MA3-024
antibody from Invitrogen Antibodies
Targeting: NFATC1
NF-ATC, NFAT2, NFATc
 Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Gel shift
Gel shift Chromatin Immunoprecipitation
Chromatin Immunoprecipitation Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [91]
- Comments [0]
- Validations
- Immunocytochemistry [6]
- Immunohistochemistry [3]
- Other assay [40]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-024 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- NFATC1 Monoclonal Antibody (7A6)
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- MA3-024 detects NFATc1 (NFAT2) from transfected human, rat, mouse, and monkey cells. This antibody does not cross react with NFATc2 (NFAT1). MA3-024 has been successfully used in Western blot, immunoprecipitation, ChIP, immunofluorescence and gel shift procedures. By Western blot, this antibody detects an ~105 kDa band representing transfected NFAT2 from COS cell lysate. The MA3-024 immunogen is bacterially expressed glutathione S-transferase (GST) fusion protein containing NF-ATc1 residues 1-654. Antibodies to this protein (and modification) were previously sold as part of a Thermo Scientific Cellomics High Content Screening Kit. This replacement antibody is now recommended for researchers who need an antibody for high content cell-based assays. It has been thoroughly tested and validated for cellular immunofluorescence (IF) applications. Further optimization, including the selection of the most appropriate fluorescent Dylight conjugated secondary antibody, may have to be performed for your high content assay.
- Reactivity
- Human, Mouse, Rat
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 7A6
- Vial size
- 100 μL
- Concentration
- Conc. Not Determined
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references MiR-1224-5p modulates osteogenesis by coordinating osteoblast/osteoclast differentiation via the Rap1 signaling target ADCY2.
DYRK1A activates NFATC1 to increase glioblastoma migration.
VEGF-C/VEGFR-3 axis protects against pressure-overload induced cardiac dysfunction through regulation of lymphangiogenesis.
PDGFRα: Expression and Function during Mitral Valve Morphogenesis.
Osteopontin isoform c promotes the survival of cisplatin-treated NSCLC cells involving NFATc2-mediated suppression on calcium-induced ROS levels.
Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation.
Combined Pharmacotherapy with Alendronate and Desferoxamine Regulate the Bone Resorption and Bone Regeneration for Preventing Glucocorticoids-Induced Osteonecrosis of the Femoral Head.
Myristoylation of LMCD1 Leads to Its Species-Specific Derepression of E2F1 and NFATc1 in the Modulation of CDC6 and IL-33 Expression During Development of Vascular Lesions.
Ubiquitin-Specific Protease 34 Inhibits Osteoclast Differentiation by Regulating NF-κB Signaling.
Cigarette Smoke Extract Activates Tartrate-Resistant Acid Phosphatase-Positive Macrophage.
Calcineurin B1 Deficiency in Glial Cells Induces Mucosal Degeneration and Inflammation in Mouse Small Intestine.
Isx9 Regulates Calbindin D28K Expression in Pancreatic β Cells and Promotes β Cell Survival and Function.
Sarcolipin deletion exacerbates soleus muscle atrophy and weakness in phospholamban overexpressing mice.
NFATc1 phosphorylation by DYRK1A increases its protein stability.
Heterodimers of the transcriptional factors NFATc3 and FosB mediate tissue factor expression for 15(S)-hydroxyeicosatetraenoic acid-induced monocyte trafficking.
p115 RhoGEF activates the Rac1 GTPase signaling cascade in MCP1 chemokine-induced vascular smooth muscle cell migration and proliferation.
Effects of sarcolipin deletion on skeletal muscle adaptive responses to functional overload and unload.
Adipocyte SIRT1 controls systemic insulin sensitivity by modulating macrophages in adipose tissue.
GDF11 decreases bone mass by stimulating osteoclastogenesis and inhibiting osteoblast differentiation.
Diaphragm assessment in mice overexpressing phospholamban in slow-twitch type I muscle fibers.
The Transcription Factor Nfatc2 Regulates β-Cell Proliferation and Genes Associated with Type 2 Diabetes in Mouse and Human Islets.
Benzyl isothiocyanate inhibits IL-13 expression in human basophilic KU812 cells.
Identification of novel regulatory NFAT and TFII-I binding elements in the calbindin-D28k promoter in response to serum deprivation.
Counter-regulation of T cell effector function by differentially activated p38.
Nuclear factor of activated T cells c1 mediates p21-activated kinase 1 activation in the modulation of chemokine-induced human aortic smooth muscle cell F-actin stress fiber formation, migration, and proliferation and injury-induced vascular wall remodeling.
Cyclosporine A impairs nucleotide binding oligomerization domain (Nod1)-mediated innate antibacterial renal defenses in mice and human transplant recipients.
Protein kinase N1 is a novel substrate of NFATc1-mediated cyclin D1-CDK6 activity and modulates vascular smooth muscle cell division and migration leading to inward blood vessel wall remodeling.
The effect of the degree of sulfation of glycosaminoglycans on osteoclast function and signaling pathways.
Constitutive nuclear localization of NFAT in Foxp3+ regulatory T cells independent of calcineurin activity.
Novel interactions between NFATc1 (Nuclear Factor of Activated T cells c1) and STAT-3 (Signal Transducer and Activator of Transcription-3) mediate G protein-coupled receptor agonist, thrombin-induced biphasic expression of cyclin D1, with first phase influencing cell migration and second phase directing cell proliferation.
Nuclear orphan receptor NR2F6 directly antagonizes NFAT and RORγt binding to the Il17a promoter.
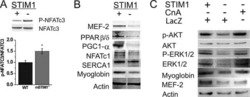
STIM1-Ca(2+) signaling is required for the hypertrophic growth of skeletal muscle in mice.
NFATc1 regulation of TRAIL expression in human intestinal cells.
Retinoic acid inhibits NFATc1 expression and osteoclast differentiation.
The amiloride derivative phenamil attenuates pulmonary vascular remodeling by activating NFAT and the bone morphogenetic protein signaling pathway.
Nuclear factor of activated T cells (NFAT) signaling regulates PTEN expression and intestinal cell differentiation.
The CCAAT/enhancer binding protein beta (C/EBPbeta) cooperates with NFAT to control expression of the calcineurin regulatory protein RCAN1-4.
Cyclin D1 is a bona fide target gene of NFATc1 and is sufficient in the mediation of injury-induced vascular wall remodeling.
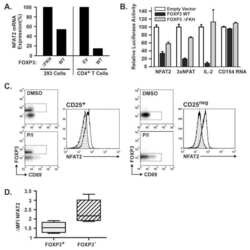
FOXP3 inhibits activation-induced NFAT2 expression in T cells thereby limiting effector cytokine expression.
NFATc1 targets cyclin A in the regulation of vascular smooth muscle cell multiplication during restenosis.
Glycogen synthase kinase 3beta regulation of nuclear factor of activated T-cells isoform c1 in the vascular smooth muscle cell response to injury.
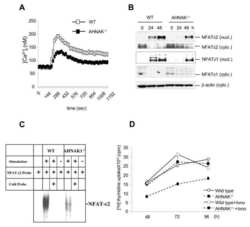
A scaffold protein, AHNAK1, is required for calcium signaling during T cell activation.
Increased oxidative properties of gastrocnemius in rats fed on a high-protein diet.
Defective IgG2a/2b class switching in PKC alpha-/- mice.
Calcineurin-mediated slow-type fiber expression and growth in reloading condition.
Activity- and calcineurin-independent nuclear shuttling of NFATc1, but not NFATc3, in adult skeletal muscle fibers.
Proteolytic regulation of nuclear factor of activated T (NFAT) c2 cells and NFAT activity by caspase-3.
Activation of the Ca(2+)/calcineurin/NFAT2 pathway controls smooth muscle cell differentiation.
Hypertrophy and transcriptional regulation induced in myogenic cell line L6-C5 by an increase of extracellular calcium.
2-Arachidonoyl-glycerol suppresses interferon-gamma production in phorbol ester/ionomycin-activated mouse splenocytes independent of CB1 or CB2.
Calcium-dependent activation of interleukin-21 gene expression in T cells.
Vasopressin-dependent myogenic cell differentiation is mediated by both Ca2+/calmodulin-dependent kinase and calcineurin pathways.
Blockade of nuclear factor of activated T cells activation signaling suppresses balloon injury-induced neointima formation in a rat carotid artery model.
Nicotine activates nuclear factor of activated T cells c2 (NFATc2) and prevents cell cycle entry in T cells.
Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis.
Interleukin (IL)-15 and IL-2 reciprocally regulate expression of the chemokine receptor CX3CR1 through selective NFAT1- and NFAT2-dependent mechanisms.
TRPC3 channels confer cellular memory of recent neuromuscular activity.
NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching.
Contribution of the calcineurin signaling pathway to overload-induced skeletal muscle fiber-type transition.
Role of NFAT proteins in IL13 gene transcription in mast cells.
Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production.
Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production.
Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells.
A potential role for nuclear factor of activated T-cells in receptor tyrosine kinase and G-protein-coupled receptor agonist-induced cell proliferation.
Maintenance of muscle mass is not dependent on the calcineurin-NFAT pathway.
The basis for TCR-mediated regulation of the IL-2 receptor alpha chain gene: role of widely separated regulatory elements.
Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation.
Cannabinol enhancement of interleukin-2 (IL-2) expression by T cells is associated with an increase in IL-2 distal nuclear factor of activated T cell activity.
Cannabinol enhancement of interleukin-2 (IL-2) expression by T cells is associated with an increase in IL-2 distal nuclear factor of activated T cell activity.
Double-stranded RNA regulates IL-4 expression.
Double-stranded RNA regulates IL-4 expression.
Preferential activation of nuclear factor of activated T cells c correlates with mouse strain susceptibility to allergic responses and interleukin-4 gene expression.
Caspase-mediated calcineurin activation contributes to IL-2 release during T cell activation.
The calcineurin-NFAT pathway and muscle fiber-type gene expression.
The calcineurin-NFAT pathway and muscle fiber-type gene expression.
c-Jun NH(2)-terminal kinase inhibits targeting of the protein phosphatase calcineurin to NFATc1.
Glucocorticoids inhibit calcium- and calcineurin-dependent activation of the human IL-4 promoter.
Evidence for suppressed activity of the transcription factor NFAT1 at its proximal binding element P0 in the IL-4 promoter associated with enhanced IL-4 gene transcription in T cells of atopic patients.
Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells.
The cyclosporin A-sensitive nuclear factor of activated T cells (NFAT) proteins are expressed in vascular smooth muscle cells. Differential localization of NFAT isoforms and induction of NFAT-mediated transcription by phospholipase C-coupled cell surface receptors.
Involvement of Jun and Rel proteins in up-regulation of interleukin-4 gene activity by the T cell accessory molecule CD28.
Histamine induces nuclear factor of activated T cell-mediated transcription and cyclosporin A-sensitive interleukin-8 mRNA expression in human umbilical vein endothelial cells.
Th2-specific protein/DNA interactions at the proximal nuclear factor-AT site contribute to the functional activity of the human IL-4 promoter.
Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway.
Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway.
Expression of NFAT-family proteins in normal human T cells.
Expression of NFAT-family proteins in normal human T cells.
Two NFAT transcription factor binding sites participate in the regulation of CD95 (Fas) ligand expression in activated human T cells.
Two NFAT transcription factor binding sites participate in the regulation of CD95 (Fas) ligand expression in activated human T cells.
NFATc3, a lymphoid-specific NFATc family member that is calcium-regulated and exhibits distinct DNA binding specificity.
NF-AT components define a family of transcription factors targeted in T-cell activation.
Hu L, Xie X, Xue H, Wang T, Panayi AC, Lin Z, Xiong Y, Cao F, Yan C, Chen L, Cheng P, Zha K, Sun Y, Liu G, Yu C, Hu Y, Tao R, Zhou W, Mi B, Liu G
Experimental & molecular medicine 2022 Jul;54(7):961-972
Experimental & molecular medicine 2022 Jul;54(7):961-972
DYRK1A activates NFATC1 to increase glioblastoma migration.
Liu H, Sun Q, Chen S, Chen L, Jia W, Zhao J, Sun X
Cancer medicine 2021 Sep;10(18):6416-6427
Cancer medicine 2021 Sep;10(18):6416-6427
VEGF-C/VEGFR-3 axis protects against pressure-overload induced cardiac dysfunction through regulation of lymphangiogenesis.
Lin QY, Zhang YL, Bai J, Liu JQ, Li HH
Clinical and translational medicine 2021 Mar;11(3):e374
Clinical and translational medicine 2021 Mar;11(3):e374
PDGFRα: Expression and Function during Mitral Valve Morphogenesis.
Moore K, Fulmer D, Guo L, Koren N, Glover J, Moore R, Gensemer C, Beck T, Morningstar J, Stairley R, Norris RA
Journal of cardiovascular development and disease 2021 Mar 13;8(3)
Journal of cardiovascular development and disease 2021 Mar 13;8(3)
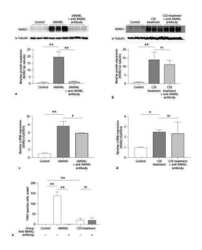
Osteopontin isoform c promotes the survival of cisplatin-treated NSCLC cells involving NFATc2-mediated suppression on calcium-induced ROS levels.
Huang J, Hu M, Niu H, Wang J, Si Y, Cheng S, Ding W
BMC cancer 2021 Jun 29;21(1):750
BMC cancer 2021 Jun 29;21(1):750
Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation.
Xian H, Liu Y, Rundberg Nilsson A, Gatchalian R, Crother TR, Tourtellotte WG, Zhang Y, Aleman-Muench GR, Lewis G, Chen W, Kang S, Luevanos M, Trudler D, Lipton SA, Soroosh P, Teijaro J, de la Torre JC, Arditi M, Karin M, Sanchez-Lopez E
Immunity 2021 Jul 13;54(7):1463-1477.e11
Immunity 2021 Jul 13;54(7):1463-1477.e11
Combined Pharmacotherapy with Alendronate and Desferoxamine Regulate the Bone Resorption and Bone Regeneration for Preventing Glucocorticoids-Induced Osteonecrosis of the Femoral Head.
Sheng H, Lao Y, Zhang S, Ding W, Lu D, Xu B
BioMed research international 2020;2020:3120458
BioMed research international 2020;2020:3120458
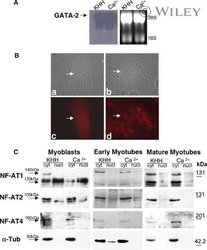
Myristoylation of LMCD1 Leads to Its Species-Specific Derepression of E2F1 and NFATc1 in the Modulation of CDC6 and IL-33 Expression During Development of Vascular Lesions.
Govatati S, Pichavaram P, Janjanam J, Guo L, Virmani R, Rao GN
Arteriosclerosis, thrombosis, and vascular biology 2020 May;40(5):1256-1274
Arteriosclerosis, thrombosis, and vascular biology 2020 May;40(5):1256-1274
Ubiquitin-Specific Protease 34 Inhibits Osteoclast Differentiation by Regulating NF-κB Signaling.
Li Q, Wang M, Xue H, Liu W, Guo Y, Xu R, Shao B, Yuan Q
Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2020 Aug;35(8):1597-1608
Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2020 Aug;35(8):1597-1608
Cigarette Smoke Extract Activates Tartrate-Resistant Acid Phosphatase-Positive Macrophage.
Igari K, Kelly MJ, Yamanouchi D
Journal of vascular research 2019;56(3):139-151
Journal of vascular research 2019;56(3):139-151
Calcineurin B1 Deficiency in Glial Cells Induces Mucosal Degeneration and Inflammation in Mouse Small Intestine.
Fujita M, Yagi T, Okura U, Tanaka J, Hirashima N, Tanaka M
Biological & pharmaceutical bulletin 2018;41(5):786-796
Biological & pharmaceutical bulletin 2018;41(5):786-796
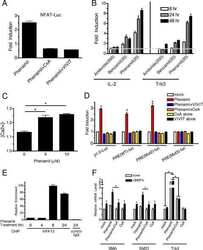
Isx9 Regulates Calbindin D28K Expression in Pancreatic β Cells and Promotes β Cell Survival and Function.
Pujol JB, Heikkila E, Savoia C, Hajibeigi A, De Marchi U, Battiprolu PK, Öz OK, Dioum EHM
International journal of molecular sciences 2018 Aug 27;19(9)
International journal of molecular sciences 2018 Aug 27;19(9)
Sarcolipin deletion exacerbates soleus muscle atrophy and weakness in phospholamban overexpressing mice.
Fajardo VA, Gamu D, Mitchell A, Bloemberg D, Bombardier E, Chambers PJ, Bellissimo C, Quadrilatero J, Tupling AR
PloS one 2017;12(3):e0173708
PloS one 2017;12(3):e0173708
NFATc1 phosphorylation by DYRK1A increases its protein stability.
Liu H, Wang K, Chen S, Sun Q, Zhang Y, Chen L, Sun X
PloS one 2017;12(2):e0172985
PloS one 2017;12(2):e0172985
Heterodimers of the transcriptional factors NFATc3 and FosB mediate tissue factor expression for 15(S)-hydroxyeicosatetraenoic acid-induced monocyte trafficking.
Kotla S, Singh NK, Kirchhofer D, Rao GN
The Journal of biological chemistry 2017 Sep 8;292(36):14885-14901
The Journal of biological chemistry 2017 Sep 8;292(36):14885-14901
p115 RhoGEF activates the Rac1 GTPase signaling cascade in MCP1 chemokine-induced vascular smooth muscle cell migration and proliferation.
Singh NK, Janjanam J, Rao GN
The Journal of biological chemistry 2017 Aug 25;292(34):14080-14091
The Journal of biological chemistry 2017 Aug 25;292(34):14080-14091
Effects of sarcolipin deletion on skeletal muscle adaptive responses to functional overload and unload.
Fajardo VA, Rietze BA, Chambers PJ, Bellissimo C, Bombardier E, Quadrilatero J, Tupling AR
American journal of physiology. Cell physiology 2017 Aug 1;313(2):C154-C161
American journal of physiology. Cell physiology 2017 Aug 1;313(2):C154-C161
Adipocyte SIRT1 controls systemic insulin sensitivity by modulating macrophages in adipose tissue.
Hui X, Zhang M, Gu P, Li K, Gao Y, Wu D, Wang Y, Xu A
EMBO reports 2017 Apr;18(4):645-657
EMBO reports 2017 Apr;18(4):645-657
GDF11 decreases bone mass by stimulating osteoclastogenesis and inhibiting osteoblast differentiation.
Liu W, Zhou L, Zhou C, Zhang S, Jing J, Xie L, Sun N, Duan X, Jing W, Liang X, Zhao H, Ye L, Chen Q, Yuan Q
Nature communications 2016 Sep 22;7:12794
Nature communications 2016 Sep 22;7:12794
Diaphragm assessment in mice overexpressing phospholamban in slow-twitch type I muscle fibers.
Fajardo VA, Smith IC, Bombardier E, Chambers PJ, Quadrilatero J, Tupling AR
Brain and behavior 2016 Jun;6(6):e00470
Brain and behavior 2016 Jun;6(6):e00470
The Transcription Factor Nfatc2 Regulates β-Cell Proliferation and Genes Associated with Type 2 Diabetes in Mouse and Human Islets.
Keller MP, Paul PK, Rabaglia ME, Stapleton DS, Schueler KL, Broman AT, Ye SI, Leng N, Brandon CJ, Neto EC, Plaisier CL, Simonett SP, Kebede MA, Sheynkman GM, Klein MA, Baliga NS, Smith LM, Broman KW, Yandell BS, Kendziorski C, Attie AD
PLoS genetics 2016 Dec;12(12):e1006466
PLoS genetics 2016 Dec;12(12):e1006466
Benzyl isothiocyanate inhibits IL-13 expression in human basophilic KU812 cells.
Tang Y, Abe N, Yoshimoto M, Zhu B, Murata Y, Nakamura Y
Bioscience, biotechnology, and biochemistry 2015;79(1):159-63
Bioscience, biotechnology, and biochemistry 2015;79(1):159-63
Identification of novel regulatory NFAT and TFII-I binding elements in the calbindin-D28k promoter in response to serum deprivation.
Hajibeigi A, Dioum EM, Guo J, Öz OK
Biochemical and biophysical research communications 2015 Sep 25;465(3):414-420
Biochemical and biophysical research communications 2015 Sep 25;465(3):414-420
Counter-regulation of T cell effector function by differentially activated p38.
Alam MS, Gaida MM, Ogawa Y, Kolios AG, Lasitschka F, Ashwell JD
The Journal of experimental medicine 2014 Jun 2;211(6):1257-70
The Journal of experimental medicine 2014 Jun 2;211(6):1257-70
Nuclear factor of activated T cells c1 mediates p21-activated kinase 1 activation in the modulation of chemokine-induced human aortic smooth muscle cell F-actin stress fiber formation, migration, and proliferation and injury-induced vascular wall remodeling.
Kundumani-Sridharan V, Singh NK, Kumar S, Gadepalli R, Rao GN
The Journal of biological chemistry 2013 Jul 26;288(30):22150-62
The Journal of biological chemistry 2013 Jul 26;288(30):22150-62
Cyclosporine A impairs nucleotide binding oligomerization domain (Nod1)-mediated innate antibacterial renal defenses in mice and human transplant recipients.
Tourneur E, Ben Mkaddem S, Chassin C, Bens M, Goujon JM, Charles N, Pellefigues C, Aloulou M, Hertig A, Monteiro RC, Girardin SE, Philpott DJ, Rondeau E, Elbim C, Werts C, Vandewalle A
PLoS pathogens 2013 Jan;9(1):e1003152
PLoS pathogens 2013 Jan;9(1):e1003152
Protein kinase N1 is a novel substrate of NFATc1-mediated cyclin D1-CDK6 activity and modulates vascular smooth muscle cell division and migration leading to inward blood vessel wall remodeling.
Singh NK, Kundumani-Sridharan V, Kumar S, Verma SK, Kotla S, Mukai H, Heckle MR, Rao GN
The Journal of biological chemistry 2012 Oct 19;287(43):36291-304
The Journal of biological chemistry 2012 Oct 19;287(43):36291-304
The effect of the degree of sulfation of glycosaminoglycans on osteoclast function and signaling pathways.
Salbach J, Kliemt S, Rauner M, Rachner TD, Goettsch C, Kalkhof S, von Bergen M, Möller S, Schnabelrauch M, Hintze V, Scharnweber D, Hofbauer LC
Biomaterials 2012 Nov;33(33):8418-29
Biomaterials 2012 Nov;33(33):8418-29
Constitutive nuclear localization of NFAT in Foxp3+ regulatory T cells independent of calcineurin activity.
Li Q, Shakya A, Guo X, Zhang H, Tantin D, Jensen PE, Chen X
Journal of immunology (Baltimore, Md. : 1950) 2012 May 1;188(9):4268-77
Journal of immunology (Baltimore, Md. : 1950) 2012 May 1;188(9):4268-77
Novel interactions between NFATc1 (Nuclear Factor of Activated T cells c1) and STAT-3 (Signal Transducer and Activator of Transcription-3) mediate G protein-coupled receptor agonist, thrombin-induced biphasic expression of cyclin D1, with first phase influencing cell migration and second phase directing cell proliferation.
Kundumani-Sridharan V, Van Quyen D, Subramani J, Singh NK, Chin YE, Rao GN
The Journal of biological chemistry 2012 Jun 29;287(27):22463-82
The Journal of biological chemistry 2012 Jun 29;287(27):22463-82
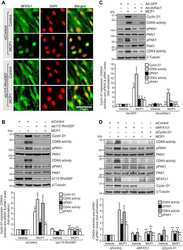
Nuclear orphan receptor NR2F6 directly antagonizes NFAT and RORγt binding to the Il17a promoter.
Hermann-Kleiter N, Meisel M, Fresser F, Thuille N, Müller M, Roth L, Katopodis A, Baier G
Journal of autoimmunity 2012 Dec;39(4):428-40
Journal of autoimmunity 2012 Dec;39(4):428-40
STIM1-Ca(2+) signaling is required for the hypertrophic growth of skeletal muscle in mice.
Li T, Finch EA, Graham V, Zhang ZS, Ding JD, Burch J, Oh-hora M, Rosenberg P
Molecular and cellular biology 2012 Aug;32(15):3009-17
Molecular and cellular biology 2012 Aug;32(15):3009-17
NFATc1 regulation of TRAIL expression in human intestinal cells.
Wang Q, Zhou Y, Weiss HL, Chow CW, Evers BM
PloS one 2011;6(5):e19882
PloS one 2011;6(5):e19882
Retinoic acid inhibits NFATc1 expression and osteoclast differentiation.
Balkan W, Rodríguez-Gonzalez M, Pang M, Fernandez I, Troen BR
Journal of bone and mineral metabolism 2011 Nov;29(6):652-61
Journal of bone and mineral metabolism 2011 Nov;29(6):652-61
The amiloride derivative phenamil attenuates pulmonary vascular remodeling by activating NFAT and the bone morphogenetic protein signaling pathway.
Chan MC, Weisman AS, Kang H, Nguyen PH, Hickman T, Mecker SV, Hill NS, Lagna G, Hata A
Molecular and cellular biology 2011 Feb;31(3):517-30
Molecular and cellular biology 2011 Feb;31(3):517-30
Nuclear factor of activated T cells (NFAT) signaling regulates PTEN expression and intestinal cell differentiation.
Wang Q, Zhou Y, Jackson LN, Johnson SM, Chow CW, Evers BM
Molecular biology of the cell 2011 Feb 1;22(3):412-20
Molecular biology of the cell 2011 Feb 1;22(3):412-20
The CCAAT/enhancer binding protein beta (C/EBPbeta) cooperates with NFAT to control expression of the calcineurin regulatory protein RCAN1-4.
Oh M, Dey A, Gerard RD, Hill JA, Rothermel BA
The Journal of biological chemistry 2010 May 28;285(22):16623-31
The Journal of biological chemistry 2010 May 28;285(22):16623-31
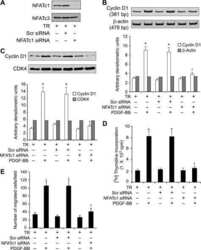
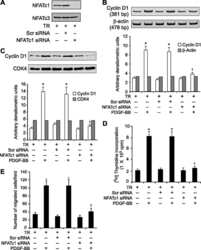
Cyclin D1 is a bona fide target gene of NFATc1 and is sufficient in the mediation of injury-induced vascular wall remodeling.
Karpurapu M, Wang D, Van Quyen D, Kim TK, Kundumani-Sridharan V, Pulusani S, Rao GN
The Journal of biological chemistry 2010 Jan 29;285(5):3510-23
The Journal of biological chemistry 2010 Jan 29;285(5):3510-23
FOXP3 inhibits activation-induced NFAT2 expression in T cells thereby limiting effector cytokine expression.
Torgerson TR, Genin A, Chen C, Zhang M, Zhou B, Añover-Sombke S, Frank MB, Dozmorov I, Ocheltree E, Kulmala P, Centola M, Ochs HD, Wells AD, Cron RQ
Journal of immunology (Baltimore, Md. : 1950) 2009 Jul 15;183(2):907-15
Journal of immunology (Baltimore, Md. : 1950) 2009 Jul 15;183(2):907-15
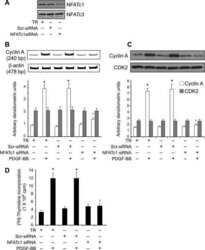
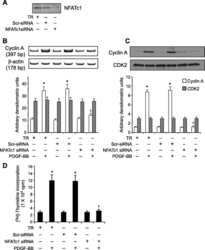
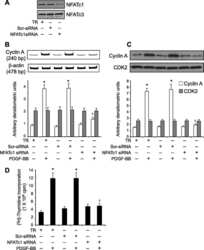
NFATc1 targets cyclin A in the regulation of vascular smooth muscle cell multiplication during restenosis.
Karpurapu M, Wang D, Singh NK, Li Q, Rao GN
The Journal of biological chemistry 2008 Sep 26;283(39):26577-90
The Journal of biological chemistry 2008 Sep 26;283(39):26577-90
Glycogen synthase kinase 3beta regulation of nuclear factor of activated T-cells isoform c1 in the vascular smooth muscle cell response to injury.
Chow W, Hou G, Bendeck MP
Experimental cell research 2008 Oct 1;314(16):2919-29
Experimental cell research 2008 Oct 1;314(16):2919-29
A scaffold protein, AHNAK1, is required for calcium signaling during T cell activation.
Matza D, Badou A, Kobayashi KS, Goldsmith-Pestana K, Masuda Y, Komuro A, McMahon-Pratt D, Marchesi VT, Flavell RA
Immunity 2008 Jan;28(1):64-74
Immunity 2008 Jan;28(1):64-74
Increased oxidative properties of gastrocnemius in rats fed on a high-protein diet.
Nakazato K, Song H
The Journal of nutritional biochemistry 2008 Jan;19(1):26-32
The Journal of nutritional biochemistry 2008 Jan;19(1):26-32
Defective IgG2a/2b class switching in PKC alpha-/- mice.
Pfeifhofer C, Gruber T, Letschka T, Thuille N, Lutz-Nicoladoni C, Hermann-Kleiter N, Braun U, Leitges M, Baier G
Journal of immunology (Baltimore, Md. : 1950) 2006 May 15;176(10):6004-11
Journal of immunology (Baltimore, Md. : 1950) 2006 May 15;176(10):6004-11
Calcineurin-mediated slow-type fiber expression and growth in reloading condition.
Miyazaki M, Hitomi Y, Kizaki T, Ohno H, Katsumura T, Haga S, Takemasa T
Medicine and science in sports and exercise 2006 Jun;38(6):1065-72
Medicine and science in sports and exercise 2006 Jun;38(6):1065-72
Activity- and calcineurin-independent nuclear shuttling of NFATc1, but not NFATc3, in adult skeletal muscle fibers.
Shen T, Liu Y, Cseresnyés Z, Hawkins A, Randall WR, Schneider MF
Molecular biology of the cell 2006 Apr;17(4):1570-82
Molecular biology of the cell 2006 Apr;17(4):1570-82
Proteolytic regulation of nuclear factor of activated T (NFAT) c2 cells and NFAT activity by caspase-3.
Wu W, Misra RS, Russell JQ, Flavell RA, Rincón M, Budd RC
The Journal of biological chemistry 2006 Apr 21;281(16):10682-90
The Journal of biological chemistry 2006 Apr 21;281(16):10682-90
Activation of the Ca(2+)/calcineurin/NFAT2 pathway controls smooth muscle cell differentiation.
Larrieu D, Thiébaud P, Duplàa C, Sibon I, Thézé N, Lamazière JM
Experimental cell research 2005 Oct 15;310(1):166-75
Experimental cell research 2005 Oct 15;310(1):166-75
Hypertrophy and transcriptional regulation induced in myogenic cell line L6-C5 by an increase of extracellular calcium.
De Arcangelis V, Coletti D, Canato M, Molinaro M, Adamo S, Reggiani C, Naro F
Journal of cellular physiology 2005 Mar;202(3):787-95
Journal of cellular physiology 2005 Mar;202(3):787-95
2-Arachidonoyl-glycerol suppresses interferon-gamma production in phorbol ester/ionomycin-activated mouse splenocytes independent of CB1 or CB2.
Kaplan BL, Ouyang Y, Rockwell CE, Rao GK, Kaminski NE
Journal of leukocyte biology 2005 Jun;77(6):966-74
Journal of leukocyte biology 2005 Jun;77(6):966-74
Calcium-dependent activation of interleukin-21 gene expression in T cells.
Kim HP, Korn LL, Gamero AM, Leonard WJ
The Journal of biological chemistry 2005 Jul 1;280(26):25291-7
The Journal of biological chemistry 2005 Jul 1;280(26):25291-7
Vasopressin-dependent myogenic cell differentiation is mediated by both Ca2+/calmodulin-dependent kinase and calcineurin pathways.
Scicchitano BM, Spath L, Musarò A, Molinaro M, Rosenthal N, Nervi C, Adamo S
Molecular biology of the cell 2005 Aug;16(8):3632-41
Molecular biology of the cell 2005 Aug;16(8):3632-41
Blockade of nuclear factor of activated T cells activation signaling suppresses balloon injury-induced neointima formation in a rat carotid artery model.
Liu Z, Zhang C, Dronadula N, Li Q, Rao GN
The Journal of biological chemistry 2005 Apr 15;280(15):14700-8
The Journal of biological chemistry 2005 Apr 15;280(15):14700-8
Nicotine activates nuclear factor of activated T cells c2 (NFATc2) and prevents cell cycle entry in T cells.
Frazer-Abel AA, Baksh S, Fosmire SP, Willis D, Pierce AM, Meylemans H, Linthicum DS, Burakoff SJ, Coons T, Bellgrau D, Modiano JF
The Journal of pharmacology and experimental therapeutics 2004 Nov;311(2):758-69
The Journal of pharmacology and experimental therapeutics 2004 Nov;311(2):758-69
Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis.
Minami T, Horiuchi K, Miura M, Abid MR, Takabe W, Noguchi N, Kohro T, Ge X, Aburatani H, Hamakubo T, Kodama T, Aird WC
The Journal of biological chemistry 2004 Nov 26;279(48):50537-54
The Journal of biological chemistry 2004 Nov 26;279(48):50537-54
Interleukin (IL)-15 and IL-2 reciprocally regulate expression of the chemokine receptor CX3CR1 through selective NFAT1- and NFAT2-dependent mechanisms.
Barlic J, McDermott DH, Merrell MN, Gonzales J, Via LE, Murphy PM
The Journal of biological chemistry 2004 Nov 19;279(47):48520-34
The Journal of biological chemistry 2004 Nov 19;279(47):48520-34
TRPC3 channels confer cellular memory of recent neuromuscular activity.
Rosenberg P, Hawkins A, Stiber J, Shelton JM, Hutcheson K, Bassel-Duby R, Shin DM, Yan Z, Williams RS
Proceedings of the National Academy of Sciences of the United States of America 2004 Jun 22;101(25):9387-92
Proceedings of the National Academy of Sciences of the United States of America 2004 Jun 22;101(25):9387-92
NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching.
McCullagh KJ, Calabria E, Pallafacchina G, Ciciliot S, Serrano AL, Argentini C, Kalhovde JM, Lømo T, Schiaffino S
Proceedings of the National Academy of Sciences of the United States of America 2004 Jul 20;101(29):10590-5
Proceedings of the National Academy of Sciences of the United States of America 2004 Jul 20;101(29):10590-5
Contribution of the calcineurin signaling pathway to overload-induced skeletal muscle fiber-type transition.
Miyazaki M, Hitomi Y, Kizaki T, Ohno H, Haga S, Takemasa T
Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 2004 Dec;55(4):751-64
Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 2004 Dec;55(4):751-64
Role of NFAT proteins in IL13 gene transcription in mast cells.
Monticelli S, Solymar DC, Rao A
The Journal of biological chemistry 2004 Aug 27;279(35):36210-8
The Journal of biological chemistry 2004 Aug 27;279(35):36210-8
Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production.
Cannon JL, Burkhardt JK
Journal of immunology (Baltimore, Md. : 1950) 2004 Aug 1;173(3):1658-62
Journal of immunology (Baltimore, Md. : 1950) 2004 Aug 1;173(3):1658-62
Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production.
Cannon JL, Burkhardt JK
Journal of immunology (Baltimore, Md. : 1950) 2004 Aug 1;173(3):1658-62
Journal of immunology (Baltimore, Md. : 1950) 2004 Aug 1;173(3):1658-62
Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells.
Pfeifhofer C, Kofler K, Gruber T, Tabrizi NG, Lutz C, Maly K, Leitges M, Baier G
The Journal of experimental medicine 2003 Jun 2;197(11):1525-35
The Journal of experimental medicine 2003 Jun 2;197(11):1525-35
A potential role for nuclear factor of activated T-cells in receptor tyrosine kinase and G-protein-coupled receptor agonist-induced cell proliferation.
Yellaturu CR, Ghosh SK, Rao RK, Jennings LK, Hassid A, Rao GN
The Biochemical journal 2002 Nov 15;368(Pt 1):183-90
The Biochemical journal 2002 Nov 15;368(Pt 1):183-90
Maintenance of muscle mass is not dependent on the calcineurin-NFAT pathway.
Dupont-Versteegden EE, Knox M, Gurley CM, Houlé JD, Peterson CA
American journal of physiology. Cell physiology 2002 Jun;282(6):C1387-95
American journal of physiology. Cell physiology 2002 Jun;282(6):C1387-95
The basis for TCR-mediated regulation of the IL-2 receptor alpha chain gene: role of widely separated regulatory elements.
Kim HP, Leonard WJ
The EMBO journal 2002 Jun 17;21(12):3051-9
The EMBO journal 2002 Jun 17;21(12):3051-9
Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation.
Diehl S, Chow CW, Weiss L, Palmetshofer A, Twardzik T, Rounds L, Serfling E, Davis RJ, Anguita J, Rincón M
The Journal of experimental medicine 2002 Jul 1;196(1):39-49
The Journal of experimental medicine 2002 Jul 1;196(1):39-49
Cannabinol enhancement of interleukin-2 (IL-2) expression by T cells is associated with an increase in IL-2 distal nuclear factor of activated T cell activity.
Jan TR, Rao GK, Kaminski NE
Molecular pharmacology 2002 Feb;61(2):446-54
Molecular pharmacology 2002 Feb;61(2):446-54
Cannabinol enhancement of interleukin-2 (IL-2) expression by T cells is associated with an increase in IL-2 distal nuclear factor of activated T cell activity.
Jan TR, Rao GK, Kaminski NE
Molecular pharmacology 2002 Feb;61(2):446-54
Molecular pharmacology 2002 Feb;61(2):446-54
Double-stranded RNA regulates IL-4 expression.
Kehoe KE, Brown MA, Imani F
Journal of immunology (Baltimore, Md. : 1950) 2001 Sep 1;167(5):2496-501
Journal of immunology (Baltimore, Md. : 1950) 2001 Sep 1;167(5):2496-501
Double-stranded RNA regulates IL-4 expression.
Kehoe KE, Brown MA, Imani F
Journal of immunology (Baltimore, Md. : 1950) 2001 Sep 1;167(5):2496-501
Journal of immunology (Baltimore, Md. : 1950) 2001 Sep 1;167(5):2496-501
Preferential activation of nuclear factor of activated T cells c correlates with mouse strain susceptibility to allergic responses and interleukin-4 gene expression.
Keen JC, Sholl L, Wills-Karp M, Georas SN
American journal of respiratory cell and molecular biology 2001 Jan;24(1):58-65
American journal of respiratory cell and molecular biology 2001 Jan;24(1):58-65
Caspase-mediated calcineurin activation contributes to IL-2 release during T cell activation.
Mukerjee N, McGinnis KM, Gnegy ME, Wang KK
Biochemical and biophysical research communications 2001 Aug 3;285(5):1192-9
Biochemical and biophysical research communications 2001 Aug 3;285(5):1192-9
The calcineurin-NFAT pathway and muscle fiber-type gene expression.
Swoap SJ, Hunter RB, Stevenson EJ, Felton HM, Kansagra NV, Lang JM, Esser KA, Kandarian SC
American journal of physiology. Cell physiology 2000 Oct;279(4):C915-24
American journal of physiology. Cell physiology 2000 Oct;279(4):C915-24
The calcineurin-NFAT pathway and muscle fiber-type gene expression.
Swoap SJ, Hunter RB, Stevenson EJ, Felton HM, Kansagra NV, Lang JM, Esser KA, Kandarian SC
American journal of physiology. Cell physiology 2000 Oct;279(4):C915-24
American journal of physiology. Cell physiology 2000 Oct;279(4):C915-24
c-Jun NH(2)-terminal kinase inhibits targeting of the protein phosphatase calcineurin to NFATc1.
Chow CW, Dong C, Flavell RA, Davis RJ
Molecular and cellular biology 2000 Jul;20(14):5227-34
Molecular and cellular biology 2000 Jul;20(14):5227-34
Glucocorticoids inhibit calcium- and calcineurin-dependent activation of the human IL-4 promoter.
Chen R, Burke TF, Cumberland JE, Brummet M, Beck LA, Casolaro V, Georas SN
Journal of immunology (Baltimore, Md. : 1950) 2000 Jan 15;164(2):825-32
Journal of immunology (Baltimore, Md. : 1950) 2000 Jan 15;164(2):825-32
Evidence for suppressed activity of the transcription factor NFAT1 at its proximal binding element P0 in the IL-4 promoter associated with enhanced IL-4 gene transcription in T cells of atopic patients.
Wierenga EA, Walchner M, Kick G, Kapsenberg ML, Weiss EH, Messer G
International immunology 1999 Feb;11(2):297-306
International immunology 1999 Feb;11(2):297-306
Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells.
Abbott KL, Friday BB, Thaloor D, Murphy TJ, Pavlath GK
Molecular biology of the cell 1998 Oct;9(10):2905-16
Molecular biology of the cell 1998 Oct;9(10):2905-16
The cyclosporin A-sensitive nuclear factor of activated T cells (NFAT) proteins are expressed in vascular smooth muscle cells. Differential localization of NFAT isoforms and induction of NFAT-mediated transcription by phospholipase C-coupled cell surface receptors.
Boss V, Abbott KL, Wang XF, Pavlath GK, Murphy TJ
The Journal of biological chemistry 1998 Jul 31;273(31):19664-71
The Journal of biological chemistry 1998 Jul 31;273(31):19664-71
Involvement of Jun and Rel proteins in up-regulation of interleukin-4 gene activity by the T cell accessory molecule CD28.
Li-Weber M, Giasi M, Krammer PH
The Journal of biological chemistry 1998 Dec 4;273(49):32460-6
The Journal of biological chemistry 1998 Dec 4;273(49):32460-6
Histamine induces nuclear factor of activated T cell-mediated transcription and cyclosporin A-sensitive interleukin-8 mRNA expression in human umbilical vein endothelial cells.
Boss V, Wang X, Koppelman LF, Xu K, Murphy TJ
Molecular pharmacology 1998 Aug;54(2):264-72
Molecular pharmacology 1998 Aug;54(2):264-72
Th2-specific protein/DNA interactions at the proximal nuclear factor-AT site contribute to the functional activity of the human IL-4 promoter.
Li-Weber M, Salgame P, Hu C, Davydov IV, Laur O, Klevenz S, Krammer PH
Journal of immunology (Baltimore, Md. : 1950) 1998 Aug 1;161(3):1380-9
Journal of immunology (Baltimore, Md. : 1950) 1998 Aug 1;161(3):1380-9
Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway.
Chow CW, Rincón M, Cavanagh J, Dickens M, Davis RJ
Science (New York, N.Y.) 1997 Nov 28;278(5343):1638-41
Science (New York, N.Y.) 1997 Nov 28;278(5343):1638-41
Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway.
Chow CW, Rincón M, Cavanagh J, Dickens M, Davis RJ
Science (New York, N.Y.) 1997 Nov 28;278(5343):1638-41
Science (New York, N.Y.) 1997 Nov 28;278(5343):1638-41
Expression of NFAT-family proteins in normal human T cells.
Lyakh L, Ghosh P, Rice NR
Molecular and cellular biology 1997 May;17(5):2475-84
Molecular and cellular biology 1997 May;17(5):2475-84
Expression of NFAT-family proteins in normal human T cells.
Lyakh L, Ghosh P, Rice NR
Molecular and cellular biology 1997 May;17(5):2475-84
Molecular and cellular biology 1997 May;17(5):2475-84
Two NFAT transcription factor binding sites participate in the regulation of CD95 (Fas) ligand expression in activated human T cells.
Latinis KM, Norian LA, Eliason SL, Koretzky GA
The Journal of biological chemistry 1997 Dec 12;272(50):31427-34
The Journal of biological chemistry 1997 Dec 12;272(50):31427-34
Two NFAT transcription factor binding sites participate in the regulation of CD95 (Fas) ligand expression in activated human T cells.
Latinis KM, Norian LA, Eliason SL, Koretzky GA
The Journal of biological chemistry 1997 Dec 12;272(50):31427-34
The Journal of biological chemistry 1997 Dec 12;272(50):31427-34
NFATc3, a lymphoid-specific NFATc family member that is calcium-regulated and exhibits distinct DNA binding specificity.
Ho SN, Thomas DJ, Timmerman LA, Li X, Francke U, Crabtree GR
The Journal of biological chemistry 1995 Aug 25;270(34):19898-907
The Journal of biological chemistry 1995 Aug 25;270(34):19898-907
NF-AT components define a family of transcription factors targeted in T-cell activation.
Northrop JP, Ho SN, Chen L, Thomas DJ, Timmerman LA, Nolan GP, Admon A, Crabtree GR
Nature 1994 Jun 9;369(6480):497-502
Nature 1994 Jun 9;369(6480):497-502
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
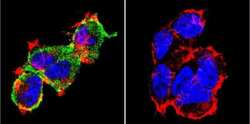
- Experimental details
- Immunofluorescent analysis of NFATc1 using NFATc1 Monoclonal Antibody (7A6) (Product # MA3-024) shows staining in 293Cells. NFATc1 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing NFATc1 (Product # MA3-024) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
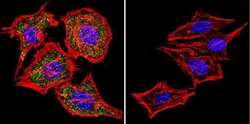
- Experimental details
- Immunofluorescent analysis of NFATc1 using NFATc1 Monoclonal Antibody (7A6) (Product # MA3-024) shows staining in Hela Cells. NFATc1 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing NFATc1 (Product # MA3-024) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
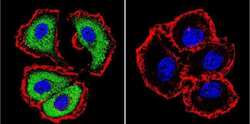
- Experimental details
- Immunofluorescent analysis of NFATc1 using NFATc1 Monoclonal Antibody (7A6) (Product # MA3-024) shows staining in MCF-7 Cells. NFATc1 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing NFATc1 (Product # MA3-024) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
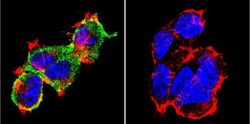
- Experimental details
- Immunofluorescent analysis of NFATc1 using NFATc1 Monoclonal Antibody (7A6) (Product # MA3-024) shows staining in 293Cells. NFATc1 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing NFATc1 (Product # MA3-024) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
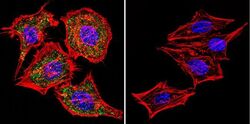
- Experimental details
- Immunofluorescent analysis of NFATc1 using NFATc1 Monoclonal Antibody (7A6) (Product # MA3-024) shows staining in Hela Cells. NFATc1 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing NFATc1 (Product # MA3-024) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
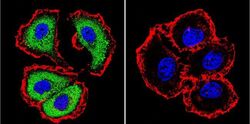
- Experimental details
- Immunofluorescent analysis of NFATc1 using NFATc1 Monoclonal Antibody (7A6) (Product # MA3-024) shows staining in MCF-7 Cells. NFATc1 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing NFATc1 (Product # MA3-024) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
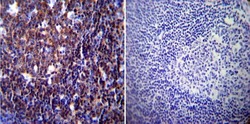
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized Human tonsil tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing NFATc1 (Product # MA3-024) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
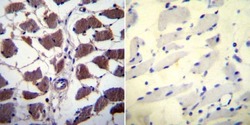
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized Human skeletal muscle tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a mouse monoclonal antibody recognizing NFATc1 (Product # MA3-024) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
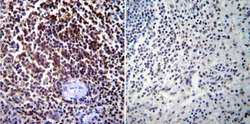
- Immunohistochemistry was performed on normal biopsies of deparaffinized Human spleen tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a mouse monoclonal antibody recognizing NFATc1 (Product # MA3-024) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
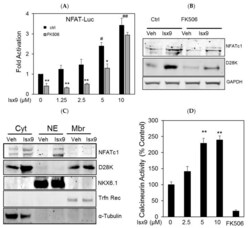
- Figure 2 Isx9 increased NFAT transcriptional activity. ( A ) Dose-dependent activation of the NFAT reporter in beta cells after 24 h treatment with increasing dose of Isx9 in the presence or absence of 0.3 uM FK506, mean +- SEM of three independent experiments in triplicates, # p < 0.05 and ## p < 0.01 Isx9 versus non-treated cells; * p < 0.05, ** p < 0.01 effect of FK506 treatment versus control for each Isx9 dose. ( B ) Immunoblotting of NFATc1 and D28K in MIN6 whole cell extract after 48 h treatment with vehicle DMSO (Veh) or 10 uM Isx9 in the presence or absence of calcineurin inhibitor FK506. ( C ) Subcellular fractionation (Pierce) of MIN6 cells treated with Isx9 or vehicle into cytoplasmic (Cyt), nuclear (NE) and membrane (Mbr) fractions followed by immunoblotting of NFATc1 and D28K. alpha-Tubulin, Nkx6.1, and Transferrin receptors are used as loading controls. ( D ) Calcineurin activity in MIN6 represented as % of untreated cells treated with increasing doses of Isx9, FK506 is used as a negative control, mean +- SEM of three independent experiments in triplicates, ** p < 0.01 vs. control. ( E ) Immunoblotting of phospho-Creb1-Ser 133, D28K, and GAPDH after increasing dose of Isx9 for 24 h or ( F ) after 8 h and 24 h treatment with 10 uM Isx9 in MIN6 cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
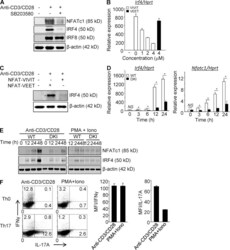
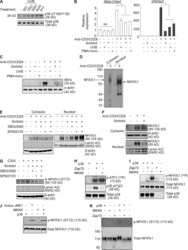
- Figure 1. p38 Alternative activation is required for TCR-induced expression of NFATc1 and IRF4 in CD4 + T cells. (A) Purified CD4 + T cells were stimulated with anti-CD3/CD28 in the presence or absence of SB203580 and immunoblotted for the indicated transcription factors 48 h later. (B and C) Purified CD4 + T cells were stimulated with anti-CD3/CD28 in the presence of 11R-VIVIT or 11R-VEET. Irf4 mRNA (B) and protein (C) were determined 24 h later. (D and E) Purified CD4 + T cells from WT and DKI mice were stimulated with anti-CD3/CD28 or PMA plus ionomycin, as indicated for the indicated times and analyzed for expression of Nfatc1 and Irf4 mRNA (D) and protein (E). Results shown in A-E are representative of three independent experiments each. (F) Naive CD4 + T cells were stimulated with either anti-CD3/CD28 or PMA and ionomycin under neutral or Th17-skewing conditions for 3 d. IL-17A and IFN-gamma expression were determined by intracellular staining and flow cytometry after restimulation with PMA and ionomycin. Results shown in the left panel are representative of three independent experiments and bar graphs in the right panel show the mean +- SEM of all three. *, P < 0.05 (unpaired two-tailed Student's t test).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 3. MAPK-cascade activated p38 inhibits functions distal to alternatively activated p38. (A) Purified CD4 + T cells were cultured overnight without stimulation, and then treated with either UV (50 J/m 2 or 100 J/m 2 or 200 J/m 2 ), 0.6 M sorbitol for 30 min, or PMA (10 ng/ml) and ionomycin (1 ug/ml) for 1 h. Cell lysates were immunoblotted with anti-phospho-p38. The results are representative of three independent experiments. (B) Purified CD4 + T cells were treated with either 0.6 M sorbitol for 30 min, 50 J/m 2 UV, or PMA and ionomycin were stimulated with anti-CD3/CD28 for 24 h. The expression of Nfatc1 and Irf4 was determined by quantitative real-time PCR. Results are the mean +- SEM of three independent experiments. *, P < 0.05 (unpaired two-tailed Student's t test). (C) Cells were treated as in B, and IRF4 expression was determined by Western blot. The results are representative of two independent experiments. (D) Freshly purified T cells were stimulated or not with anti-CD3/CD28 for 48 h. Cytosolic and nuclear fractions were separated in a low percentage SDS-PAGE (8%) gel and immunoblotted for NFATc1. The results are representative of three independent experiments. (E) CD4 + T cells were stimulated for 48 h with anti-CD3/CD28, and then treated with sorbitol in the presence or absence of SB203580 and/or SP600125 for 30 min, rested for 1 h, then immunoblotted for NFATc1 in cytosolic and nuclear fractions. Results are representative of three independent experime
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
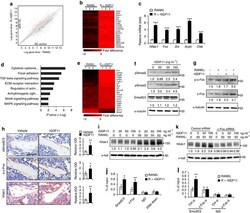
- Figure 4 GDF11 activates Smad2/3-dependent TGF-beta pathway. ( a ) Gene expression levels of the BMMs stimulated without or with 100 ng ml -1 rGDF11 for 24 h (three biological replicates per group). 467 genes (red) were upregulated and 679 genes (black) were downregulated. 100 ng ml -1 M-CSF was present in all settings. ( b ) Heatmap of the osteoclastogenesis associated genes. ( c ) Quantitative RT-PCR confirmed the increased expression of osteoclast key marker genes. Results are shown as mean+-s.d.; n =3. *** P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
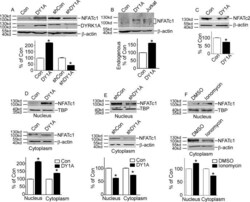
- Fig 1 DYRK1A increased NFATc1 protein expression and its nuclear translocation. A. HEK293 cells were transfected with p-NFATc1mycFLAG and pCMV-DYRK1A or pGFP-V-RS-shDYRK1A. NFATc1 expression was detected by mouse anti-FLAG antibody immunoblotting. DYRK1A was blotted with anti-DYRK1A antibody. B. Endogenous NFATc1 was detected by anti-NFATc1 antibody in HEK293 cells transfected with pCMV-DYRK1A. An activated Jurkat T cells treated with 5uM ionomycin plus 500uM calcium chloride for 12 hours was used as positive control. C. HEK293 cells were co-transfected with pWT-NFATc2 and pCMV-DYRK1A. Anti-HA antibody was used to detect NFATc2. D and E. HEK293 cells were co-transfected with p-NFATc1mycFLAG and pCMV-DYRK1A orpGFP-V-RS-shDYRK1A. Mouse anti-FLAG antibody was used to detect NFATc1 protein in nucleus or cytoplasm.Beta-actin was used as cytoplasmic control and TBP was used as nuclear protein loading control. F. HEK293 cells were treated with ionomycin. Mouse anti-FLAG antibody was used to detect NFATc1 protein in nucleus or cytoplasm beta-actin was used as cytoplasmic control and TBP was used as nuclear protein loading control. Values represent means+-SD, n = 3. DY1A means DYRK1A. shDY1A means shDYRK1A.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig 4 Identification of DYRK1A-targeted motifs in NFATc1 protein. A. Diagram showed NFATs sequence alignment. Sequences of NFATc1 (NP_765978), NFATc2 (NP_775114), NFATc3 (NP_775118), and NFATc4 (NP_001129494) were aligned using ClustalW2 software. Conserved regions among NFATs are underlined. Conserved serine residues in four NFATs are shown in blue. The DYRK1A target motifs are labeled as yellow highlights. The DYRK1A phosphorylation serine residues were in red and bold. B. Schematic diagram represents the domain structure of NFATc1 and location of five deletion constructs. AD: activation domain. SPRIEIT: calcineurin binding motif. SP: sp repeats region; SRR: serine-rich region; NLS: nuclear localization sequence. C. Five deletion constructs of NFATc1 were co-transfected with pCMV-DYRK1A in HEK293 cells. D. Deletionconstructs of NFATc1 was co-transfected with pCMV-DYRK1A in HEK293 cells. Anti-DYRK1A antibody was used as the immunoprecipitation antibody, and anti-FLAG antibody was used to detect NFATc1 in immunoblotting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 5 Immunofluorescence images of GSK-3 beta ((a) red: nucleus; green: GSK-3 beta ) and beta -catenin ((b) blue: nucleus; green: beta -catenin). Scale bar = 200 mu m.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
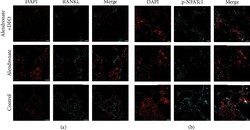
- Figure 6 Immunofluorescence images of RANKL ((a) red: nucleus; light blue: RANKL) and NFATc1 ((b) blue: nucleus; light blue: NFATc1). Scale bar = 200 mu m.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
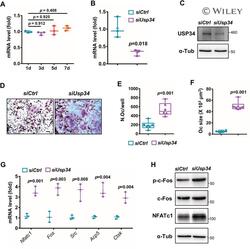
- Experimental details
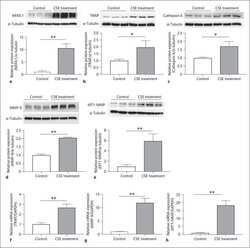
- Usp34 knockdown results in enhanced osteoclast differentiation of RAW264.7 cells. ( A ) qRT-PCR analysis of the expression of Usp34 during osteoclast induction of RAW264.7 cells. n = 3. ( B ) qRT-PCR and ( C ) Western blot confirmed successful knockdown of Usp34 in RAW264.7 cells. n = 3. ( D ) Representative images of TRAP staining. RAW264.7 cells were transfected with siRNA and stimulated with 50 ng/mL RANKL for 3 days. Scale bar = 100 mum. ( E ) Quantification of osteoclast number and ( F ) quantification of osteoclast size. No less than three nuclei in TRAP-positive cells were counted as osteoclasts. n = 6. ( G ) qRT-PCR detected the mRNA expression of osteoclast markers in the presence of RANKL for 2 days. n = 3. ( H ) Western blot analysis of indicated osteoclast markers after 2-day osteoclast induction. All quantified data are presented by box-plot with median value (cross), interquartile range (box), minimum/maximum (whiskers), and symbols representing all data points. The p values were calculated by one-way ANOVA with Tukey's post hoc test ( A ) or two-tailed Student's t test ( B , E , F , G ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
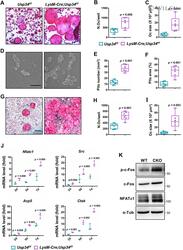
- Experimental details
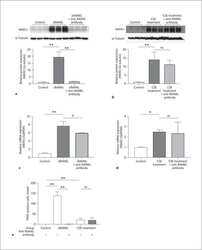
- Loss of USP34 increases osteoclastogenesis in vitro. ( A ) TRAP staining of multinucleated cells. Scale bar = 100 mum. ( B , C ) Quantification of osteoclasts number and size after 6-day osteoclast induction. n = 6. ( D ) Representative images of bone resorption pits after 7-day M-CSF (50 ng/mL) and RANKL (50 ng/mL) treatment. Scale bar = 100 mum. ( E , F ) Quantification of pits number and pits area after osteoclast induction. n = 6. ( G ) TRAP staining after BMMs and calvarial osteoblasts coculture. Cells were cultured with 1,25-(OH) 2 D 3 and PGE2 for 5 days. Scale bar = 100 mum. ( H , I ) Quantification of osteoclasts number and size after 5-day induction. n = 6. ( J ) qRT-PCR analysis of osteoclast markers for the indicated time periods. n = 3. ( K ) BMMs were treated with RANKL for 2 days and immunoblotted with indicated antibodies. All quantified data are presented by box-plot with median value (cross), interquartile range (box), minimum/maximum (whiskers), and symbols representing all data points. The p values were calculated by two-tailed Student's t test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
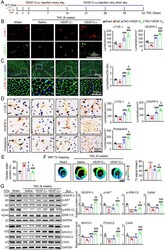
- Experimental details
- FIGURE 4 Administration of VEGF-C 156S improves cardiac lymphangiogenesis and edema after pressure overload. (A) Schematic of the methods for treatment of WT mice with saline or VEGF-C 156S at doses of 33 (VEGF-C -L ) and 100 (VEGF-C -H ) ng/g and TAC for 6 weeks. (B) Heart sections stained with an anti-LYVE-1 (red) or anti-VEGFR-3 antibody (green) (left, scale bar: 50 mum) and quantification of LYVE-1 + or VEGFR-3 + lymphatics (right, n = 6). (C) Heart sections stained with an anti-LYVE-1 antibody (red), TRITC-labeled WGA (green) and DAPI (blue) (left, scale bar: 50 mum) and the LYVE-1 + vessel to CM ratio (right, n = 6). (D) Immunohistochemical staining of heart sections with an antibody against LYVE-1, VEGFR-3 or Podoplanin (left, scale bar: 50 mum) and quantification of LYVE-1 + , VEGFR-3 + , and Podoplanin + lymphatic vessels (right, n = 6). (E) Gravimetric assessment of cardiac water content (%) as determined by the cardiac dry weight to wet weight ( n = 6). (F) Representative color-coded images of cardiac MRI T2 mapping in which the turquoise/blue areas are normal tissues and the red/yellow areas exhibit cardiac edema (left) and MRI-based quantification of cardiac water content (T2 map signal intensity, msec) (right, n = 4). (G) Immunoblot analysis of the VEGFR-3, p-AKT, AKT, p-ERK1/2, ERK1/2, CaNA, NFATc1, FOX2C, and CX43 proteins in the heart (left) and quantification of these proteins (right, n = 4). GAPDH was used as an internal control. The data are presented as t
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
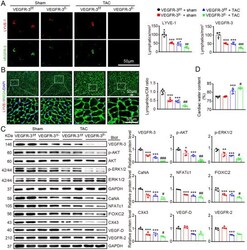
- Experimental details
- FIGURE 2 Knockdown of VEGFR-3 impairs cardiac lymphangiogenesis and causes edema after pressure overload. VEGFR-3 knockdown (VEGFR-3 f/- ) mice and their WT (VEGFR-3 f/f ) littermates were subjected to sham or TAC surgery and remained under sham or TAC conditions for 6 weeks. (A) Heart sections stained with an antibody against LYVE-1 (red) or VEGFR-3 (green) (left, scale bar: 50 mum) and quantification of LYVE-1 + or VEGFR-3 + lymphatic vessels (right, n = 6). (B) Heart sections stained with an anti-LYVE-1 antibody (red), TRITC-labeled WGA (green) and DAPI (blue) (left, scale bar: 50 mum) and the LYVE-1 + vessel to CM ratio (right, n = 6). (C) Immunoblot analysis of the VEGFR-3, p-AKT, AKT, p-ERK1/2, ERK1/2, calcineurin A (CaNA), NFATc1, FOXC2, CX43, VEGF-D, and VEGFR-2 proteins in the heart (left) and quantification of these proteins (right, n = 4). GAPDH was used as an internal control. (D) Gravimetric assessment of the cardiac water content (%) as determined by the cardiac dry weight-to-wet weight ratio ( n = 6). The data are presented as the mean +- SD, and n represents the number of animals per group. Statistical analysis was performed with one-way ANOVA; * p < 0.05, ** p < 0.01, and *** p < 0.001 versus VEGFR-3 f/f + sham; # p < 0.05, ## p < 0.01, and ### p < 0.001 versus VEGFR-3 f/f + TAC
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
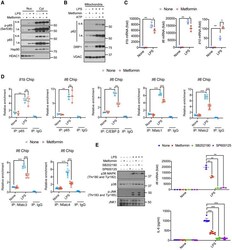
- Figure 1 Metformin attenuates Il1b and Il6 mRNA induction but does not inhibit NF-kappaB activation (A) BMDM pretreated -/+ metformin (0.5 mM, 16 h) were harvested 30 min after LPS (100 ng/mL) addition. Nuclear (Nuc) and cytosolic (Cyt) fractions were analyzed for the indicated proteins. s.e., short exposure; l.e., long exposure. One representative immunoblot (IB) out of 2. (B) IB analysis of p62, DRP1, and VDAC in mitochondria from BMDM pretreated -/+ metformin, primed with LPS, and challenged with ATP (4 mM, 1 h). (C) Q-PCR quantitation of Il1b , Il6 and Il10 mRNAs before or after LPS stimulation -/+ metformin pre-treatment (n = 3). (D) p65, C/EBPbeta or NFATc1-4 recruitments to the Il1 and Il6 promoters in BMDM before or after LPS stimulation was analyzed by ChIP-qPCR assay (n = 3-7). (E) IB analysis of p38 MAPK and JNK phosphorylation (left) and quantitation of Il6 mRNA (top) and IL-6 secretion (bottom) from BMDM pretreated with metformin, SB202190 (10 muM) or SP600125 (40 muM) for 16 h and stimulated with LPS for 30 min. All IB show one representative out of 3. Results in (C-E) are averages +-SD. * p < 0.05; ** p < 0.01; *** p < 0.001. Two-sided unpaired t test. See also Figure S1. Figure S1
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
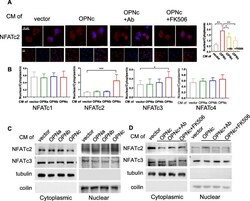
- Fig. 3 Nuclear accumulation of NFATc2 in cells treated with OPN-CMs. a Immunofluorescent images for NFATc2 subcellular localization in cells induced by OPNc-CM and CM with addition of OPN-neutralizing antibody or FK506 as an inhibitor to calcineurin. Twenty individual cells were selected for quantitative analyses of the ratio between nuclear localization and cytoplasmic localization for each NFATc2 as shown in the plot. b Quantification of NFATc1-c4 nuclear localization from immunofluorescence in cells treated with OPN-CMs for 3 h. c Western blot analyses for cytoplasm or nuclear distribution of NFATc2 and NFATc3 proteins in cells treated with OPN-CMs, where tubulin and coilin were used as cytoplasm and nuclear makers respectively. d Western blot analyses for cytoplasm or nuclear distribution of NFATc2 and NFATc3 proteins in cells treated with OPNc-CM and CM with addition of OPN-neutralizing antibody or FK506. * p < 0.05, ** p < 0.01, *** p < 0.001
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
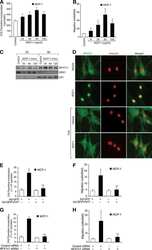
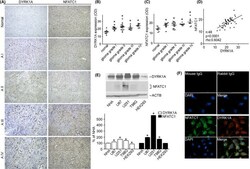
- FIGURE 1 DYRK1A and NFATC1 protein expression were coordinately increased in brain gliomas. A Representative images for the immunohistochemical staining of DYRK1A and NFATC1 in four different grades of gliomas and normal tissues. DYRK1A was detected using a polyclonal anti-DYRK1A antibody (CST, #2771) and NFATC1 was detected using a monoclonal antibody (Thermo Fisher, #MA3-024) in the microarray (Biomax, #GL481 and T173a). B. DYRK1A protein expression was increased in gliomas. The expression of DYRK1A was quantified by ImageJ software as described in the methods section. C. NFATC1 protein levels were increased in gliomas. The expression of NFATC1 was quantified by ImageJ software as described in the methods section. D. Correlation between protein levels of NFATC1 and DYRK1A in tissues was analyzed by Spearman's rank correlation test. p < 0.0001. rho = 0.0642. n =48. E. NFATC1 and DYRK1A were detected by WB in the cell lysate of NHA, U87, U251, T98G, and HEK293 cell lines. NFATC1 monoclonal antibody and DYRK1A polyclonal antibody were used as described in the methods section. F. Colocalization images for the immunofluorescent staining of DYRK1A and NFATC1 in U251 cells. DYRK1A was detected using a polyclonal anti-DYRK1A antibody (CST, #2771), and NFATC1 was detected using a monoclonal antibody (Thermo Fisher, #MA3-024), mouse IgG (Santa Cruz, sc-2025), and rabbit IgG (Proteintech, B900610) as negative controls. Values represent means +-SD, n = 3.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
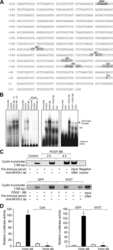
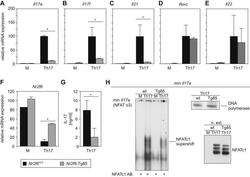
- Fig. 3 Nr2f6 overexpression suppresses Th17 differentiation in vitro . (A) Wildtype ( Nr2f6 +/+ ) or Nr2f6- Tg85 (Tg85) CD4 + cells were differentiated under Th17 conditions, and Il17a , (B) Il17f , (C) Il21 , (D) Rorc , (E) and Il22 transcript levels from both genotypes were determined by qRT-PCR. The data shown are derived from three independent experiments and are normalized to the amounts of Gapdh expression. Expression in the Th17 Nr2f6 +/+ differentiated cells subset was arbitrarily set to 100 in order to facilitate comparison between the different cytokines. Error bars denote the mean +- s.e.m. * p < 0.05. The error bars denote mean +- s.e.m. * p < 0.05. (F) Expression of Nr2f6 in Nr2f6 +/+ or Nr2f6- Tg85 un-stimulated or Th17-differentiated CD4 + T cells. Note the approximate 4-fold increase in expression in the Tg85 transgenic NR2F6 cells. (G) Supernatants from wildtype ( Nr2f6 +/+ ) or Nr2f6- Tg85 (Tg85) CD4 + Th17-differentiated cells were used to measure IL-17 secretion via BioPlex. (H) EMSA analysis of nuclear extracts prepared from wildtype ( Nr2f6 +/+ ) or Tg85 CD4 + Th17-differentiated cells hybridized to the min Il17a (NFAT o3) oligonucleotide and supershifted with an NFATc1 antibody. Equal NFAT nuclear translocation was observed by western blot, and DNA polymerase delta was used as the loading control. One of three independent experiments is shown.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
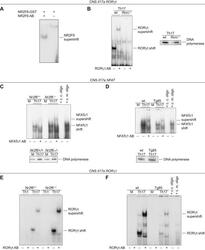
- Fig. 6 NR2F6 suppresses NFAT and RORgammat binding to the CNS2 region of the Il17a promoter. (A) GST-NR2F6 binds to the CNS2 Il17a RORgammat oligonucleotide [16] of the Il17a CNS2 promoter region in EMSA analysis. (B) EMSA analysis of nuclear extracts from wildtype (wt) or Rorc -/- CD4 + T cells stimulated under Th17 conditions for 3 days and hybridized to the CNS2 Il17a ROR oligonucleotide. The specific binding of RORgammat was confirmed using a RORgammat supershift antibody loading was controlled by performing western blots with nuclear extracts probed with DNA polymerase delta no gross differences could be observed between the genotypes within the same stimulation conditions. (C) EMSA analysis of nuclear extracts of Nr2f6 +/+ or Nr2f6 -/- CD4 + T cells either left unstimulated (M) or stimulated under Th17 conditions for 3 days and hybridized to a CNS2 Il17a NFAT-specific oligonucleotide. (D) EMSA analysis of nuclear extracts of wildtype ( Nr2f6 +/+ ) or Nr2f6- Tg85 CD4 + T cells stimulated under Th17 conditions for 3 days and hybridized to a CNS2 IL17a NFAT-specific oligonucleotide. The specific binding of NFATc1 was confirmed using the NFATc1 supershift antibody and cold (c.c. oligo) and mutant competition oligonucleotides (c.m. oligo). (E) EMSA analysis of nuclear extracts from Nr2f6 +/+ or Nr2f6 -/- CD4 + T cells stimulated under Th1 or under Th17 conditions for 3 days and hybridized to the CNS2 Il17a RORgamma oligonucleotide. (F) EMSA analysis of nuclear extracts from
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
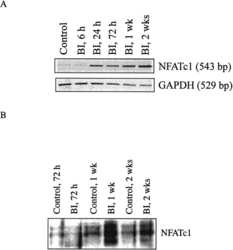
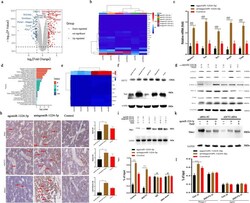
- MiRNA-1224-5p inactivates the ADCY2-dependent Rap1 signaling pathway. a Gene expression levels of BMMs using agomiRNA-1224 and antagomiRNA-1224 24 h after transfection. The expression of 460 genes was upregulated (red), and the expression of 670 genes was downregulated (blue). Each group was treated with 30 ng/ml M-CSF. b Heatmap showing genes related to osteoclasts. c RT-PCR quantitatively confirmed that the expression of key genes in osteoclasts was upregulated. d KEGG pathway analysis predicts that the function of the Rap1 signaling pathway will change. e Heatmap shows genes related to the Rap1 pathway. f Western blot analysis showed that 6 h after treatment with agomiRNA-1224 in BMMs, the level of Rap1 decreased significantly. g Western blotting confirmed that treatment of BMMs with agomiRNA-1224 decreased the RANKL-induced protein expression of CDC42, p-Rac1, p-Rho-A, FAK, and Rac1 for 24 h. h Representative immunohistochemical staining showing that agomiRNA-1224 injection decreased ADCY2 and Rap1 levels in vivo. Femurs were collected within 24 h of miRNA after injection. Scale bar, 50 um. i Western blotting confirmed that treatment of BMMs with agomiRNA-1224 decreased the expression of NFATC1 protein induced by RANKL for 48 h. j ChIP experiment results show that agomiRNA-1224 induces ADCY2 to occupy the Nfatc1-binding region together. k Western blot analysis of Nfatc1. The removal of ADCY2 weakened the expression of Nfatc1 induced by agomiRNA-1224. l ChIP experiment res
 Explore
Explore Validate
Validate Learn
Learn