MA3-061
antibody from Invitrogen Antibodies
Targeting: AP2A1
ADTAA, CLAPA1
 Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [25]
- Comments [0]
- Validations
- Western blot [2]
- Immunocytochemistry [4]
- Immunohistochemistry [1]
- Flow cytometry [1]
- Other assay [5]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-061 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- alpha Adaptin Monoclonal Antibody (AC1-M11)
- Antibody type
- Monoclonal
- Antigen
- Purifed from natural sources
- Description
- MA3-061 detects alpha-adaptin from bovine, chicken, frog, human, monkey, mouse, pig and rat. This antibody detects the products of both alpha-adaptin genes, alphaA and alphaC, as well as an alternatively spliced isoform of alphaA found in neurons. MA3-061 has been successfully used in Western blot, immunofluorescence, and immunoprecipitation procedures. By Western blot, this antibody recognizes an ~100 kDa doublet representing the two isoforms of alpha-adaptin from pig brain extract. Immunofluorescence staining of alpha-adaptin in transfected COS cells with MA3-061A yields distinct punctate dots on the plasma membrane. The MA3-061 antigen is purified bovine brain adaptor complexes.
- Reactivity
- Human, Mouse, Rat, Bovine, Chicken/Avian, Porcine
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- AC1-M11
- Vial size
- 500 μL
- Concentration
- 2 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Indoleamine 2,3-Dioxygenase 2 Immunohistochemical Expression in Resected Human Non-small Cell Lung Cancer: A Potential New Prognostic Tool.
An internally eGFP-tagged α-adaptin is a fully functional and improved fiduciary marker for clathrin-coated pit dynamics.
Cell-specific non-canonical amino acid labelling identifies changes in the de novo proteome during memory formation.
DASC, a sensitive classifier for measuring discrete early stages in clathrin-mediated endocytosis.
A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery.
Ikarugamycin: A Natural Product Inhibitor of Clathrin-Mediated Endocytosis.
Clathrin and phosphatidylinositol-4,5-bisphosphate regulate autophagic lysosome reformation.
Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission.
Endocytosis in cultured neurons is altered by chronic alcohol exposure.
Synaptic and vesicular coexistence of VGLUT and VGAT in selected excitatory and inhibitory synapses.
Silencing of fas-associated death domain protects mice from septic lung inflammation and apoptosis.
ARAP1 regulates endocytosis of EGFR.
A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis.
Interaction of SPIN90 with syndapin is implicated in clathrin-mediated endocytic pathway in fibroblasts.
Death-receptor activation halts clathrin-dependent endocytosis.
Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment.
Xenopus autosomal recessive hypercholesterolemia protein couples lipoprotein receptors with the AP-2 complex in oocytes and embryos and is required for vitellogenesis.
Tensile stress induces alpha-adaptin C production in mouse calvariae in an organ culture: possible involvement of endocytosis in mechanical stress-stimulated osteoblast differentiation.
Cytoplasmic transport of Stat3 by receptor-mediated endocytosis.
The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro.
A beta-turn endocytic code is required for optimal internalization of the growth hormone receptor but not for alpha-adaptin association.
Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic.
Adaptor self-aggregation, adaptor-receptor recognition and binding of alpha-adaptin subunits to the plasma membrane contribute to recruitment of adaptor (AP2) components of clathrin-coated pits.
100-kD proteins of Golgi- and trans-Golgi network-associated coated vesicles have related but distinct membrane binding properties.
100-kD proteins of Golgi- and trans-Golgi network-associated coated vesicles have related but distinct membrane binding properties.
Mandarano M, Bellezza G, Belladonna ML, Vannucci J, Gili A, Ferri I, Lupi C, Ludovini V, Falabella G, Metro G, Mondanelli G, Chiari R, Cagini L, Stracci F, Roila F, Puma F, Volpi C, Sidoni A
Frontiers in immunology 2020;11:839
Frontiers in immunology 2020;11:839
An internally eGFP-tagged α-adaptin is a fully functional and improved fiduciary marker for clathrin-coated pit dynamics.
Mino RE, Chen Z, Mettlen M, Schmid SL
Traffic (Copenhagen, Denmark) 2020 Sep;21(9):603-616
Traffic (Copenhagen, Denmark) 2020 Sep;21(9):603-616
Cell-specific non-canonical amino acid labelling identifies changes in the de novo proteome during memory formation.
Evans HT, Bodea LG, Götz J
eLife 2020 Jan 6;9
eLife 2020 Jan 6;9
DASC, a sensitive classifier for measuring discrete early stages in clathrin-mediated endocytosis.
Wang X, Chen Z, Mettlen M, Noh J, Schmid SL, Danuser G
eLife 2020 Apr 30;9
eLife 2020 Apr 30;9
A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery.
Wolfram J, Nizzero S, Liu H, Li F, Zhang G, Li Z, Shen H, Blanco E, Ferrari M
Scientific reports 2017 Oct 23;7(1):13738
Scientific reports 2017 Oct 23;7(1):13738
Ikarugamycin: A Natural Product Inhibitor of Clathrin-Mediated Endocytosis.
Elkin SR, Oswald NW, Reed DK, Mettlen M, MacMillan JB, Schmid SL
Traffic (Copenhagen, Denmark) 2016 Oct;17(10):1139-49
Traffic (Copenhagen, Denmark) 2016 Oct;17(10):1139-49
Clathrin and phosphatidylinositol-4,5-bisphosphate regulate autophagic lysosome reformation.
Rong Y, Liu M, Ma L, Du W, Zhang H, Tian Y, Cao Z, Li Y, Ren H, Zhang C, Li L, Chen S, Xi J, Yu L
Nature cell biology 2012 Sep;14(9):924-34
Nature cell biology 2012 Sep;14(9):924-34
Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission.
Milosevic I, Giovedi S, Lou X, Raimondi A, Collesi C, Shen H, Paradise S, O'Toole E, Ferguson S, Cremona O, De Camilli P
Neuron 2011 Nov 17;72(4):587-601
Neuron 2011 Nov 17;72(4):587-601
Endocytosis in cultured neurons is altered by chronic alcohol exposure.
Marín MP, Esteban-Pretel G, Ponsoda X, Romero AM, Ballestín R, López C, Megías L, Timoneda J, Molowny A, Canales JJ, Renau-Piqueras J
Toxicological sciences : an official journal of the Society of Toxicology 2010 May;115(1):202-13
Toxicological sciences : an official journal of the Society of Toxicology 2010 May;115(1):202-13
Synaptic and vesicular coexistence of VGLUT and VGAT in selected excitatory and inhibitory synapses.
Zander JF, Münster-Wandowski A, Brunk I, Pahner I, Gómez-Lira G, Heinemann U, Gutiérrez R, Laube G, Ahnert-Hilger G
The Journal of neuroscience : the official journal of the Society for Neuroscience 2010 Jun 2;30(22):7634-45
The Journal of neuroscience : the official journal of the Society for Neuroscience 2010 Jun 2;30(22):7634-45
Silencing of fas-associated death domain protects mice from septic lung inflammation and apoptosis.
Matsuda N, Yamamoto S, Takano K, Kageyama S, Kurobe Y, Yoshihara Y, Takano Y, Hattori Y
American journal of respiratory and critical care medicine 2009 May 1;179(9):806-15
American journal of respiratory and critical care medicine 2009 May 1;179(9):806-15
ARAP1 regulates endocytosis of EGFR.
Yoon HY, Lee JS, Randazzo PA
Traffic (Copenhagen, Denmark) 2008 Dec;9(12):2236-52
Traffic (Copenhagen, Denmark) 2008 Dec;9(12):2236-52
A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis.
Ferguson SM, Brasnjo G, Hayashi M, Wölfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, O'toole E, Flavell R, Cremona O, Miesenböck G, Ryan TA, De Camilli P
Science (New York, N.Y.) 2007 Apr 27;316(5824):570-4
Science (New York, N.Y.) 2007 Apr 27;316(5824):570-4
Interaction of SPIN90 with syndapin is implicated in clathrin-mediated endocytic pathway in fibroblasts.
Kim SH, Choi HJ, Lee KW, Hong NH, Sung BH, Choi KY, Kim SM, Chang S, Eom SH, Song WK
Genes to cells : devoted to molecular & cellular mechanisms 2006 Oct;11(10):1197-211
Genes to cells : devoted to molecular & cellular mechanisms 2006 Oct;11(10):1197-211
Death-receptor activation halts clathrin-dependent endocytosis.
Austin CD, Lawrence DA, Peden AA, Varfolomeev EE, Totpal K, De Mazière AM, Klumperman J, Arnott D, Pham V, Scheller RH, Ashkenazi A
Proceedings of the National Academy of Sciences of the United States of America 2006 Jul 5;103(27):10283-10288
Proceedings of the National Academy of Sciences of the United States of America 2006 Jul 5;103(27):10283-10288
Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment.
Ivanov AI, Nusrat A, Parkos CA
Molecular biology of the cell 2004 Jan;15(1):176-88
Molecular biology of the cell 2004 Jan;15(1):176-88
Xenopus autosomal recessive hypercholesterolemia protein couples lipoprotein receptors with the AP-2 complex in oocytes and embryos and is required for vitellogenesis.
Zhou Y, Zhang J, King ML
The Journal of biological chemistry 2003 Nov 7;278(45):44584-92
The Journal of biological chemistry 2003 Nov 7;278(45):44584-92
Tensile stress induces alpha-adaptin C production in mouse calvariae in an organ culture: possible involvement of endocytosis in mechanical stress-stimulated osteoblast differentiation.
Shimomura J, Ishibashi O, Ikegame M, Yoshizawa T, Ejiri S, Noda T, Kawashima H
Journal of cellular physiology 2003 Jun;195(3):488-96
Journal of cellular physiology 2003 Jun;195(3):488-96
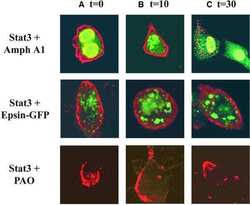
Cytoplasmic transport of Stat3 by receptor-mediated endocytosis.
Bild AH, Turkson J, Jove R
The EMBO journal 2002 Jul 1;21(13):3255-63
The EMBO journal 2002 Jul 1;21(13):3255-63
The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro.
Engqvist-Goldstein AE, Warren RA, Kessels MM, Keen JH, Heuser J, Drubin DG
The Journal of cell biology 2001 Sep 17;154(6):1209-23
The Journal of cell biology 2001 Sep 17;154(6):1209-23
A beta-turn endocytic code is required for optimal internalization of the growth hormone receptor but not for alpha-adaptin association.
Vleurick L, Pezet A, Kühn ER, Decuypere E, Edery M
Molecular endocrinology (Baltimore, Md.) 1999 Nov;13(11):1823-31
Molecular endocrinology (Baltimore, Md.) 1999 Nov;13(11):1823-31
Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic.
Tebar F, Bohlander SK, Sorkin A
Molecular biology of the cell 1999 Aug;10(8):2687-702
Molecular biology of the cell 1999 Aug;10(8):2687-702
Adaptor self-aggregation, adaptor-receptor recognition and binding of alpha-adaptin subunits to the plasma membrane contribute to recruitment of adaptor (AP2) components of clathrin-coated pits.
Chang MP, Mallet WG, Mostov KE, Brodsky FM
The EMBO journal 1993 May;12(5):2169-80
The EMBO journal 1993 May;12(5):2169-80
100-kD proteins of Golgi- and trans-Golgi network-associated coated vesicles have related but distinct membrane binding properties.
Wong DH, Brodsky FM
The Journal of cell biology 1992 Jun;117(6):1171-9
The Journal of cell biology 1992 Jun;117(6):1171-9
100-kD proteins of Golgi- and trans-Golgi network-associated coated vesicles have related but distinct membrane binding properties.
Wong DH, Brodsky FM
The Journal of cell biology 1992 Jun;117(6):1171-9
The Journal of cell biology 1992 Jun;117(6):1171-9
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
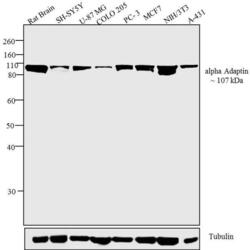
- Experimental details
- Western blot analysis was performed on tissue and membrane enriched extract (30 µg) of Rat Brain (Lane 1), SH-SY5Y (Lane 2), U-87 MG (Lane 3), COLO 205 (Lane 4), PC-3 (Lane 5), MCF7 (Lane 6), NIH/3T3 (Lane 7) and A-431 (Lane 8). The blots were probed with Anti-alpha Adaptin Mouse Monoclonal Antibody (Product # MA3-061, 2 µg/mL) and detected by chemiluminescence using Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, HRP conjugate (Product # A28177, 0.4 µg/mL, 1:2500 dilution). A ~ 107 kDa band corresponding to alpha Adaptin was observed across all cell lines and tissues tested. Known quantity of protein samples were electrophoresed using Novex® NuPAGE® 10 % Bis-Tris gel (Product # NP0302BOX), XCell SureLock™ Electrophoresis System (Product # EI0002) and Novex® Sharp Pre-Stained Protein Standard (Product # LC5800). Resolved proteins were then transferred onto a nitrocellulose membrane with Pierce™ Power Blotter System (22834). The membrane was probed with the relevant primary and secondary Antibody using iBind™ Flex Western Starter Kit (Product # SLF2000S). Chemiluminescent detection was performed using Pierce™ ECL Western Blotting Substrate (Product # 32106).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
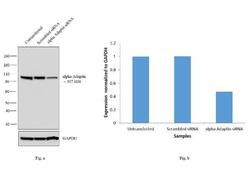
- Experimental details
- Knockdown of alpha Adaptin was achieved by transfecting U-87 MG cells with alpha Adaptin specific siRNAs (Silencer® select Product # s184). Western blot analysis (Fig a) was performed using membrane enriched extracts from the alpha Adaptin knockdown cells (lane 3), non-specific scrambled siRNA transfected cells (lane 2) and untransfected cells (lane 1). The blots were probed with Anti-alpha Adaptin Monoclonal Antibody (Product # MA3-061, 2 µg/mL) and Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, HRP conjugate (Product # A28177, 0.25 µg/mL, 1:4000 dilution). Densitometric analysis of this western blot is shown in histogram (Fig b). Decrease in signal upon siRNA mediated knock down confirms that antibody is specific to alpha Adaptin.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
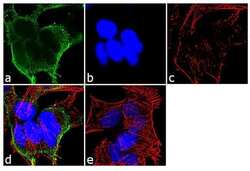
- Experimental details
- Immunofluorescent analysis of alpha Adaptin was performed using 70% confluent log phase SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with alpha Adaptin (AC1-M11) Mouse Monoclonal Antibody (Product # MA3-061) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunolocalization of alpha-adaptin in NRK cells using Product # MA3-061.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
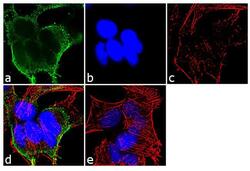
- Experimental details
- Immunofluorescent analysis of alpha Adaptin was performed using 70% confluent log phase SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with alpha Adaptin (AC1-M11) Mouse Monoclonal Antibody (Product # MA3-061) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
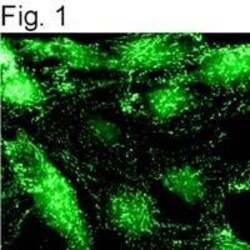
- Experimental details
- Immunolocalization of alpha-adaptin in NRK cells using Product # MA3-061.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
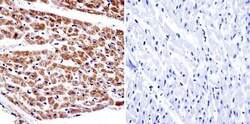
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized human heart tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a Mouse Monoclonal Antibody recognizing alpha Adaptin (Product # MA3-061) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
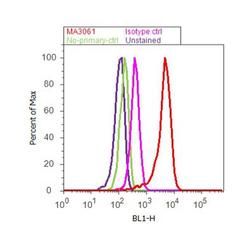
- Experimental details
- Flow cytometry analysis of alpha Adaptin was done on SH-SY5Y cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with alpha Adaptin Mouse Monoclonal Antibody (MA3-061, red histogram) or with mouse isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Rabbit Anti-Mouse Secondary Antibody (A11059) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10, 000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
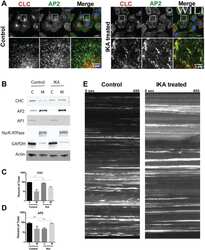
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
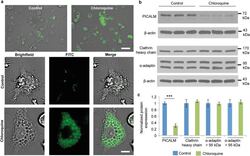
- Figure 4 Chloroquine-induced changes in Kupffer cells. ( a ) Microscopy images of chloroquine-induced vacuole formation in live cells. Cells were pretreated with 100 muM of chloroquine. Lysotracker, green. Scale bar, 50 mum (upper), 10 mum (lower). ( b ) Western blot analysis of phosphatidylinositol-binding clathrin assembly protein (PICALM), alpha-adaptin, and clathrin heavy chain expression in cells. beta-actin was used as a loading control. ( c ) Densitometric analysis of western blot results. Results represent the ratio between the protein of interest and beta-actin (mean +- s.d. of three samples), and are normalized to control cells. Statistics by Student's t -test. *** P < 0.001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
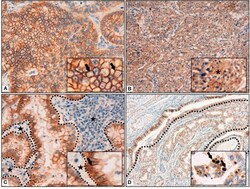
- Figure 1 (A) Membrane reinforcement of IDO2, as black arrows shown in the inset. ( B) Cytoplasmic expression of IDO2, as indicated in the inset by an asterisk. (C) IDO2 staining reinforcement at tumor-stroma interface. Dotted lines circumscribe the stroma and the black stars highlight it; IDO2 staining reinforcement is shown by the black arrow in the inset. (D) IDO2 bronchial epithelium staining (top left of the longer dotted line and circumscribed by the shorter dotted lines) and membranous tumoral staining (bottom right of the longer dotted line). Arrows in the inset highlight the nuclear staining of IDO2. Original magnification 400 x (A,C) , 200 x (B) , 100 x (D) ; insets: 600 x (A,C) , 400 x (B,D) .
 Explore
Explore Validate
Validate Learn
Learn