Antibody data
- Antibody Data
- Antigen structure
- References [51]
- Comments [0]
- Validations
- Immunocytochemistry [6]
- Flow cytometry [1]
- Other assay [22]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-062 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Furin Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA1-062 detects furin convertase from canine and mouse cells as well as transfected human furin. This antibody does not detect endogenous furin from BSC-40, HeLa, J774A.1 BPAEC, or CHO cells, or from rat skeletal muscle, spleen, kidney, ovary, testes, heart, or brain tissue. PA1-062 has been successfully used in Western blot, immunocytochemistry, immunoprecipitation, and immunofluorescence procedures. By Western blot, this antibody detects an ~100 kDa protein representing furin in BSC-40 cells transfected with the human furin gene. In addition, PA1-062 detects human furin transfected into 3T3 cells. Immunofluorescence staining of endogenous furin convertase in 3T3 cells with PA1-062 yields a pattern consistent with paranuclear staining. The PA1-062 immunogen is a synthetic peptide corresponding to residues R(780) G E R T A F I K D Q S A L(793) of human Furin. The PA1-062 immunizing peptide (Cat. # PEP-010) is available for use in neutralization and control experiments.
- Reactivity
- Human, Mouse, Rat, Canine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- 2 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Generation of Mature DENVs via Genetic Modification and Directed Evolution.
The proprotein convertase furin inhibits IL-13-induced inflammation in airway smooth muscle by regulating integrin-associated signaling complexes.
Integrative oncogene-dependency mapping identifies RIT1 vulnerabilities and synergies in lung cancer.
TGF-β1 Increases GDNF Production by Upregulating the Expression of GDNF and Furin in Human Granulosa-Lutein Cells.
FlexiBAC: a versatile, open-source baculovirus vector system for protein expression, secretion, and proteolytic processing.
Amino acids stimulate the endosome-to-Golgi trafficking through Ragulator and small GTPase Arl5.
Proprotein convertase furin regulates osteocalcin and bone endocrine function.
The novel monoclonal antibody 9F5 reveals expression of a fragment of GPNMB/osteoactivin processed by furin-like protease(s) in a subpopulation of microglia in neonatal rat brain.
Posttranslational processing of FGF23 in osteocytes during the osteoblast to osteocyte transition.
Furin Cleavage of L2 during Papillomavirus Infection: Minimal Dependence on Cyclophilins.
Recombinant BMP4 and BMP7 increase activin A production by up-regulating inhibin βA subunit and furin expression in human granulosa-lutein cells.
Proteolysis during tumor cell extravasation in vitro: metalloproteinase involvement across tumor cell types.
Proprotein convertases play an important role in regulating PKGI endoproteolytic cleavage and nuclear transport.
Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane.
Hypoxia enhances cancer cell invasion through relocalization of the proprotein convertase furin from the trans-Golgi network to the cell surface.
Basolateral sorting of syntaxin 4 is dependent on its N-terminal domain and the AP1B clathrin adaptor, and required for the epithelial cell polarity.
HIV-1 Nef binds a subpopulation of MHC-I throughout its trafficking itinerary and down-regulates MHC-I by perturbing both anterograde and retrograde trafficking.
A novel transmembrane domain mediating retention of a highly motile herpesvirus glycoprotein in the endoplasmic reticulum.
Oligemic hypoperfusion differentially affects tau and amyloid-{beta}.
OCRL1 function in renal epithelial membrane traffic.
Human testicular orphan receptor 4 enhances thyroid hormone receptor signaling.
Rab13 regulates membrane trafficking between TGN and recycling endosomes in polarized epithelial cells.
Identification of a pH sensor in the furin propeptide that regulates enzyme activation.
Analysis of furin ectodomain shedding in epididymal fluid of mammals: demonstration that shedding of furin occurs in vivo.
Increased production of functional recombinant human clotting factor IX by baby hamster kidney cells engineered to overexpress VKORC1, the vitamin K 2,3-epoxide-reducing enzyme of the vitamin K cycle.
Distinct Golgi populations of phosphatidylinositol 4-phosphate regulated by phosphatidylinositol 4-kinases.
MARCH-II is a syntaxin-6-binding protein involved in endosomal trafficking.
Sorting of furin in polarized epithelial and endothelial cells: expression beyond the Golgi apparatus.
Co-recycling of MT1-MMP and MT3-MMP through the trans-Golgi network. Identification of DKV582 as a recycling signal.
Proprotein convertase furin interacts with and cleaves pro-ADAMTS4 (Aggrecanase-1) in the trans-Golgi network.
The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains.
The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells.
Mechanism of constitutive export from the golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-golgi network tubular domains.
Activation of the proteolytic activity of ADAMTS4 (aggrecanase-1) by C-terminal truncation.
Intracellular activation of human adamalysin 19/disintegrin and metalloproteinase 19 by furin occurs via one of the two consecutive recognition sites.
Biosynthetic processing of the pro-alpha 1(V)2pro-alpha 2(V) collagen heterotrimer by bone morphogenetic protein-1 and furin-like proprotein convertases.
Activation of membrane-type matrix metalloproteinase 3 zymogen by the proprotein convertase furin in the trans-Golgi network.
The ordered and compartment-specfific autoproteolytic removal of the furin intramolecular chaperone is required for enzyme activation.
Shedding of membrane type matrix metalloproteinase 5 by a furin-type convertase: a potential mechanism for down-regulation.
Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells.
Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells.
A protein-based therapeutic for human cytomegalovirus infection.
A furin-like convertase mediates propeptide cleavage of BACE, the Alzheimer's beta -secretase.
The PC6B cytoplasmic domain contains two acidic clusters that direct sorting to distinct trans-Golgi network/endosomal compartments.
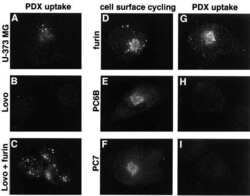
Localization of endogenous furin in cultured cell lines.
Localization of endogenous furin in cultured cell lines.
Cytoskeletal protein ABP-280 directs the intracellular trafficking of furin and modulates proprotein processing in the endocytic pathway.
Cytoskeletal protein ABP-280 directs the intracellular trafficking of furin and modulates proprotein processing in the endocytic pathway.
Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface.
Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface.
Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo.
Tse LV, Meganck RM, Dong S, Adams LE, White LJ, Mallory ML, Jadi R, de Silva AM, Baric RS
mBio 2022 Jun 28;13(3):e0038622
mBio 2022 Jun 28;13(3):e0038622
The proprotein convertase furin inhibits IL-13-induced inflammation in airway smooth muscle by regulating integrin-associated signaling complexes.
Wu Y, Huang Y, Zhang W, Gunst SJ
American journal of physiology. Lung cellular and molecular physiology 2021 Jul 1;321(1):L102-L115
American journal of physiology. Lung cellular and molecular physiology 2021 Jul 1;321(1):L102-L115
Integrative oncogene-dependency mapping identifies RIT1 vulnerabilities and synergies in lung cancer.
Vichas A, Riley AK, Nkinsi NT, Kamlapurkar S, Parrish PCR, Lo A, Duke F, Chen J, Fung I, Watson J, Rees M, Gabel AM, Thomas JD, Bradley RK, Lee JK, Hatch EM, Baine MK, Rekhtman N, Ladanyi M, Piccioni F, Berger AH
Nature communications 2021 Aug 9;12(1):4789
Nature communications 2021 Aug 9;12(1):4789
TGF-β1 Increases GDNF Production by Upregulating the Expression of GDNF and Furin in Human Granulosa-Lutein Cells.
Yin J, Chang HM, Yi Y, Yao Y, Leung PCK
Cells 2020 Jan 10;9(1)
Cells 2020 Jan 10;9(1)
FlexiBAC: a versatile, open-source baculovirus vector system for protein expression, secretion, and proteolytic processing.
Lemaitre RP, Bogdanova A, Borgonovo B, Woodruff JB, Drechsel DN
BMC biotechnology 2019 Mar 29;19(1):20
BMC biotechnology 2019 Mar 29;19(1):20
Amino acids stimulate the endosome-to-Golgi trafficking through Ragulator and small GTPase Arl5.
Shi M, Chen B, Mahajan D, Boh BK, Zhou Y, Dutta B, Tie HC, Sze SK, Wu G, Lu L
Nature communications 2018 Nov 26;9(1):4987
Nature communications 2018 Nov 26;9(1):4987
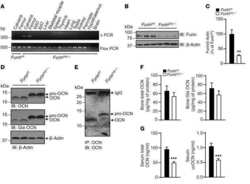
Proprotein convertase furin regulates osteocalcin and bone endocrine function.
Al Rifai O, Chow J, Lacombe J, Julien C, Faubert D, Susan-Resiga D, Essalmani R, Creemers JW, Seidah NG, Ferron M
The Journal of clinical investigation 2017 Nov 1;127(11):4104-4117
The Journal of clinical investigation 2017 Nov 1;127(11):4104-4117
The novel monoclonal antibody 9F5 reveals expression of a fragment of GPNMB/osteoactivin processed by furin-like protease(s) in a subpopulation of microglia in neonatal rat brain.
Kawahara K, Hirata H, Ohbuchi K, Nishi K, Maeda A, Kuniyasu A, Yamada D, Maeda T, Tsuji A, Sawada M, Nakayama H
Glia 2016 Nov;64(11):1938-61
Glia 2016 Nov;64(11):1938-61
Posttranslational processing of FGF23 in osteocytes during the osteoblast to osteocyte transition.
Yamamoto H, Ramos-Molina B, Lick AN, Prideaux M, Albornoz V, Bonewald L, Lindberg I
Bone 2016 Mar;84:120-130
Bone 2016 Mar;84:120-130
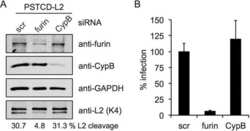
Furin Cleavage of L2 during Papillomavirus Infection: Minimal Dependence on Cyclophilins.
Bronnimann MP, Calton CM, Chiquette SF, Li S, Lu M, Chapman JA, Bratton KN, Schlegel AM, Campos SK
Journal of virology 2016 Jul 15;90(14):6224-6234
Journal of virology 2016 Jul 15;90(14):6224-6234
Recombinant BMP4 and BMP7 increase activin A production by up-regulating inhibin βA subunit and furin expression in human granulosa-lutein cells.
Chang HM, Cheng JC, Klausen C, Leung PC
The Journal of clinical endocrinology and metabolism 2015 Mar;100(3):E375-86
The Journal of clinical endocrinology and metabolism 2015 Mar;100(3):E375-86
Proteolysis during tumor cell extravasation in vitro: metalloproteinase involvement across tumor cell types.
Voura EB, English JL, Yu HY, Ho AT, Subarsky P, Hill RP, Hojilla CV, Khokha R
PloS one 2013;8(10):e78413
PloS one 2013;8(10):e78413
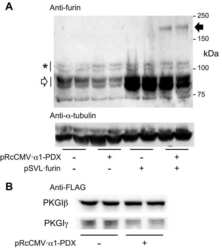
Proprotein convertases play an important role in regulating PKGI endoproteolytic cleavage and nuclear transport.
Kato S, Zhang R, Roberts JD Jr
American journal of physiology. Lung cellular and molecular physiology 2013 Jul 15;305(2):L130-40
American journal of physiology. Lung cellular and molecular physiology 2013 Jul 15;305(2):L130-40
Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane.
Gerl MJ, Sampaio JL, Urban S, Kalvodova L, Verbavatz JM, Binnington B, Lindemann D, Lingwood CA, Shevchenko A, Schroeder C, Simons K
The Journal of cell biology 2012 Jan 23;196(2):213-21
The Journal of cell biology 2012 Jan 23;196(2):213-21
Hypoxia enhances cancer cell invasion through relocalization of the proprotein convertase furin from the trans-Golgi network to the cell surface.
Arsenault D, Lucien F, Dubois CM
Journal of cellular physiology 2012 Feb;227(2):789-800
Journal of cellular physiology 2012 Feb;227(2):789-800
Basolateral sorting of syntaxin 4 is dependent on its N-terminal domain and the AP1B clathrin adaptor, and required for the epithelial cell polarity.
Reales E, Sharma N, Low SH, Fölsch H, Weimbs T
PloS one 2011;6(6):e21181
PloS one 2011;6(6):e21181
HIV-1 Nef binds a subpopulation of MHC-I throughout its trafficking itinerary and down-regulates MHC-I by perturbing both anterograde and retrograde trafficking.
Yi L, Rosales T, Rose JJ, Chowdhury B, Knutson JR, Venkatesan S
The Journal of biological chemistry 2010 Oct 1;285(40):30884-905
The Journal of biological chemistry 2010 Oct 1;285(40):30884-905
A novel transmembrane domain mediating retention of a highly motile herpesvirus glycoprotein in the endoplasmic reticulum.
Däubner T, Fink A, Seitz A, Tenzer S, Müller J, Strand D, Seckert CK, Janssen C, Renzaho A, Grzimek NK, Simon CO, Ebert S, Reddehase MJ, Oehrlein-Karpi SA, Lemmermann NA
The Journal of general virology 2010 Jun;91(Pt 6):1524-34
The Journal of general virology 2010 Jun;91(Pt 6):1524-34
Oligemic hypoperfusion differentially affects tau and amyloid-{beta}.
Koike MA, Green KN, Blurton-Jones M, Laferla FM
The American journal of pathology 2010 Jul;177(1):300-10
The American journal of pathology 2010 Jul;177(1):300-10
OCRL1 function in renal epithelial membrane traffic.
Cui S, Guerriero CJ, Szalinski CM, Kinlough CL, Hughey RP, Weisz OA
American journal of physiology. Renal physiology 2010 Feb;298(2):F335-45
American journal of physiology. Renal physiology 2010 Feb;298(2):F335-45
Human testicular orphan receptor 4 enhances thyroid hormone receptor signaling.
Huang YH, Liao CH, Chen RN, Liao CJ, Lin KH
Journal of cellular physiology 2010 Feb;222(2):347-56
Journal of cellular physiology 2010 Feb;222(2):347-56
Rab13 regulates membrane trafficking between TGN and recycling endosomes in polarized epithelial cells.
Nokes RL, Fields IC, Collins RN, Fölsch H
The Journal of cell biology 2008 Sep 8;182(5):845-53
The Journal of cell biology 2008 Sep 8;182(5):845-53
Identification of a pH sensor in the furin propeptide that regulates enzyme activation.
Feliciangeli SF, Thomas L, Scott GK, Subbian E, Hung CH, Molloy SS, Jean F, Shinde U, Thomas G
The Journal of biological chemistry 2006 Jun 9;281(23):16108-16
The Journal of biological chemistry 2006 Jun 9;281(23):16108-16
Analysis of furin ectodomain shedding in epididymal fluid of mammals: demonstration that shedding of furin occurs in vivo.
Thimon V, Belghazi M, Dacheux JL, Gatti JL
Reproduction (Cambridge, England) 2006 Dec;132(6):899-908
Reproduction (Cambridge, England) 2006 Dec;132(6):899-908
Increased production of functional recombinant human clotting factor IX by baby hamster kidney cells engineered to overexpress VKORC1, the vitamin K 2,3-epoxide-reducing enzyme of the vitamin K cycle.
Wajih N, Hutson SM, Owen J, Wallin R
The Journal of biological chemistry 2005 Sep 9;280(36):31603-7
The Journal of biological chemistry 2005 Sep 9;280(36):31603-7
Distinct Golgi populations of phosphatidylinositol 4-phosphate regulated by phosphatidylinositol 4-kinases.
Weixel KM, Blumental-Perry A, Watkins SC, Aridor M, Weisz OA
The Journal of biological chemistry 2005 Mar 18;280(11):10501-8
The Journal of biological chemistry 2005 Mar 18;280(11):10501-8
MARCH-II is a syntaxin-6-binding protein involved in endosomal trafficking.
Nakamura N, Fukuda H, Kato A, Hirose S
Molecular biology of the cell 2005 Apr;16(4):1696-710
Molecular biology of the cell 2005 Apr;16(4):1696-710
Sorting of furin in polarized epithelial and endothelial cells: expression beyond the Golgi apparatus.
Mayer G, Boileau G, Bendayan M
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2004 May;52(5):567-79
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2004 May;52(5):567-79
Co-recycling of MT1-MMP and MT3-MMP through the trans-Golgi network. Identification of DKV582 as a recycling signal.
Wang X, Ma D, Keski-Oja J, Pei D
The Journal of biological chemistry 2004 Mar 5;279(10):9331-6
The Journal of biological chemistry 2004 Mar 5;279(10):9331-6
Proprotein convertase furin interacts with and cleaves pro-ADAMTS4 (Aggrecanase-1) in the trans-Golgi network.
Wang P, Tortorella M, England K, Malfait AM, Thomas G, Arner EC, Pei D
The Journal of biological chemistry 2004 Apr 9;279(15):15434-40
The Journal of biological chemistry 2004 Apr 9;279(15):15434-40
The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains.
Fölsch H, Pypaert M, Maday S, Pelletier L, Mellman I
The Journal of cell biology 2003 Oct 27;163(2):351-62
The Journal of cell biology 2003 Oct 27;163(2):351-62
The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells.
Ang AL, Fölsch H, Koivisto UM, Pypaert M, Mellman I
The Journal of cell biology 2003 Oct 27;163(2):339-50
The Journal of cell biology 2003 Oct 27;163(2):339-50
Mechanism of constitutive export from the golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-golgi network tubular domains.
Polishchuk EV, Di Pentima A, Luini A, Polishchuk RS
Molecular biology of the cell 2003 Nov;14(11):4470-85
Molecular biology of the cell 2003 Nov;14(11):4470-85
Activation of the proteolytic activity of ADAMTS4 (aggrecanase-1) by C-terminal truncation.
Gao G, Westling J, Thompson VP, Howell TD, Gottschall PE, Sandy JD
The Journal of biological chemistry 2002 Mar 29;277(13):11034-41
The Journal of biological chemistry 2002 Mar 29;277(13):11034-41
Intracellular activation of human adamalysin 19/disintegrin and metalloproteinase 19 by furin occurs via one of the two consecutive recognition sites.
Kang T, Zhao YG, Pei D, Sucic JF, Sang QX
The Journal of biological chemistry 2002 Jul 12;277(28):25583-91
The Journal of biological chemistry 2002 Jul 12;277(28):25583-91
Biosynthetic processing of the pro-alpha 1(V)2pro-alpha 2(V) collagen heterotrimer by bone morphogenetic protein-1 and furin-like proprotein convertases.
Unsöld C, Pappano WN, Imamura Y, Steiglitz BM, Greenspan DS
The Journal of biological chemistry 2002 Feb 15;277(7):5596-602
The Journal of biological chemistry 2002 Feb 15;277(7):5596-602
Activation of membrane-type matrix metalloproteinase 3 zymogen by the proprotein convertase furin in the trans-Golgi network.
Kang T, Nagase H, Pei D
Cancer research 2002 Feb 1;62(3):675-81
Cancer research 2002 Feb 1;62(3):675-81
The ordered and compartment-specfific autoproteolytic removal of the furin intramolecular chaperone is required for enzyme activation.
Anderson ED, Molloy SS, Jean F, Fei H, Shimamura S, Thomas G
The Journal of biological chemistry 2002 Apr 12;277(15):12879-90
The Journal of biological chemistry 2002 Apr 12;277(15):12879-90
Shedding of membrane type matrix metalloproteinase 5 by a furin-type convertase: a potential mechanism for down-regulation.
Wang X, Pei D
The Journal of biological chemistry 2001 Sep 21;276(38):35953-60
The Journal of biological chemistry 2001 Sep 21;276(38):35953-60
Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells.
Yeaman C, Grindstaff KK, Wright JR, Nelson WJ
The Journal of cell biology 2001 Nov 12;155(4):593-604
The Journal of cell biology 2001 Nov 12;155(4):593-604
Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells.
Fölsch H, Pypaert M, Schu P, Mellman I
The Journal of cell biology 2001 Feb 5;152(3):595-606
The Journal of cell biology 2001 Feb 5;152(3):595-606
A protein-based therapeutic for human cytomegalovirus infection.
Jean F, Thomas L, Molloy SS, Liu G, Jarvis MA, Nelson JA, Thomas G
Proceedings of the National Academy of Sciences of the United States of America 2000 Mar 14;97(6):2864-9
Proceedings of the National Academy of Sciences of the United States of America 2000 Mar 14;97(6):2864-9
A furin-like convertase mediates propeptide cleavage of BACE, the Alzheimer's beta -secretase.
Bennett BD, Denis P, Haniu M, Teplow DB, Kahn S, Louis JC, Citron M, Vassar R
The Journal of biological chemistry 2000 Dec 1;275(48):37712-7
The Journal of biological chemistry 2000 Dec 1;275(48):37712-7
The PC6B cytoplasmic domain contains two acidic clusters that direct sorting to distinct trans-Golgi network/endosomal compartments.
Xiang Y, Molloy SS, Thomas L, Thomas G
Molecular biology of the cell 2000 Apr;11(4):1257-73
Molecular biology of the cell 2000 Apr;11(4):1257-73
Localization of endogenous furin in cultured cell lines.
Shapiro J, Sciaky N, Lee J, Bosshart H, Angeletti RH, Bonifacino JS
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 1997 Jan;45(1):3-12
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 1997 Jan;45(1):3-12
Localization of endogenous furin in cultured cell lines.
Shapiro J, Sciaky N, Lee J, Bosshart H, Angeletti RH, Bonifacino JS
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 1997 Jan;45(1):3-12
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 1997 Jan;45(1):3-12
Cytoskeletal protein ABP-280 directs the intracellular trafficking of furin and modulates proprotein processing in the endocytic pathway.
Liu G, Thomas L, Warren RA, Enns CA, Cunningham CC, Hartwig JH, Thomas G
The Journal of cell biology 1997 Dec 29;139(7):1719-33
The Journal of cell biology 1997 Dec 29;139(7):1719-33
Cytoskeletal protein ABP-280 directs the intracellular trafficking of furin and modulates proprotein processing in the endocytic pathway.
Liu G, Thomas L, Warren RA, Enns CA, Cunningham CC, Hartwig JH, Thomas G
The Journal of cell biology 1997 Dec 29;139(7):1719-33
The Journal of cell biology 1997 Dec 29;139(7):1719-33
Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface.
Molloy SS, Thomas L, VanSlyke JK, Stenberg PE, Thomas G
The EMBO journal 1994 Jan 1;13(1):18-33
The EMBO journal 1994 Jan 1;13(1):18-33
Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface.
Molloy SS, Thomas L, VanSlyke JK, Stenberg PE, Thomas G
The EMBO journal 1994 Jan 1;13(1):18-33
The EMBO journal 1994 Jan 1;13(1):18-33
Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo.
Bresnahan PA, Leduc R, Thomas L, Thorner J, Gibson HL, Brake AJ, Barr PJ, Thomas G
The Journal of cell biology 1990 Dec;111(6 Pt 2):2851-9
The Journal of cell biology 1990 Dec;111(6 Pt 2):2851-9
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
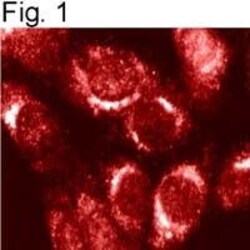
- Experimental details
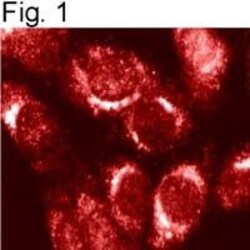
- Immunolocalization of endogenous furin in mouse 3T3 cells with PA1-062.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
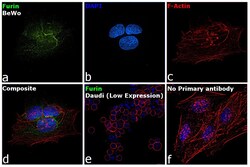
- Experimental details
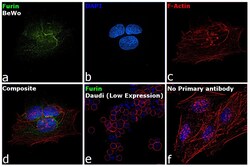
- Immunofluorescence analysis of Furin was performed using 70% confluent log phase BeWo cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with Furin Polyclonal Antibody (Product # PA1-062) at 2 µg/mL dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32790), (1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing predominantly Golgi Complex and cytoplasmic localization. Panel e represents Daudi cells with lower expression of Furin (DOI: 10.1099/0022-1317-75-10-2821). Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
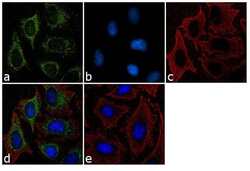
- Experimental details
- Immunofluorescent analysis of Furin Convertase was performed using 70% confluent log phase HeLa cells. The cells were fixed with Methanol for 5 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with Furin Convertase Rabbit Polyclonal Antibody (Product # PA1-062) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Cytoskeleton was stained with alpha-Tubulin Monoclonal Antibody (Product # 32-2500, 1 µg/mL) followed by Goat anti-Mouse IgG Secondary Antibody, Alexa Fluor® 594 conjugate (Product # A-11032, 1:400) (Panel c: red). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). Panel d represents the merged image showing cytoplasmic localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
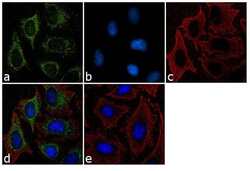
- Experimental details
- Immunofluorescent analysis of Furin Convertase was performed using 70% confluent log phase HeLa cells. The cells were fixed with Methanol for 5 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with Furin Convertase Rabbit Polyclonal Antibody (Product # PA1-062) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Cytoskeleton was stained with alpha-Tubulin Monoclonal Antibody (Product # 32-2500, 1 µg/mL) followed by Goat anti-Mouse IgG Secondary Antibody, Alexa Fluor® 594 conjugate (Product # A-11032, 1:400) (Panel c: red). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). Panel d represents the merged image showing cytoplasmic localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunolocalization of endogenous furin in mouse 3T3 cells with PA1-062.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescence analysis of Furin was performed using 70% confluent log phase BeWo cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with Furin Polyclonal Antibody (Product # PA1-062) at 2 µg/mL dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32790), (1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing predominantly Golgi Complex and cytoplasmic localization. Panel e represents Daudi cells with lower expression of Furin (DOI: 10.1099/0022-1317-75-10-2821). Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
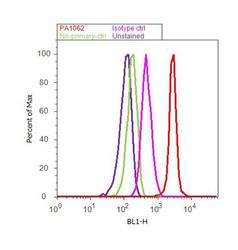
- Flow cytometry analysis of Furin Convertase was done on HeLa cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with Furin Convertase Rabbit Polyclonal Antibody (PA1-062, red histogram) or with rabbit isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10, 000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
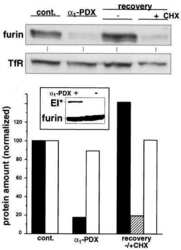
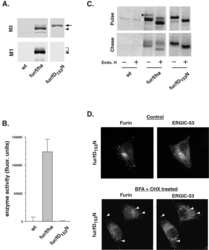
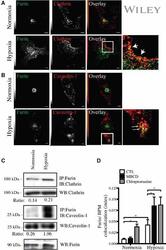
- 4 Furin internalization in hypoxic cells involves both clathrin and lipid rafts. Cells were seeded on collagen type IV-coated slides, cultured in normoxic or hypoxic chambers for 4 h. A : Micrographs of furin (eGFP), clathrin (red), and merged images. B : Micrographs of furin (eGFP), caveolin-1 (red), and merged images. C : Co-immunoprecipitation of furin with clathrin and caveolin-1 in HT-1080 cells incubated in normoxia or hypoxia. D : Graph showing the percentage of furin with the basal plasma membrane. Cells were seeded on collagen type IV-coated slides, left either untreated (control; CTL), or pretreated with MbetaCD (300 uM) or chlorpromazine (5 uM) prior to culture in normoxic or hypoxic chambers for 4 h. Cells were then labeled with DiI processed for confocal microscopy and fluorogram analysis were performed as described under Materials and Methods section. Column, mean; bar, SE; BPM, basal plasma membrane; * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
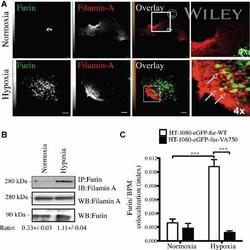
- 6 Filamin-A is essential for hypoxia-driven relocalization of furin to the plasma membrane. Cells were seeded on collagen type IV-coated slides and cultured in normoxic or hypoxic chambers for 4 h. A : Micrographs of furin (eGFP), filamin-A (red), and merged images. B : Co-immunoprecipitation of furin and filamin-A in normoxic or hypoxic cells. C : Graph showing the percentage of furin with the basal plasma membrane. HT-1080-eGFP-fur and HT-1080-eGFP-fur-VA 750 were seeded on top of collagen IV-coated slides and cultured either in normoxic or hypoxic chambers for 4 h and labeled with DiI. Fluorogram analysis were performed as described under Materials and Methods section. Column, mean; bar, SE; BPM, basal plasma membrane; *** P < 0.0007; scale bars correspond to 5 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
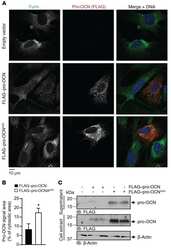
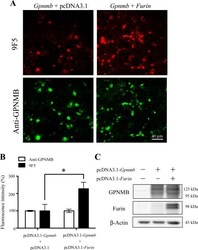
- Figure 7 Overexpression of furin cDNA increases the expression level of 9F5 antigen. COS-7 cells were cotransfected with pcDNA3.1- Gpnmb /pcDNA3.1 or pcDNA3.1- Gpnmb /pcDNA3.1-furin vector. A : At 72 hr after transfection, cells were double-stained with 9F5 (red) and anti-GPNMB (green) antibodies. B : The results shown in A were quantified and are given as means +- SEM ( n = 3). Fluorescence intensities (9F5 and anti-GPNMB) in cells transfected with pcDNA3.1- Gpnmb /pcDNA3.1were set at 100%. * P < 0.05 by Dunnett's multiple comparison test. C : Cell lysates were also subjected to immunoblot analysis for GPNMB, furin, and beta-actin. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com .]
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
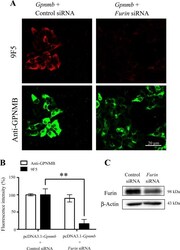
- Figure 9 A furin siRNA inhibits the expression level of 9F5 antigen. HEK293 cells were cotransfected with pcDNA3.1- Gpnmb /control small interfering RNA (siRNA) or pcDNA3.1- Gpnmb /furin siRNA. A : At 72 hr after transfection, cells were double-stained with 9F5 (red) and anti-GPNMB (green) antibodies. B : The results shown in A were quantified and are given as means +- SEM ( n = 3-4). Fluorescence intensities (9F5 and anti-GPNMB) in cells transfected with pcDNA3.1- Gpnmb /pcDNA3.1 were set at 100%. ** P < 0.01 by Dunnett's multiple comparison test. C : Cell lysates were also subjected to immunoblot analysis for furin and beta-actin. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com .]
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
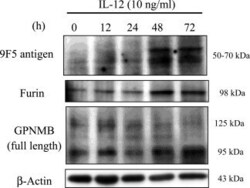
- Figure 10 IL-12 increases both furin and 9F5 antigen of rat type 1 MG with similar kinetics. Rat type 1 MG were treated with rat recombinant IL-12 (10 ng ml -1 ) at the indicated hours, and cell lysates were subjected to immunoblot analysis for 9F5 antigen, furin, GPNMB, and beta-actin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
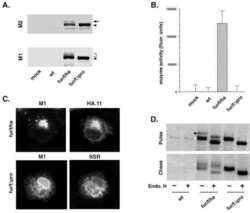
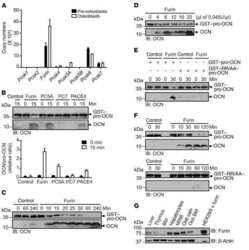
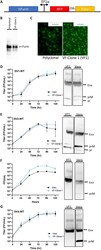
- FIG 2 Growth kinetics and maturation status of DENVs on Vero and VF1 cells. (A) Schematic of the sleeping beauty-based transposon cassette for ectopic expression of human furin (hFurin). A bidirectional EF1a promoter was used to drive the expression of hFurin and red fluorescent protein (RFP) with a puromycin resistance gene (Puro) linked by a 2A self-cleaving peptide (P2A). (B and C) Western blot (B) and immunofluorescence (C) images of polyclonal and clonal VF1 using anti-furin antibodies. (D to G) Growth kinetics and degree of maturation of DENV1-WT (D), DENV2-WT (E), DENV3-WT (F), and DENV4-WT (G) in unmodified Vero cells (black circles) and VF1 cells (blue triangles). Cells were infected with DENV at an MOI of 0.01 to 0.05 for 120 h. Supernatants were harvested every 24 h for 120 h, and the 120-h supernatants were analyzed by Western blotting for DENV maturation using anti-Env and anti-prM antibodies. All assays were performed with at least two biological repeats with two technical replicates. Growth kinetics of DENV variants were compared to their corresponding wild type using two-way ANOVA multiple comparisons.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
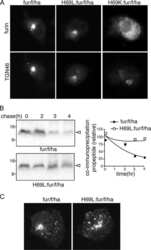
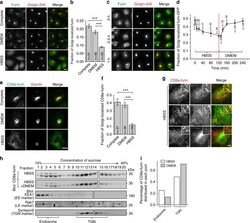
- Fig. 1 Starvation translocates TGN membrane proteins to endosomes. All cells are HeLa cells. a , b Furin loses its Golgi localization during starvation. Cells treated with indicated medium for 1 h and endogenous furin and Golgin-245 were stained. The fraction of Golgi-localized furin is quantified in b . c The recovery of Golgi localization of furin after supplying nutrient. After starvation in HBSS for 2 h, cells were treated with DMEM for indicated time and stained as in a . d Kinetics of Golgi-localized furin-GFP during HBSS and subsequent DMEM treatment. Cells expressing furin-GFP were first starved in HBSS for 2 h and subsequently stimulated by DMEM for 2 h. At indicated time, cells were stained for endogenous Giantin and the fraction of Golgi-localized furin-GFP is quantified. e , f Nutrient starvation significantly reduces the Golgi localization of CD8a-furin. Cells transiently expressing CD8a-furin were treated by indicated medium for 2 h and stained as in e . The fraction of Golgi-localized CD8a-furin is quantified in f . g The translocation of CD8a-furin to the endosome during nutrient starvation. Cells transiently expressing indicated constructs were treated with HBSS for 2 h and stained. Boxed regions are enlarged at the upper right corner. Arrows indicate colocalization. h , i The endosomal pool of CD8a-furin increases during nutrient starvation. Similar results have been observed in four independent experiments. Cells stably expressing CD8a-fu
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
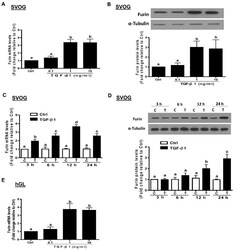
- Figure 2 TGF-beta1 upregulates the expression of furin in human granulosa-lutein cells. ( A , B ) SVOG cells were treated with vehicle control (PBS) or various concentrations (0.1, 1, or 10 ng/mL) of TGF-beta1 for 12 h ( A ) and 24 h ( B ), and the mRNA ( A ) and protein ( B ) levels of furin were examined using RT-PCR ( A ) and Western blot ( B ), respectively. ( C , D ) SVOG cells were treated with 1 ng/mL TGF-beta1 for 3, 6, 12 or 24 h, and the mRNA ( C ) and protein ( D ) levels of furin were examined using RT-PCR ( C ) and Western blot analysis ( D ), respectively. ( E ) hGL cells were treated with vehicle control or various concentrations (0.1, 1, or 10 ng/mL) of TGF-beta1 for 12 h, and the mRNA levels of furin were examined using RT-PCR. The results are expressed as the mean +- SE of at least three independent experiments. Values with different lower-case letters are significantly different ( p < 0.05). If a pair of values is significantly different ( p < 0.05), the values have different subscript letters (a vs. b or b vs. c) assigned to them.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
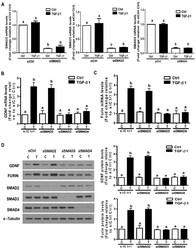
- Figure 5 The SMAD3-SMAD4 signaling pathway is required for the TGF-beta1-induced upregulation of GDNF and furin in SVOG cells. ( A ) SVOG cells were transfected with 25 nM siCtrl, 25 nM siSMAD2 25 nM siSMAD3 or 25 nM siSMAD4 for 48 h. The mRNA levels of SMAD2, SMAD3 and SMAD4 were examined using RT-qPCR. ( B , C ) SVOG cells were transfected for 48 h with 25 nM siCtrl, 25 nM siSMAD2, 25 nM siSMAD3 or 25 nM siSMAD4 and then treated with the vehicle control or 1 ng/mL TGF-beta1 for an additional 12 h. The mRNA levels of GDNF ( B ) and furin ( C ) were examined using RT-qPCR. ( D ) SVOG cells were transfected for 48 h with 25 nM siCtrl, 25 nM siSMAD2, 25 nM siSMAD3 or 25 nM siSMAD4 and then treated with the vehicle control or 1 ng/mL TGF-beta1 for an additional 24 h. The protein levels of GDNF, furin, SMAD2, SMAD3 and SMAD4 were examined using Western blot analysis. The results are expressed as the mean +- SE. Values with different lower-case letters are significantly different ( p < 0.05). If a pair of values is significantly different ( p < 0.05), the values have different subscript letters (a vs. b or b vs. c) assigned to them.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
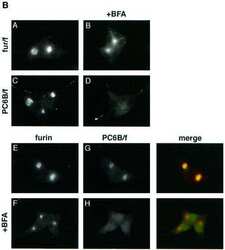
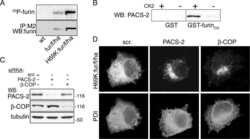
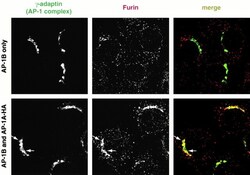
- Figure 6 AP-1A is necessary for furin localization at the TGN. mu1A -/- fibroblasts stably transfected with mu1B alone (top) or with mu1B and mu1A-HA (bottom) were double-labeled with anti-gamma-adaptin antibodies (left) and antifurin antibodies (middle) in combination with Alexa 488-labeled (anti-gamma staining) and Alexa 594-labeled (antifurin staining) secondary antibodies. Specimens were analyzed by confocal microscopy and representative images are shown. The arrows denote regions in the AP-1B/AP-1A-HA transfectants that are positive for AP-1 but negative for furin.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation