, Vahed H, Pham T, Yu HT, Nesburn AB, BenMohamed L
Journal of virology 2017 Jan 15;91(2)
Journal of virology 2017 Jan 15;91(2)
Blocking the recruitment of naive CD4(+) T cells reverses immunosuppression in breast cancer.
Su S, Liao J, Liu J, Huang D, He C, Chen F, Yang L, Wu W, Chen J, Lin L, Zeng Y, Ouyang N, Cui X, Yao H, Su F, Huang JD, Lieberman J, Liu Q, Song E
Cell research 2017 Apr;27(4):461-482
Cell research 2017 Apr;27(4):461-482
Peritoneal carcinomatosis of colorectal cancer is characterized by structural and functional reorganization of the tumor microenvironment inducing senescence and proliferation arrest in cancer cells.
Seebauer CT, Brunner S, Glockzin G, Piso P, Ruemmele P, Schlitt HJ, Geissler EK, Fichtner-Feigl S, Kesselring R
Oncoimmunology 2016;5(12):e1242543
Oncoimmunology 2016;5(12):e1242543
Regulatory T Cell Modulation by CBP/EP300 Bromodomain Inhibition.
Ghosh S, Taylor A, Chin M, Huang HR, Conery AR, Mertz JA, Salmeron A, Dakle PJ, Mele D, Cote A, Jayaram H, Setser JW, Poy F, Hatzivassiliou G, DeAlmeida-Nagata D, Sandy P, Hatton C, Romero FA, Chiang E, Reimer T, Crawford T, Pardo E, Watson VG, Tsui V, Cochran AG, Zawadzke L, Harmange JC, Audia JE, Bryant BM, Cummings RT, Magnuson SR, Grogan JL, Bellon SF, Albrecht BK, Sims RJ 3rd, Lora JM
The Journal of biological chemistry 2016 Jun 17;291(25):13014-27
The Journal of biological chemistry 2016 Jun 17;291(25):13014-27
IL12-mediated sensitizing of T-cell receptor-dependent and -independent tumor cell killing.
Braun M, Ress ML, Yoo YE, Scholz CJ, Eyrich M, Schlegel PG, Wölfl M
Oncoimmunology 2016 Jul;5(7):e1188245
Oncoimmunology 2016 Jul;5(7):e1188245
Cutting edge: identification and characterization of human intrahepatic CD49a+ NK cells.
Marquardt N, Béziat V, Nyström S, Hengst J, Ivarsson MA, Kekäläinen E, Johansson H, Mjösberg J, Westgren M, Lankisch TO, Wedemeyer H, Ellis EC, Ljunggren HG, Michaëlsson J, Björkström NK
Journal of immunology (Baltimore, Md. : 1950) 2015 Mar 15;194(6):2467-71
Journal of immunology (Baltimore, Md. : 1950) 2015 Mar 15;194(6):2467-71
Differentiation and functional regulation of human fetal NK cells.
Ivarsson MA, Loh L, Marquardt N, Kekäläinen E, Berglin L, Björkström NK, Westgren M, Nixon DF, Michaëlsson J
The Journal of clinical investigation 2013 Sep;123(9):3889-901
The Journal of clinical investigation 2013 Sep;123(9):3889-901
Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma.
Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD
Science translational medicine 2013 Feb 27;5(174):174ra26
Science translational medicine 2013 Feb 27;5(174):174ra26
Common gamma chain cytokines promote rapid in vitro expansion of allo-specific human CD8+ suppressor T cells.
Yu Y, Zitzner JR, Houlihan J, Herrera N, Xu L, Miller J, Mathew JM, Tambur AR, Luo X
PloS one 2011;6(12):e28948
PloS one 2011;6(12):e28948
Transfer of regulatory properties from tolerogenic to proinflammatory dendritic cells via induced autoreactive regulatory T cells.
Kleijwegt FS, Laban S, Duinkerken G, Joosten AM, Koeleman BP, Nikolic T, Roep BO
Journal of immunology (Baltimore, Md. : 1950) 2011 Dec 15;187(12):6357-64
Journal of immunology (Baltimore, Md. : 1950) 2011 Dec 15;187(12):6357-64
The lytic potential of human liver NK cells is restricted by their limited expression of inhibitory killer Ig-like receptors.
Burt BM, Plitas G, Zhao Z, Bamboat ZM, Nguyen HM, Dupont B, DeMatteo RP
Journal of immunology (Baltimore, Md. : 1950) 2009 Aug 1;183(3):1789-96
Journal of immunology (Baltimore, Md. : 1950) 2009 Aug 1;183(3):1789-96
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
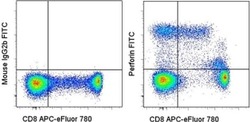
- Experimental details
- Intracellular staining of normal human peripheral blood cells with Anti-Human CD8 APC-eFluor® 780 (Product # 47-0088-42) and Mouse IgG2b K Isotype Control FITC (Product # 11-4732-42) (left) or Anti-Human Perforin FITC (right). Cells in the lymphocyte gate were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
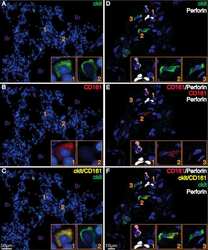
- Figure 3 Proliferation with IL-15 induces cytotoxic mediators in CD10 + PD-1 + IELps. ( A ) fold expansion of TCRgammadelta + , conventional and IELp cells cultured for 8 days under various conditions (n = 2); ( B ) IFN-gamma, granzyme B and perforin production by unstimulated TCRgammadelta + , conventional and IELp cells incubated for 5 days in the presence of IL-7, IL-15 or IL-15 + IL-12 + IL-18. IFN-gamma, granzyme B, and perforin production measured via intracellular flow cytometry. Bottom: Cytokine production in IELps, plotted for the first and sixth/last generation ( Figure S5 ; mean +- SEM, n = 3); ( C ) flow cytometric analysis of IFN-gamma, granzyme B and perforin production by TCRgammadelta + cells and IELps proliferated for 6 days with IL-15, followed by stimulation with IL-12 + IL-18 for 48 h or with PMA + ionomycin for 4 h (mean +- SEM, n = 3). Dunnett''s multiple comparisons test was used to assess statistically significant differences in cytokine production between the different populations and different interleukins. p -value < 0.05 (*), p < 0.01 (**), and p < 0.001 (***).
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
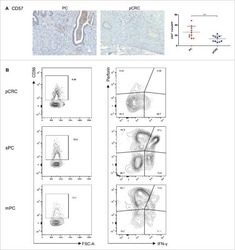
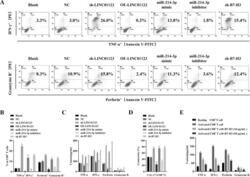
- Fig. 4 Upregulation of LINC01123 or B7-H3 or downregulation of miR-214-3p induces dysfunction of CD8 + T cells. CD8 + T cells were isolated from PBMCs using immunomagnetic beads. After activation and amplification, CD8 + T cells were cocultured with different CAL-27 cell treatment groups. A Expression of TNF-alpha, IFN-gamma, perforin, and granzyme B in CD8 + T-cell population was analyzed with flow cytometry. B Statistical analysis of the results from flow cytometry of CD8 + T cells. C Expression levels of TNF-alpha, IFN-gamma, perforin, and granzyme B in CAL-27-CD8 + T cocultured supernatants, as detected by ELISA. D CCK8 cytotoxicity test of CD8 + T cells. E ELISA was used to assess the immune activity of CD8 + T cells after recombinant human B7-H3 treatment. * P < 0.05 compared with cells without treatment. The experiment was repeated three times.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
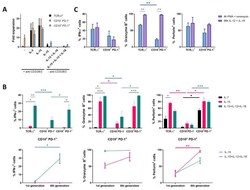
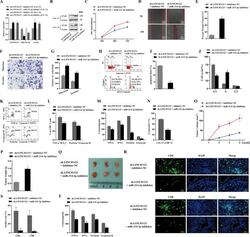
- Fig. 6 The function of LINC01123 in HNSCC can be reversed by miR-214-3p silencing. HNSCC cells were transfected with sh-LINC01123 in the presence of inhibitor-NC or miR-214-3p inhibitor. A Relative expression levels of LINC01123, miR-214-3p, and B7-H3 were determined by qRT-PCR. B B7-H3 protein expression was determined by western blot analysis. C CAL-27 cell viability was measured using a CCK8 assay. D Migration of CAL-27 cells was determined by scratch wound-healing assay. E Statistical results of the scratch-healing test. F Migration and invasion of CAL-27 cells, as determined by transwell migration and invasion assay. G Number of migration and invasion cells. H Apoptosis rate and cell-cycle stage were detected by flow cytometry. I Statistical analysis of apoptosis results. J Statistical analysis of cell cycle stage results. K CD8 + T cells were cocultured with CAL-27 cells that had been transfected with plasmids, and the expression of TNF-alpha, IFN-gamma, perforin, and granzyme B in the CD8 + T cells was analyzed by flow cytometry. L Statistical analysis of CD8 + T-cell flow cytometry results. M After different treatments, CAL-27 cells were cocultured with CD8 + T cells, and the expression levels of TNF-alpha, IFN-gamma, perforin, and granzyme B in coculture supernatants were detected by ELISA. N Killing effect of CD8 + T cells on CAL-27 cells, as assessed by CCK8. O Tumor-volume growth curves of the sh-LINC01123 + inhibitor-NC group and sh-LINC01123 + miR-214-3p-inhibit
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
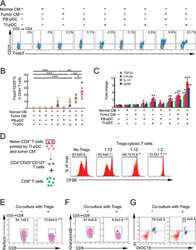
- Figure 3 Naive CD4 + T cells are converted to functional Tregs by tumor-infiltrating DCs and tumor conditioned medium (CM). (A-C) Naive CD4 + T cells from peripheral blood of patients with invasive breast carcinoma were co-cultured with or without autologous pDCs isolated from tumor (TI) or peripheral blood (PB) for 9 days in the presence or absence of 30% CM from autologous tumor slices or adjacent normal tissue slices. (A , B) Non-adherent cells from co-cultures were stained for CD3, CD4, CD25 and intracellular Foxp3, and analyzed by flow cytometry. Representative plots of gated CD3 + CD4 + cells (A) and quantification of percentage of Foxp3 + CD25 + cells among CD3 + CD4 + cells (B) are shown (mean +- SEM, n = 19; * P < 0.05, ** P < 0.01, *** P < 0.001 by Student's t -test). (C) Expression of Treg-associated genes, assessed by qRT-PCR normalized to GAPDH , in sorted CD4 + T cells, relative to expression in cultures without DCs or CM (mean +- SEM, n = 19; * P < 0.05, ** P < 0.01, *** P < 0.001 compared with naive CD4 + T cells cultured alone by Student's t -test). (D-G) Effect of naive CD4 + T cell-derived Tregs, obtained by co-culture with TI pDCs and tumor CM as above, on function of autologous tumor-specific CD8 + T cells. Tumor-specific CD8 + T cells were generated for each subject by stimulating autologous PB CD8 + T cells with autologous tumor lysate-pulsed autologous DCs. Tregs were recovered from co-cultures by magnetic sorting. (D) CFSE-labeled CD8 + T ce
- Conjugate
- Green dye
 Explore
Explore Validate
Validate Learn
Learn Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry