PA1-46189
antibody from Invitrogen Antibodies
Targeting: YAP1
YAP65
 Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Chromatin Immunoprecipitation
Chromatin Immunoprecipitation Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [9]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [1]
- Chromatin Immunoprecipitation [2]
- Other assay [12]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-46189 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- YAP1 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Recombinant full-length protein
- Description
- Suggested positive control: brain and kidney lysates, and HEK 293 cell lysate, 293 whole cell lysate, antigen standard for YAP1 (transient overexpression lysate), human kidney protein, human brain protein.
- Reactivity
- Human, Mouse, Rat, Canine, Zebrafish
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- 1.0 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Directed invasion of cancer cell spheroids inside 3D collagen matrices oriented by microfluidic flow in experiment and simulation.
Radiation therapy affects YAP expression and intracellular localization by modulating lamin A/C levels in breast cancer.
Aberrant transcriptional and post-transcriptional regulation of SPAG5, a YAP-TAZ-TEAD downstream effector, fuels breast cancer cell proliferation.
Role of the Hippo signaling pathway in safflower yellow pigment treatment of paraquat-induced pulmonary fibrosis.
PI3K Inhibitors Curtail MYC-Dependent Mutant p53 Gain-of-Function in Head and Neck Squamous Cell Carcinoma.
Alginate oligosaccharide attenuates α2,6-sialylation modification to inhibit prostate cancer cell growth via the Hippo/YAP pathway.
NSCLC depend upon YAP expression and nuclear localization after acquiring resistance to EGFR inhibitors.
The clinical significance of forkhead box protein A1 and its role in colorectal cancer.
Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma.
Geiger F, Schnitzler LG, Brugger MS, Westerhausen C, Engelke H
PloS one 2022;17(3):e0264571
PloS one 2022;17(3):e0264571
Radiation therapy affects YAP expression and intracellular localization by modulating lamin A/C levels in breast cancer.
La Verde G, Artiola V, Pugliese M, La Commara M, Arrichiello C, Muto P, Netti PA, Fusco S, Panzetta V
Frontiers in bioengineering and biotechnology 2022;10:969004
Frontiers in bioengineering and biotechnology 2022;10:969004
Aberrant transcriptional and post-transcriptional regulation of SPAG5, a YAP-TAZ-TEAD downstream effector, fuels breast cancer cell proliferation.
Canu V, Donzelli S, Sacconi A, Lo Sardo F, Pulito C, Bossel N, Di Benedetto A, Muti P, Botti C, Domany E, Bicciato S, Strano S, Yarden Y, Blandino G
Cell death and differentiation 2021 May;28(5):1493-1511
Cell death and differentiation 2021 May;28(5):1493-1511
Role of the Hippo signaling pathway in safflower yellow pigment treatment of paraquat-induced pulmonary fibrosis.
Li H, Kan B, Song L, Liu Y, Jian X
The Journal of international medical research 2020 Sep;48(9):300060520905425
The Journal of international medical research 2020 Sep;48(9):300060520905425
PI3K Inhibitors Curtail MYC-Dependent Mutant p53 Gain-of-Function in Head and Neck Squamous Cell Carcinoma.
Ganci F, Pulito C, Valsoni S, Sacconi A, Turco C, Vahabi M, Manciocco V, Mazza EMC, Meens J, Karamboulas C, Nichols AC, Covello R, Pellini R, Spriano G, Sanguineti G, Muti P, Bicciato S, Ailles L, Strano S, Fontemaggi G, Blandino G
Clinical cancer research : an official journal of the American Association for Cancer Research 2020 Jun 15;26(12):2956-2971
Clinical cancer research : an official journal of the American Association for Cancer Research 2020 Jun 15;26(12):2956-2971
Alginate oligosaccharide attenuates α2,6-sialylation modification to inhibit prostate cancer cell growth via the Hippo/YAP pathway.
Han Y, Zhang L, Yu X, Wang S, Xu C, Yin H, Wang S
Cell death & disease 2019 May 10;10(5):374
Cell death & disease 2019 May 10;10(5):374
NSCLC depend upon YAP expression and nuclear localization after acquiring resistance to EGFR inhibitors.
McGowan M, Kleinberg L, Halvorsen AR, Helland Å, Brustugun OT
Genes & cancer 2017 Mar;8(3-4):497-504
Genes & cancer 2017 Mar;8(3-4):497-504
The clinical significance of forkhead box protein A1 and its role in colorectal cancer.
Ma W, Jiang J, Li M, Wang H, Zhang H, He X, Huang L, Zhou Q
Molecular medicine reports 2016 Sep;14(3):2625-31
Molecular medicine reports 2016 Sep;14(3):2625-31
Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma.
Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C, Yao Y, Liu Q
Molecular cancer 2014 May 17;13:110
Molecular cancer 2014 May 17;13:110
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
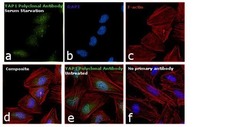
- Experimental details
- Immunofluorescence analysis of YAP1 was performed using 70% confluent log phase HeLa cells treated with serum starvation for 12 hours. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with YAP1 Polyclonal Antibody (Product # PA1-46189) at 1:250 dilution in 0.1% BSA, incubated at 4 degree Celsius overnight and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic and nuclear localization. Panel e shows untreated cells with predominant nuclear localization. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
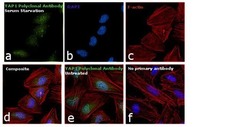
- Experimental details
- Immunofluorescence analysis of YAP1 was performed using 70% confluent log phase HeLa cells treated with serum starvation for 12 hours. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with YAP1 Polyclonal Antibody (Product # PA1-46189) at 1:250 dilution in 0.1% BSA, incubated at 4 degree Celsius overnight and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic and nuclear localization. Panel e shows untreated cells with predominant nuclear localization. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
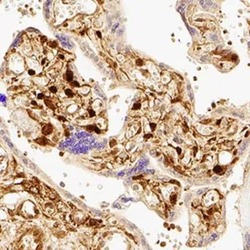
- Experimental details
- Immunohistochemical analysis of YAP1 in immersion fixed paraffin-embedded sections of human placenta. Samples were incubated in YAP1 polyclonal antibody (Product # PA1-46189) using a dilution of 1:200 for 1 hour at room temperature followed by the Anti-Rabbit IgG VisUCyte™ HRP Polymer Antibody. Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to nuclear and cytoplasm in trophoblast cells.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
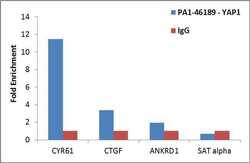
- Experimental details
- Enrichment of endogenous YAP1 protein at specific gene loci using Anti-YAP1 Antibody: Chromatin Immunoprecipitation (ChIP) was performed using Anti-YAP1 Rabbit Polyclonal Antibody (Product # PA1-46189, 3 ug) on sheared chromatin from 2 million HeLa cells using the MAGnify ChIP System (Product # 49-2024). Normal Rabbit IgG was used as a negative IP control. The purified DNA was analyzed by qPCR with PCR primer pairs over CYR61, CTGF, ANKRD1 (active) and SAT alpha (inactive). Data is presented as fold enrichment of the antibody signal versus the negative control IgG using the comparative CT method.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
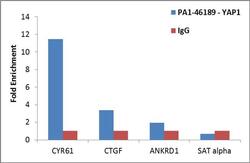
- Experimental details
- Enrichment of endogenous YAP1 protein at specific gene loci using Anti-YAP1 Antibody: Chromatin Immunoprecipitation (ChIP) was performed using Anti-YAP1 Rabbit Polyclonal Antibody (Product # PA1-46189, 3 ug) on sheared chromatin from 2 million HeLa cells using the MAGnify ChIP System (Product # 49-2024). Normal Rabbit IgG was used as a negative IP control. The purified DNA was analyzed by qPCR with PCR primer pairs over CYR61, CTGF, ANKRD1 (active) and SAT alpha (inactive). Data is presented as fold enrichment of the antibody signal versus the negative control IgG using the comparative CT method.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
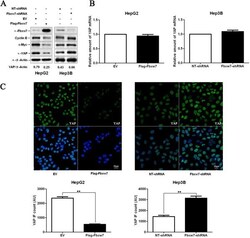
- Figure 4 Fbxw7 regulates the stability of the YAP protein in HCC cells. A) HepG2 and Hep3B cells that were transfected with Flag-Fbxw7 and Fbxw7-shRNA, respectively, and subjected to Western blotting for Fbxw7, c-Myc, Cyclin E and YAP. Fbxw7 overexpression decreased c-Myc, Cyclin E and YAP protein levels in HepG2 cells, whereas Fbxw7 knockdown led to c-Myc, Cyclin E and YAP accumulation in Hep3B cells. Data are representative of multiple repeats with similar results. B) HepG2 cells transfected with EV or Flag-Fbxw7 and Hep3B cells transfected with non-targeting (NT)-shRNA or Fbxw7-shRNA were harvested for RNA extraction and real time RT-PCR. Fbxw7 overexpression or knockdown did not change YAP mRNA levels. n = 3 independent experiment; Values are depicted as the Mean +- SEM. C) HCC cells that were treated as above were subjected to IF for YAP. Quantification of YAP immunofluorescence revealed that the average level of YAP in the control cells was significantly higher than that in the Fbxw7 overexpressing HepG2 cells and lower than that in the Fbxw7 shRNA transfected Hep3B cells. Scale bar: 20 mum, n = 6; Values are depicted as the Mean +- SEM; ** P < 0.01 by t test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
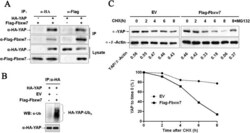
- Figure 5 Fbxw7 binds to YAP and promotes the ubiquitin-mediated proteolysis of YAP. A) YAP and Fbxw7 coimmunoprecipitate with each other. Flag-Fbxw7 and HA-YAP plasmids were transfected into HEK293 cells. YAP or Fbxw7 was immunoprecipitated with anti-HA or anti-Flag antibody. Western blotting was performed to detect the specific proteins indicated on the left side of each panel. B) HA-YAP was precipitated from HA-YAP overexpressing HepG2 cells by immunoprecipitation using an anti-HA antibody; YAP ubiquitination was detected by western blotting. Fbxw7 overexpression markedly promoted YAP ubiquitination. C) The Fbxw7 turnover rate was shorter in Fbxw7 overexpressing HepG2 cells. The protein half-life of YAP was analyzed following treatment with cycloheximide. The YAP band intensity was normalized to GAPDH and then normalized to t = 100 controls. The half-life of YAP in Flag-Fbxw7 = 5.2 h (R 2 = 0.96) and in EV = 16.5 h (R 2 = 0.92). Additionally, treatment with MG132 (a proteasome inhibitor) inhibited Fbxw7 induced YAP degradation in HepG2 cells. The data are representative of multiple independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
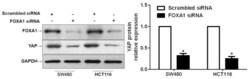
- Experimental details
- Figure 5 Downregulation of forkhead box protein A1 (FOXA1) reduces the expression of yes-associated protein (YAP) in colorectal cancer cells. SW480 and HCT116 cells were transfected with FOXA1 small interfering (si)RNA or scrambled siRNA, and were subjected to western blotting to determine YAP expression. Semi-quantitative analysis revealed that FOXA1 knockdown significantly reduced the protein expression levels of YAP. n=3 independent repeats with similar results. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
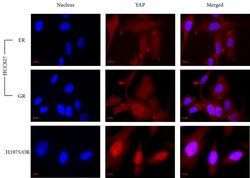
- Experimental details
- Figure 3 An immunocytochemistry staining showing the localization of YAP in drug-resistant sub-lines Both HCC827/ER and GR sub-lines show YAP expression distributed in the cytoplasm and nucleus. The H1975/OR sub-line shows YAP staining more focused in the nucleus than the cytoplasm. Scale bar was 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
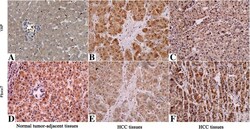
- Experimental details
- Figure 3 Immunohistochemical analyses of YAP and its correlation with Fbxw7 protein in HCC. In cases of high Fbxw7 protein expression (D, F) , there was no detectable YAP protein expression (A, C) in the same tissue section. In contrast, in the case of low Fbxw7 protein expression (E) , there was strong YAP protein expression (B) . The fibrotic septa (B, E) simply represent fibrotic tumor stroma with normal background liver. Scale bar: 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
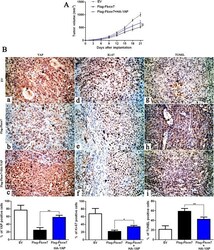
- Experimental details
- Figure 7 YAP partially abolishes Fbxw7's suppression of tumor growth. A) Control Hep3B cells (EV, n = 6), Fbxw7 overexpressing Hep3B cells (Flag-Fbxw7, n = 6) and co-expressing Hep3B cells (Flag-Fbxw7 + HA-YAP, n = 6), respectively, were implanted into nude mice via subcutaneous injection. Tumor nodules were measured using a caliper at different times after implantation. Fbxw7 overexpressing Hep3B cells exhibited a greater tumor-inhibiting effect compared with control cells; however, restoring YAP expression accelerated tumor growth, compared with the Flag-Fbxw7 group. ** P < 0.01 by two-way ANOVA. B) Tumor nodules were subjected to immunohistochemical staining for YAP and Ki-67, TUNEL assays and quantitative analysis. Representative immunostaining and TUNEL assays revealed that Fbxw7 overexpression significantly reduced the number of YAP and Ki-67 positive cells and increased the number of apoptotic cells. However, the percentage of YAP and Ki-67 positive cells in tumors arising from the Flag-Fbxw7 + HA-YAP group was significantly higher than that in the tumors from the Flag-Fbxw7 group and the percentage of apoptotic cells in the Flag-Fbxw7 + HA-YAP group was significantly lower than that in the Flag-Fbxw7 group. Black arrows indicate positive cells in each photomicrograph. Scale bar: 100 mum; n = 6; Values are depicted as the Mean +- SEM; * P < 0.05 and ** P < 0.01 by one-way ANOVA.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
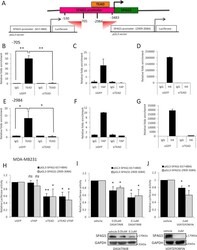
- Fig. 6 TEAD and coactivator YAP directly bind the SPAG5 promoter. A Schematic representation of the SPAG5 promoter with the putative TEAD-binding sites (LASAGNA Search 2.0). The promoter regions 657-884 and 2909-3084 were amplified and cloned into pGL3 vector. B , C , E , F Chip analysis of the TEAD and YAP binding on SPAG5 promoter in MDA-MB-231 cell line after TEAD interference detected by qRT-PCR analysis. D - G Transcriptional active chromatin on SPAG5 promoter evidenced by anti-H4-Acetylate antibody. Data are shown as the mean of three independent replicates with the a relative P value. H - J Luciferase assay pGL3 vector carrying SPAG5 657-884 and SPAG5 2909-3084 promoter regions was transfected in MDA-MB-231 cell line after YAP and/or TEAD interference. H MDA-MB-231 cell lines were treated with 0.05-0.1 uM of Dasatinib ( I ) or with 2 uM of Verteporfin ( J ) and transfected with pGL3 vector carrying SPAG5 657-884 and SPAG5 2909-3084 promoter regions. Normalized luciferase-activity values and P value from three independent experiments are shown. In the lower panel, western blot shows SPAG5 expression after 0.05-0.1 uM of Dasatinib ( I ) or with 2 uM of Verteporfin ( J ) treatments. ( ns nonsignificant; * P value
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
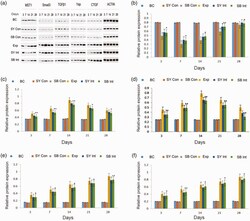
- Figure 4. Immunoblotting analysis of MST, TGF-beta1, Smad3, Yap and CTGF among groups over time. Actin was used as an internal reference. a. Protein expression levels of MST, TGF-beta1, Smad3, Yap, CTGF and actin. b. Quantification of MST protein expression in lung tissue. c. Quantification of Smad3 protein expression in lung tissue. d. Quantification of TGF-beta1 protein expression in lung tissue. e. Quantification of Yap protein expression in lung tissue. f. Quantification of CTGF protein expression in lung tissue. Exp vs BC: aa (P < 0.01), a (P < 0.05); Exp vs SY Int: bb (P < 0.01), b (P < 0.05); Exp vs SB Int: **(P < 0.01), *(P < 0.05).Abbreviations: BC, blank control; SY Con, safflower yellow pigment control; SB Con, SB431542 control; Exp, exposure; SY Int, safflower yellow pigment intervention; SB Int, SB431542 intervention; MST, mammalian STE20-like; TGF-beta1, transforming growth factor-beta1; CTGF, connective tissue growth factor.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
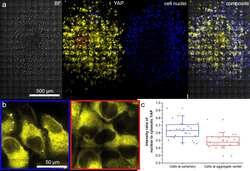
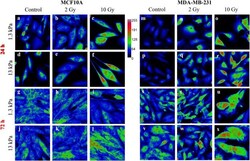
- FIGURE 1 Sum intensity projections of z-stack images taken from YAP immunofluorescence in MCF10A (A-L) and MDA-MB-231 (M-X) , shown as rainbow RGB look-up table. Colour bar: YAP intensity (A-U) . Scale bar, 50 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
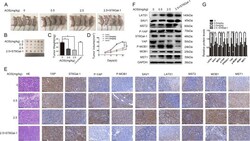
- Fig. 7 AOS inhibits the prostate tumor formation in nude mice through the Hippo/YAP pathway. a Morphological images of tumors that were engrafted and AOS treated. b Solid tumors were isolated from nude mice. c , d Average tumor weights ( c ) and sizes ( d ) were measured in different groups. e , f Western blot assay ( e ) and IHC ( f ) were used to detect the expression levels of the main signaling molecules of the Hippo/YAP in tumor tissues. * P < 0.05
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
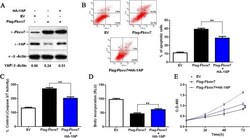
- Figure 6 Fbxw7's suppression of Hep3B cell growth was partially reverted by YAP. A) Flag-Fbxw7 transfected Hep3B cells successfully up-regulated Fbxw7 protein expression as shown by western blot. Fbxw7 overexpression in the same cell line could reduce the levels of YAP. Fbxw7 over-expressing cells that were transfected with HA-YAP partially rescued the phenotype, showing higher YAP levels. The data are representative of multiple repeats with similar results. B) Apoptotic cells were measured by flow cytometry. Restoring YAP expression decreased the percentage of apoptotic cells in Flag-Fbxw7 transfected Hep3B cells. ** P < 0.01 by one-way ANOVA; n = 3 repeats with similar results. C) The activity of the pro-apoptotic caspases 3 and 7 in Fbxw7 overexpressing Hep3B cells was decreased by HA-YAP transfection. ** P < 0.01 by one-way ANOVA; n = 3 repeats with similar results. D) A BrdU assay showed that YAP promotes proliferation in Fbxw7 overexpressing Hep3B cells. ** P < 0.01 by one-way ANOVA; n = 3 repeats with similar results. E) YAP was found to enhance the viability of Fbxw7 over-expressing Hep3B cells (MTT assay). ** P < 0.01 by two-way ANOVA; n = 3 repeats with similar results. Values are depicted as the Mean +- SEM.
 Explore
Explore Validate
Validate Learn
Learn