37-9400
antibody from Invitrogen Antibodies
Targeting: HSP90AB1
HSPC2, HSPCB
 Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [20]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [1]
- Flow cytometry [1]
- Other assay [22]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 37-9400 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- HSP90 beta Monoclonal Antibody (H9010)
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Reactivity
- Human, Mouse, Chicken/Avian, Rabbit
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- H9010
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- -20°C
Submitted references Cell-free DNA topology depends on its subcellular and cellular origins in cancer.
HSP90 promotes radioresistance of cervical cancer cells via reducing FBXO6-mediated CD147 polyubiquitination.
Heat shock protein HSP90 immunoexpression in equine endometrium during oestrus, dioestrus and anoestrus.
Time-of-day specificity of anticancer drugs may be mediated by circadian regulation of the cell cycle.
MAL2 mediates the formation of stable HER2 signaling complexes within lipid raft-rich membrane protrusions in breast cancer cells.
Grp94 Regulates the Recruitment of Aneural AChR Clusters for the Assembly of Postsynaptic Specializations by Modulating ADF/Cofilin Activity and Turnover.
Inhibition of ezrin causes PKCα-mediated internalization of erbb2/HER2 tyrosine kinase in breast cancer cells.
Effects of Pseudomonas aeruginosa on Microglial-Derived Extracellular Vesicle Biogenesis and Composition.
HER2 signaling regulates HER2 localization and membrane retention.
Neural Precursor-Derived Pleiotrophin Mediates Subventricular Zone Invasion by Glioma.
PMCA2 regulates HER2 protein kinase localization and signaling and promotes HER2-mediated breast cancer.
The Inhibition of Heat Shock Protein 90 Facilitates the Degradation of Poly-Alanine Expanded Poly (A) Binding Protein Nuclear 1 via the Carboxyl Terminus of Heat Shock Protein 70-Interacting Protein.
Histone Deacetylases Positively Regulate Transcription through the Elongation Machinery.
A C-terminal HSP90 inhibitor restores glucocorticoid sensitivity and relieves a mouse allograft model of Cushing disease.
Heat shock protein 90β stabilizes focal adhesion kinase and enhances cell migration and invasion in breast cancer cells.
Dissecting cellular responses to irradiation via targeted disruptions of the ATM-CHK1-PP2A circuit.
Proteomic identification of putative biomarkers of radiotherapy resistance: a possible role for the 26S proteasome?
Proteomic identification of putative biomarkers of radiotherapy resistance: a possible role for the 26S proteasome?
HSP90 beta regulates rapsyn turnover and subsequent AChR cluster formation and maintenance.
Pseudovacuoles--immobilized by high-pressure freezing--are associated with blebbing in walker carcinosarcoma cells.
Malkin EZ, De Michino S, Lambie M, Gill R, Zhao Z, Rostami A, Arruda A, Minden MD, Bratman SV
JCI insight 2022 Oct 24;7(20)
JCI insight 2022 Oct 24;7(20)
HSP90 promotes radioresistance of cervical cancer cells via reducing FBXO6-mediated CD147 polyubiquitination.
Song Q, Wen J, Li W, Xue J, Zhang Y, Liu H, Han J, Ning T, Lu Z
Cancer science 2022 Apr;113(4):1463-1474
Cancer science 2022 Apr;113(4):1463-1474
Heat shock protein HSP90 immunoexpression in equine endometrium during oestrus, dioestrus and anoestrus.
Camacho Benítez A, Vasconcellos R, Lombide P, Viotti H, Pérez W, Cazales N, Cavestany D, Martin GB, Pedrana G
Anatomia, histologia, embryologia 2021 Jan;50(1):50-57
Anatomia, histologia, embryologia 2021 Jan;50(1):50-57
Time-of-day specificity of anticancer drugs may be mediated by circadian regulation of the cell cycle.
Lee Y, Fong SY, Shon J, Zhang SL, Brooks R, Lahens NF, Chen D, Dang CV, Field JM, Sehgal A
Science advances 2021 Feb;7(7)
Science advances 2021 Feb;7(7)
MAL2 mediates the formation of stable HER2 signaling complexes within lipid raft-rich membrane protrusions in breast cancer cells.
Jeong J, Shin JH, Li W, Hong JY, Lim J, Hwang JY, Chung JJ, Yan Q, Liu Y, Choi J, Wysolmerski J
Cell reports 2021 Dec 28;37(13):110160
Cell reports 2021 Dec 28;37(13):110160
Grp94 Regulates the Recruitment of Aneural AChR Clusters for the Assembly of Postsynaptic Specializations by Modulating ADF/Cofilin Activity and Turnover.
Chan ZC, Deng L, Lee CW
eNeuro 2020 Sep Oct;7(5)
eNeuro 2020 Sep Oct;7(5)
Inhibition of ezrin causes PKCα-mediated internalization of erbb2/HER2 tyrosine kinase in breast cancer cells.
Jeong J, Choi J, Kim W, Dann P, Takyar F, Gefter JV, Friedman PA, Wysolmerski JJ
The Journal of biological chemistry 2019 Jan 18;294(3):887-901
The Journal of biological chemistry 2019 Jan 18;294(3):887-901
Effects of Pseudomonas aeruginosa on Microglial-Derived Extracellular Vesicle Biogenesis and Composition.
Jones LB, Kumar S, Bell CR, Peoples VA, Crenshaw BJ, Coats MT, Scoffield JA, Rowe GC, Sims B, Matthews QL
Pathogens (Basel, Switzerland) 2019 Dec 14;8(4)
Pathogens (Basel, Switzerland) 2019 Dec 14;8(4)
HER2 signaling regulates HER2 localization and membrane retention.
Jeong J, Kim W, Kim LK, VanHouten J, Wysolmerski JJ
PloS one 2017;12(4):e0174849
PloS one 2017;12(4):e0174849
Neural Precursor-Derived Pleiotrophin Mediates Subventricular Zone Invasion by Glioma.
Qin EY, Cooper DD, Abbott KL, Lennon J, Nagaraja S, Mackay A, Jones C, Vogel H, Jackson PK, Monje M
Cell 2017 Aug 24;170(5):845-859.e19
Cell 2017 Aug 24;170(5):845-859.e19
PMCA2 regulates HER2 protein kinase localization and signaling and promotes HER2-mediated breast cancer.
Jeong J, VanHouten JN, Dann P, Kim W, Sullivan C, Yu H, Liotta L, Espina V, Stern DF, Friedman PA, Wysolmerski JJ
Proceedings of the National Academy of Sciences of the United States of America 2016 Jan 19;113(3):E282-90
Proceedings of the National Academy of Sciences of the United States of America 2016 Jan 19;113(3):E282-90
The Inhibition of Heat Shock Protein 90 Facilitates the Degradation of Poly-Alanine Expanded Poly (A) Binding Protein Nuclear 1 via the Carboxyl Terminus of Heat Shock Protein 70-Interacting Protein.
Shi C, Huang X, Zhang B, Zhu D, Luo H, Lu Q, Xiong WC, Mei L, Luo S
PloS one 2015;10(9):e0138936
PloS one 2015;10(9):e0138936
Histone Deacetylases Positively Regulate Transcription through the Elongation Machinery.
Greer CB, Tanaka Y, Kim YJ, Xie P, Zhang MQ, Park IH, Kim TH
Cell reports 2015 Nov 17;13(7):1444-1455
Cell reports 2015 Nov 17;13(7):1444-1455
A C-terminal HSP90 inhibitor restores glucocorticoid sensitivity and relieves a mouse allograft model of Cushing disease.
Riebold M, Kozany C, Freiburger L, Sattler M, Buchfelder M, Hausch F, Stalla GK, Paez-Pereda M
Nature medicine 2015 Mar;21(3):276-80
Nature medicine 2015 Mar;21(3):276-80
Heat shock protein 90β stabilizes focal adhesion kinase and enhances cell migration and invasion in breast cancer cells.
Xiong X, Wang Y, Liu C, Lu Q, Liu T, Chen G, Rao H, Luo S
Experimental cell research 2014 Aug 1;326(1):78-89
Experimental cell research 2014 Aug 1;326(1):78-89
Dissecting cellular responses to irradiation via targeted disruptions of the ATM-CHK1-PP2A circuit.
Palii SS, Cui Y, Innes CL, Paules RS
Cell cycle (Georgetown, Tex.) 2013 Apr 1;12(7):1105-18
Cell cycle (Georgetown, Tex.) 2013 Apr 1;12(7):1105-18
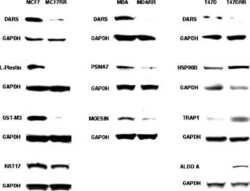
Proteomic identification of putative biomarkers of radiotherapy resistance: a possible role for the 26S proteasome?
Smith L, Qutob O, Watson MB, Beavis AW, Potts D, Welham KJ, Garimella V, Lind MJ, Drew PJ, Cawkwell L
Neoplasia (New York, N.Y.) 2009 Nov;11(11):1194-207
Neoplasia (New York, N.Y.) 2009 Nov;11(11):1194-207
Proteomic identification of putative biomarkers of radiotherapy resistance: a possible role for the 26S proteasome?
Smith L, Qutob O, Watson MB, Beavis AW, Potts D, Welham KJ, Garimella V, Lind MJ, Drew PJ, Cawkwell L
Neoplasia (New York, N.Y.) 2009 Nov;11(11):1194-207
Neoplasia (New York, N.Y.) 2009 Nov;11(11):1194-207
HSP90 beta regulates rapsyn turnover and subsequent AChR cluster formation and maintenance.
Luo S, Zhang B, Dong XP, Tao Y, Ting A, Zhou Z, Meixiong J, Luo J, Chiu FC, Xiong WC, Mei L
Neuron 2008 Oct 9;60(1):97-110
Neuron 2008 Oct 9;60(1):97-110
Pseudovacuoles--immobilized by high-pressure freezing--are associated with blebbing in walker carcinosarcoma cells.
Vanhecke D, Bellmann R, Baum O, Graber W, Eggli P, Keller H, Studer D
Journal of microscopy 2008 May;230(Pt 2):253-62
Journal of microscopy 2008 May;230(Pt 2):253-62
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
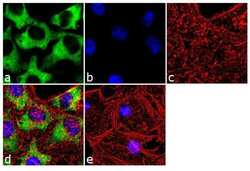
- Experimental details
- Immunofluorescence analysis of Hsp90 beta was done on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with Hsp90 beta (H9010) Mouse Monoclonal Antibody (Product # 37-9400) at 1:250 dilution in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
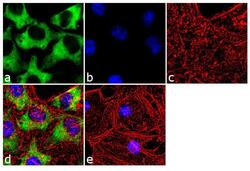
- Experimental details
- Immunofluorescence analysis of Hsp90 beta was done on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with Hsp90 beta (H9010) Mouse Monoclonal Antibody (Product # 37-9400) at 1:250 dilution in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
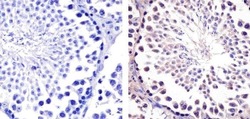
- Experimental details
- Immunohistochemistry analysis of Hsp90-beta showing staining in the cytoplasm of paraffin-embedded mouse testis tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Anti- nHsp90-beta Monoclonal Antibody (H9010) (Product # 37-9400) diluted in 3% BSA-PBS at a dilution of 1:20 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
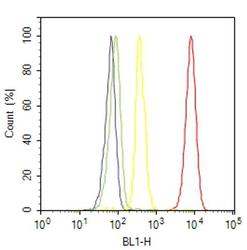
- Experimental details
- Flow cytometry analysis of Hsp90-beta was done on HeLa cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with Hsp90-beta Mouse Monoclonal Antibody (379400, red histogram) or with mouse isotype control (yellow histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Rabbit Anti-Mouse Secondary Antibody (A11059) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
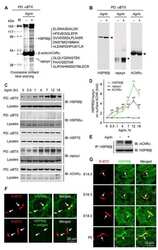
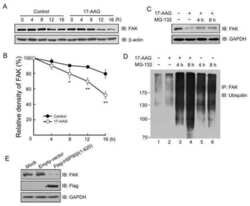
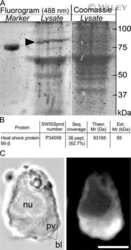
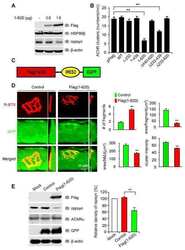
- Fig 3 Disruption of HSP90 function induces PABPN1 degradation. (A) Reduction of A17-PABPN1 in 17-AAG-treated muscle cells. C2C12 myoblasts were transfected with HA-tagged A17-PABPN1 constructs. Twenty-four hours post-transfection, cells were treated with CHX (10 mug/ml) alone or together with 17-AAG (2 muM) or DMSO (2 muM) for the indicated times at 37degC. Lysates were blotted to show the expression of the proteins of interest. Band density was quantified and is shown in the line graph (bottom panel). Data are shown as the mean +- SEM (n = 5); **, P < 0.01. (B) Real-time PCR for mutant A17-PABPN1 mRNA. The mRNA levels of mutant A17-PABPN1 were similar with or without 17-AAG treatment. (C, D) Degradation of PABPN1 in 17-AAG treated myotubes cultured at 40degC. C2C12 myoblasts were transfected with GFP-tagged A10-PABPN1 and A17-PABPN1, and the cells were chased at 40degC in the presence of 17-AAG (2 muM) and CHX (10 mug/ml) for the indicated times 24 hr post-transfection. Lysates were blotted to show the expression of the proteins of interest. Band density was quantified and is shown in the line graphs (bottom panels). Values are mean +- SEM (n = 5). (E) 17-AAG reduced PABPN1 levels by 65% in cells transfected with non-silencing shRNA, whereas the suppression of HSP90 expression abrogated the 17-AAG-mediated reductions in PABPN1 expression. (F) . The disruption of A17-PABPN1/HSP90 complex by 17-AAG. HEK293 cells were transfected with A17-PABPN1, and the cells were treated with
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
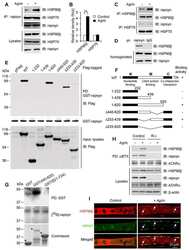
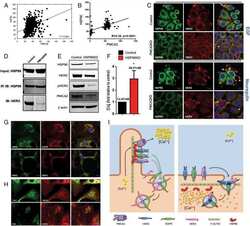
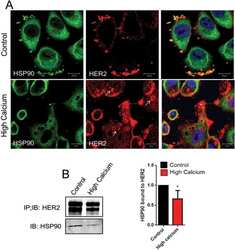
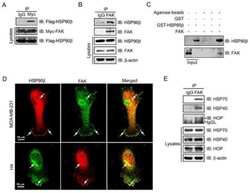
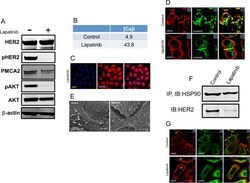
- Fig 4 Lapatinib increases intracellular calcium and inhibits membrane protrusions. A) Immunoblot showing HER2 signaling components in SKBR3 cells +- lapatinib ( 2 micromolar for 48-hours). B) Intracellular calcium measurements in control and lapatinib-treated SKBR3 cells. Numbers are mean calcium concentrations estimated by FURA2 measurements. C) Con-focal images of immunofluorescence for NFATc1 (red) and DAPI (blue) in SKBR3 cells treated with lapatinib. D) Con-focal images of immunofluorescence for HER2 (red) and phalloidin (actin, green) in control and lapatinib-treated SKBR3 cells. Insets represent Z-stack images in 2 different orientations. E) Scanning electron microscopy of control and lapatinib-treated SKBR3 cells. Arrows point to larger membrane protrusions. F) Co-IP for HER2 and HSP90 from control and lapatinib-treated SKBR3 cells. Cell lysates were immunoprecipitated with antibodies to HSP90 and blotted for HSP90 or HER2. G) Con-focal images of immunofluorescence for HER2 (red) and HSP90 (green) in control (top row) and lapatinib-treated (bottom row) SKBR3 cells. Insets represent Z-stack images in 2 different orientations and white arrows indicate internalized HER2. Scale bars in C, D and G represent 10mum. Scale bars in E represent 20muM.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
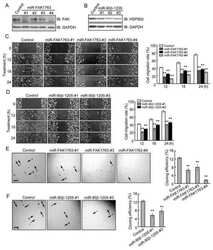
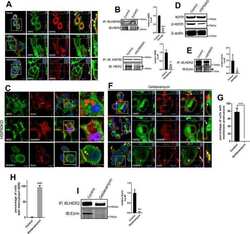
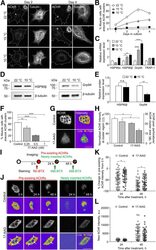
- Figure 1. Temperature stress-modulated expression and pharmacological inhibition of HSP90 regulate the formation and stability of aneural AChR clusters. A , Representative images showing the inhibition of aneural AChR cluster formation in cultured Xenopus muscle cells treated with lower temperatures. Tubulin immunostaining indicated that cytoskeletal structures were largely unaffected in muscle cells cultured at different temperatures, ranging from 10-22degC. B , Quantification showing the percentage of cultured muscle cells with bottom aneural AChR clusters at different culturing temperatures over 4 d; n = 150 cells in each condition from three independent experiments. C , Quantification showing the relative mRNA levels of HSP90alpha, HSP90beta, Grp94, and TRAP-1 in 2-d-old Xenopus muscle cells cultured at different temperatures; n = 3 independent experiments. D , E , Western blot analysis ( D ) and quantification ( E ) showing the protein expression level of HSP90beta and Grp94 in Xenopus muscle cells cultured at 22degC or 10degC for 2 d. beta-Tubulin was used as the loading control for normalization. F , Quantification showing the dose-dependent effects of 17-AAG on aneural AChR cluster formation in cultured Xenopus muscle cells; n = 191 (Control), n = 198 (0.25 n m 17-AAG), n = 199 (0.5 n m 17-AAG), and n = 200 (1 n m 17-AAG) muscle cells from four independent experiments. G , Representative images showing the organization and intensity of aneural AChR clusters in respons
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
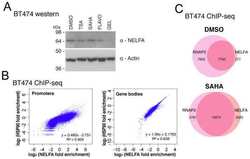
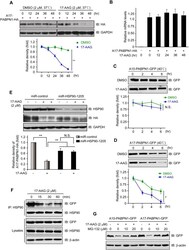
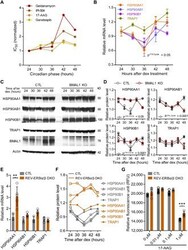
- Fig. 3 Rhythmic effect of HSP90 inhibitors is associated with circadian expression of HSP90 genes. ( A ) Line graphs are replotted on the basis of the primary screening results in Fig. 1 to show that several HSP90 inhibitors are more effective at inhibiting cell proliferation at a specific time of day. The x axis depicts circadian phase as determined by the timing of dex addition (see Materials and Methods). ( B ) Temporal mRNA expression of the indicated HSP90 isoforms of dex-synchronized U2OS cells across circadian time (). P JTKCycle < 0.05 indicates significant rhythms of HSP90AA1, HSP90AB1, and HSP90B1 (details are in data file S3). ( C ) Western blot analysis of the indicated HSP90 isoforms and BMAL1 in control (CTL) and BMAL1 knockout U2OS (BMAL1 KO) cells collected every 6 hours for 24 hours after dex stimulation. ( D ) Statistical analysis of the Western blot data in (C) showing protein abundance over time in CTL (gray circle) and BMAL1 KO (red brown circle) cells. JTKCycle identified a significant 24-hour rhythm in the expression of HSP90AA1, HSP90AB1, and HSP90B1 in CTL cells. Data were normalized to actin and are represented as means +- SEM from n = 3 independent experiments. ( E ) mRNA expression of the indicated HSP90 isoforms in control (CTL) and REV-ERB alpha/beta double-knockout U2OS cells (REV-ERBalpha/beta DKO). ** P < 0.005; two-way ANOVA with Bonferroni's multiple comparisons test. Data are shown with the means +- SD; n = 3 to 6 in each genes. ( F ) Line
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
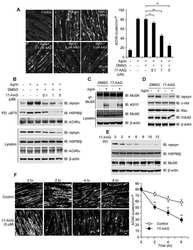
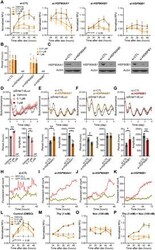
- Fig. 4 Isoforms of HSP90 that mediate temporal drug sensitivity affect circadian rhythms and the cell cycle. ( A ) Graphs show time-of-day-dependent cytotoxic effect of 17-AAG delivered to dex-synchronized U2OS cells treated with control ( si-CTL ) or siRNAs targeting the indicated HSP90 isoforms. JTKCycle detected a significant 24-hour rhythm in 1 muM drug-treated si-CTL and si-HSP90AB1 samples (details are in data file S3). ( B ) Period analysis of temporal 17-AAG cytotoxicity data shown in (A) using the Biodare2 rhythm analysis program ( https://biodare2.ed.ac.uk/ ). AR, arrhythmic. ( C ) Western blot analysis to verify efficiency of siRNAs targeting the indicated HSP90 isoforms. ( D to G ) Bioluminescence recordings of a Bmal1 promoter-luciferase reporter (p Bmal1 -dsLuc) in U2OS cells treated with vehicle (gray) or 17-AAG at the indicated doses (D) or 48 hours after transfection of control siRNA ( si-CTL , gray) or siRNAs targeting the indicated HSP90 isoforms (E to G) (top). Graphs below present period (left) and amplitude (right) analysis results of the top data panels. * P < 0.05, ** P < 0.005, and **** P < 0.00001 by Biodare2 analysis. Data show means +- SD; n = 3 for all samples. ( H to K ) Real-time cell analysis of U2OS cells stably expressing FUCCI cell cycle indicators [RFP (G 1 ), dark pink; GFP (S-G 2 -M), gray green) 48 hours after transfection of control siRNA ( si-CTL ) (H) or siRNAs targeting the indicated HSP90 isoforms (I to K) (top). ( L to P ) DMSO (co
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
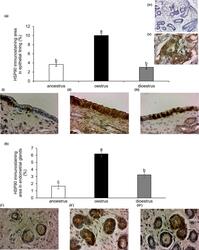
- 2 Figure Variation with phase of reproduction in the immunoexpression of heat shock protein HSP90 in the endometrium of the mare. Quantitative image analyses of immunostained area in: (a) endometrial lining epithelium; and (b) endometrial glands in anoestrus (i, i'), oestrus (ii, ii') and dioestrus (iii, iii'). Magnification 400. Scale bar = 10um. Columns show immunostaining area (%) mean +- SEM . Letters that differ among columns indicate significant differences ( p < .0001). Controls for immunohistochemical technique: upper right image iv) negative control (mare endometrium); v) positive control (canine mammary gland carcinoma)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
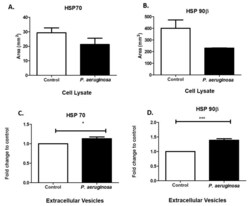
- Figure 4 Stress-induced protein expression within infected BV-2 cell lysates and BV-2-derived EVs. HSP70 and HSP90beta were found in BV-2 lysates after 72 h P. aeruginosa infection using dot blot analysis ( A , B ). HSP70 ( C ) and HSP90beta ( D ) were detected in EVs at 72 h post-inoculation and confirmed using ELISA. Experiments were performed using four or five experiments. Statistical significance is indicated as (*) p
 Explore
Explore Validate
Validate Learn
Learn