Antibody data
- Antibody Data
- Antigen structure
- References [7]
- Comments [0]
- Validations
- Immunocytochemistry [3]
- Immunohistochemistry [3]
- Other assay [6]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-20390 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- IFN beta Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- A suggested positive control is A-20 cell lysate. PA5-20390 can be used with blocking peptide PEP-0507.
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- 4°C
Submitted references Klotho deficiency intensifies hypoxia-induced expression of IFN-α/β through upregulation of RIG-I in kidneys.
Inhibition of interferon-signalling halts cancer-associated fibroblast-dependent protection of breast cancer cells from chemotherapy.
Nanoparticle-enhanced chemo-immunotherapy to trigger robust antitumor immunity.
IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus.
Transgenic tomatoes expressing the 6F peptide and ezetimibe prevent diet-induced increases of IFN-β and cholesterol 25-hydroxylase in jejunum.
RIPK3 interacts with MAVS to regulate type I IFN-mediated immunity to Influenza A virus infection.
STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity.
Urabe A, Doi S, Nakashima A, Ike T, Morii K, Sasaki K, Doi T, Arihiro K, Masaki T
PloS one 2021;16(10):e0258856
PloS one 2021;16(10):e0258856
Inhibition of interferon-signalling halts cancer-associated fibroblast-dependent protection of breast cancer cells from chemotherapy.
Broad RV, Jones SJ, Teske MC, Wastall LM, Hanby AM, Thorne JL, Hughes TA
British journal of cancer 2021 Mar;124(6):1110-1120
British journal of cancer 2021 Mar;124(6):1110-1120
Nanoparticle-enhanced chemo-immunotherapy to trigger robust antitumor immunity.
Liang J, Wang H, Ding W, Huang J, Zhou X, Wang H, Dong X, Li G, Chen E, Zhou F, Fan H, Xia J, Shen B, Cai D, Lan P, Jiang H, Ling J, Cheng Z, Liu X, Sun J
Science advances 2020 Aug;6(35):eabc3646
Science advances 2020 Aug;6(35):eabc3646
IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus.
Shao S, Tsoi LC, Sarkar MK, Xing X, Xue K, Uppala R, Berthier CC, Zeng C, Patrick M, Billi AC, Fullmer J, Beamer MA, Perez-White B, Getsios S, Schuler A, Voorhees JJ, Choi S, Harms P, Kahlenberg JM, Gudjonsson JE
Science translational medicine 2019 Sep 25;11(511)
Science translational medicine 2019 Sep 25;11(511)
Transgenic tomatoes expressing the 6F peptide and ezetimibe prevent diet-induced increases of IFN-β and cholesterol 25-hydroxylase in jejunum.
Mukherjee P, Hough G, Chattopadhyay A, Navab M, Fogelman HR, Meriwether D, Williams K, Bensinger S, Moller T, Faull KF, Lusis AJ, Iruela-Arispe ML, Bostrom KI, Tontonoz P, Reddy ST, Fogelman AM
Journal of lipid research 2017 Aug;58(8):1636-1647
Journal of lipid research 2017 Aug;58(8):1636-1647
RIPK3 interacts with MAVS to regulate type I IFN-mediated immunity to Influenza A virus infection.
Downey J, Pernet E, Coulombe F, Allard B, Meunier I, Jaworska J, Qureshi S, Vinh DC, Martin JG, Joubert P, Divangahi M
PLoS pathogens 2017 Apr;13(4):e1006326
PLoS pathogens 2017 Apr;13(4):e1006326
STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity.
Demaria O, De Gassart A, Coso S, Gestermann N, Di Domizio J, Flatz L, Gaide O, Michielin O, Hwu P, Petrova TV, Martinon F, Modlin RL, Speiser DE, Gilliet M
Proceedings of the National Academy of Sciences of the United States of America 2015 Dec 15;112(50):15408-13
Proceedings of the National Academy of Sciences of the United States of America 2015 Dec 15;112(50):15408-13
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
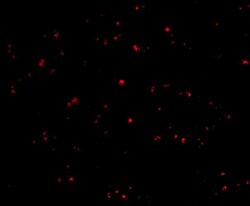
- Immunofluorescent analysis of human liver cells using a IFN-beta polyclonal antibody (Product # PA5-20390) at a 20 µg/mL dilution.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
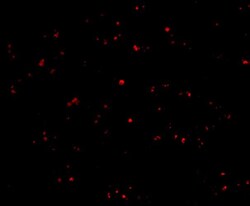
- Experimental details
- Immunofluorescent analysis of 4% paraformaldehyde-fixed human liver cells labeling IFN-beta with IFN beta Polyclonal Antibody (Product # PA5-20390) at 20 µg/mL, followed by goat anti-rabbit IgG secondary antibody at 1:500 dilution (red).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of 4% paraformaldehyde-fixed human liver cells labeling IFN-beta with IFN beta Polyclonal Antibody (Product # PA5-20390) at 20 µg/mL, followed by goat anti-rabbit IgG secondary antibody at 1:500 dilution (red).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
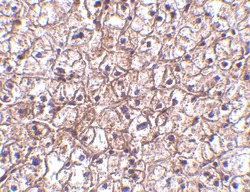
- Experimental details
- Immunohistochemical analysis of paraffin-embedded human liver tissue using IFN beta Polyclonal Antibody (Product # PA5-20390) at 5 µg/mL. Tissue was fixed with formaldehyde and blocked with 0.1 serum for 1 h at RT; antigen retrieval was by heat mediation with a citrate buffer (pH6). Samples were incubated with primary antibody overnight at 4˚C. A goat anti-rabbit IgG H&L (HRP) at 1/250 was used as secondary. Counter stained with Hematoxylin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
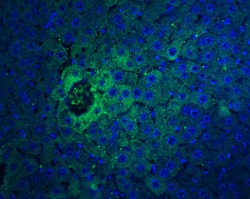
- Experimental details
- Immunofluorescent analysis of 4% paraformaldehyde-fixed mouse liver tissue labeling IFN-beta with IFN beta Polyclonal Antibody (Product # PA5-20390) at 20 µg/mL, followed by goat anti-rabbit IgG secondary antibody at 1:500 dilution (green) and DAPI staining (blue).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
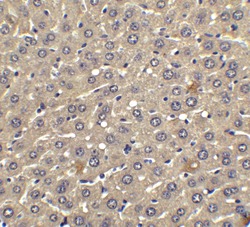
- Experimental details
- Immunohistochemical analysis of paraffin-embedded human liver tissue using IFN beta Polyclonal Antibody (Product # PA5-20390) at 2.5 µg/mL. Tissue was fixed with formaldehyde and blocked with 0.1 serum for 1 h at RT; antigen retrieval was by heat mediation with a citrate buffer (pH6). Samples were incubated with primary antibody overnight at 4˚C. A goat anti-rabbit IgG H&L (HRP) at 1/250 was used as secondary. Counter stained with Hematoxylin.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
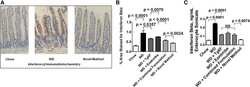
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
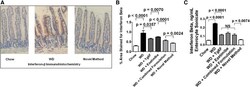
- Experimental details
- Fig. 3. WD induced increased levels of IFN-beta in the enterocytes of the jejunum of the mice described in Fig. 2 , which was better ameliorated by the novel method for adding Tg6F and ezetimibe compared with adding the same doses of Tg6F and ezetimibe to WD by the combined formulation. The induction of IFN-beta by WD in the enterocytes of the jejunum was determined by IHC and ELISA as described in Materials and Methods. A: An example of IHC showing induction of IFN-beta expression by WD and amelioration by the novel method. B: Quantification of IHC was performed as described in Materials and Methods. C: The results of IHC were confirmed by ELISA as described in Materials and Methods. The results shown are mean +- SEM. Abbreviations are the same as in the Fig. 2 legend.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
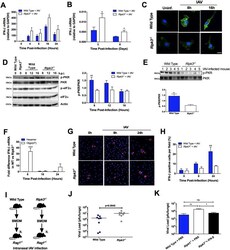
- Experimental details
- Fig 4 RIPK3 regulates IFN-beta mRNA integrity through activation of PKR. Total RNA was extracted from IAV-infected BMD-Mphi (MOI 1) (A) or cells of the BAL (50 pfu) (B) and the expression of IFN-beta mRNA was determined by qPCR. (C) Phosphorylation of PKR (green) was analyzed by immunofluorescence in WT and Ripk3 -/- BMD-Mphi at different time point post IAV-infection. Nuclei were stained with Hoechst (blue). The scale bars represent 10mum. (D) Phosphorylated and total forms of PKR and eIF2alpha in whole-cell lysates were analysed by immunoblotting. beta-Actin was used as a loading control. One representative blot is shown (left panel). Densitometry analysis to quantify the ratio of phosphorylated PKR relative to total PKR (n = 4, right panel). (E) Cells were harvested from the BAL of infected (50 pfu) WT or RIPK3-deficient mice and levels of phosphorylated and total PKR were determined by western blot (top panel). Densitometry analysis to quantify the ratio of phosphorylated PKR relative to total PKR is shown in the bottom panel. (F) Difference in the expression of IFN-beta mRNA between WT and Ripk3 -/- BMD-Mphi infected with IAV. Gene expression was analyzed by qPCR following cDNA generation using random hexamers (blue bars) or oligo(dT) primers (white bars). (G) Confocal images showing IFN-beta production in IAV-infected BMD-Mphi. Cells were stained with a rabbit polyclonal antibody specific for IFN-beta (red) as well as nuclear dye Hoechst (blue). (H) Percentage of cells
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
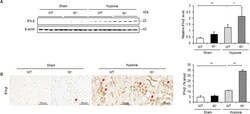
- 10.1371/journal.pone.0258856.g007 Fig 7 IFN-beta is increased under hypoxic conditions in Kl -/- mice. (A) Western blot analysis demonstrating IFN-beta expression in WT and Kl -/- mice. Protein levels were normalized to beta-actin levels (n = 5 in each group). (B) Representative immunohistochemical staining images showing IFN-beta expression in WT and Kl -/- mice. Values are mean +- SD. * P < 0.05, ** P < 0.01. Bar = 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
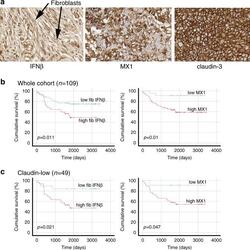
- Fig. 5 In primary cancers, IFNbeta1 in CAFs and MX1 in cancer cells correlate with each other and with poor survival. TMAs of tissue from 109 TNBC resections were assembled and expression of IFNbeta1 in fibroblasts, and MX1 and claudin-3 in tumour cells was determined using immunohistochemistry. a Representative images of immunohistochemistry, showing tissue scored '3' for IFNbeta in fibroblasts (left), '3' for MX1, and 'positive' for claudin-3. b The cohort was split into groups with high or low expression of IFNbeta1 in fibroblasts (left) or MX1 in tumour cells (right) using ROC analyses. Cumulative disease-free survival in the groups was compared using Kaplan-Meier analyses and log rank tests. c The cohort was split into claudin-low or claudin-high groups, based on expression levels of claudin-3 (positive or negative). The claudin-low group ( n = 49) were analysed as in b .
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry