Antibody data
- Antibody Data
- Antigen structure
- References [6]
- Comments [0]
- Validations
- Flow cytometry [1]
- Other assay [6]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-812 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- RORA Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA1-812 detects human ROR alpha protein in transfected cell samples. PA1-812 is specific for alpha isotypes. PA1-812 has successfully been used in Western blot procedures. By Western blot, this antibody detects an ~56 kDa protein representing ROR alpha in transfected BL21 cells using a pGEX-hROR alpha1-GST vector. The PA1-812 immunogen is a synthetic peptide corresponding to residues K(541) E L F T S E F E P A M Q I D G(556) of human ROR alpha. This peptide (Cat. # PEP-230) is available for use in neutralization and control experiments.
- Reactivity
- Human, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Rora Regulates Neutrophil Migration and Activation in Zebrafish.
CYP11A1‑derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas.
Nobiletin Alleviates Non-alcoholic Steatohepatitis in MCD-Induced Mice by Regulating Macrophage Polarization.
A maresin 1/RORα/12-lipoxygenase autoregulatory circuit prevents inflammation and progression of nonalcoholic steatohepatitis.
RORα switches transcriptional mode of ERRγ that results in transcriptional repression of CYP2E1 under ethanol-exposure.
Expression of retinoic acid-related orphan receptor alpha and its responsive genes in human endometrium regulated by cholesterol sulfate.
Hsu AY, Wang T, Syahirah R, Liu S, Li K, Zhang W, Wang J, Cao Z, Tian S, Matosevic S, Staiger CJ, Wan J, Deng Q
Frontiers in immunology 2022;13:756034
Frontiers in immunology 2022;13:756034
CYP11A1‑derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas.
Slominski AT, Brożyna AA, Kim TK, Elsayed MM, Janjetovic Z, Qayyum S, Slominski RM, Oak ASW, Li C, Podgorska E, Li W, Jetten AM, Tuckey RC, Tang EKY, Elmets C, Athar M
International journal of oncology 2022 Aug;61(2)
International journal of oncology 2022 Aug;61(2)
Nobiletin Alleviates Non-alcoholic Steatohepatitis in MCD-Induced Mice by Regulating Macrophage Polarization.
Wang SW, Lan T, Sheng H, Zheng F, Lei MK, Wang LX, Chen HF, Xu CY, Zhang F
Frontiers in physiology 2021;12:687744
Frontiers in physiology 2021;12:687744
A maresin 1/RORα/12-lipoxygenase autoregulatory circuit prevents inflammation and progression of nonalcoholic steatohepatitis.
Han YH, Shin KO, Kim JY, Khadka DB, Kim HJ, Lee YM, Cho WJ, Cha JY, Lee BJ, Lee MO
The Journal of clinical investigation 2019 Mar 11;129(4):1684-1698
The Journal of clinical investigation 2019 Mar 11;129(4):1684-1698
RORα switches transcriptional mode of ERRγ that results in transcriptional repression of CYP2E1 under ethanol-exposure.
Han YH, Kim DK, Na TY, Ka NL, Choi HS, Lee MO
Nucleic acids research 2016 Feb 18;44(3):1095-104
Nucleic acids research 2016 Feb 18;44(3):1095-104
Expression of retinoic acid-related orphan receptor alpha and its responsive genes in human endometrium regulated by cholesterol sulfate.
Zenri F, Hiroi H, Momoeda M, Tsutsumi R, Hosokawa Y, Koizumi M, Nakae H, Osuga Y, Yano T, Taketani Y
The Journal of steroid biochemistry and molecular biology 2012 Jan;128(1-2):21-8
The Journal of steroid biochemistry and molecular biology 2012 Jan;128(1-2):21-8
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
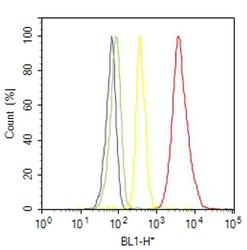
- Experimental details
- Flow cytometry analysis of ROR alpha was done on SH-SY5Y cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with ROR alpha Rabbit Polyclonal Antibody (PA1812, red histogram) or with rabbit isotype control (yellow histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control..
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
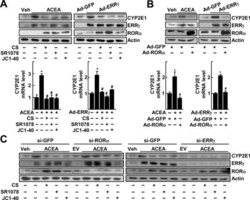
- Experimental details
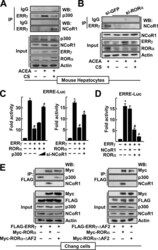
- Figure 1. RORalpha suppresses the ERRgamma-mediated induction of CYP2E1 gene expression. ( A ) Primary cultures of mouse hepatocytes were treated with 20 muM ACEA in the presence or absence of 20 muM CS, 5 muM SR1078 or 20 muM JC1-40 for 24 h. Or the hepatocytes were infected by Ad-GFP or Ad-ERRgamma and then underwent the same treatment. The protein (top) and mRNA (bottom) levels were measured by western blotting and qRT-PCR, respectively. ( B ) Hepatocytes were infected by Ad-GFP or Ad-ERRgamma and then treated with 20 muM ACEA for 24 h. Or the hepatocytes were infected by Ad-GFP or Ad-ERRgamma together with Ad-GFP or Ad-RORalpha for 24 h. The protein (top) and mRNA (bottom) levels were measured by western blotting and qRT-PCR, respectively. ( C ) Hepatocytes were transfected with si-GFP, si-RORalpha or si-ERRgamma for 24 h and then treated with 20 muM ACEA, 20 muM CS, 5 muM SR1078 and/or 20 muM JC1-40 for 24 h as indicated. The protein level was measured by western blotting. The data represent mean +- SD. * P < 0.05 versus vehicle treatment or Ad-GFP infection; # P < 0.05 versus ACEA treatment or Ad-ERRgamma infection (n = 3). Representatives of at least three independent experiments with similar results are shown.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
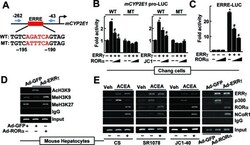
- Experimental details
- Figure 2. CYP2E1 expression is controlled by RORalpha through an ERRE in the CYP2E1 promoter. ( A ) Schematic representation of the mouse CYP2E1 promoter with the wild-type (WT) or the mutant (MT) ERRE. ( B ) Chang liver cells were transfected with the WT or the MT CYP2E1 promoter-Luc reporter with empty vector, FLAG-ERRgamma or FLAG-RORalpha for 24 h. Or the reporter transfected cells were treated with 10 or 20 muM JC1-40 (JC1) for additional 24 h. ( C ) Chang liver cells were transfected with the ERRE-Luc reporter with EV, FLAG-ERRgamma or FLAG-RORalpha for 24 h. Luciferase activity that normalized to the corresponding beta-galactosidase activity was converted to fold activity with no treatment as 1. The data represent mean +- SD. * P < 0.05 versus EV; # P < 0.05 versus ERRgamma (n = 3). ( D ) The hepatocytes were infected by Ad-GFP or Ad-ERRgamma with Ad-RORalpha for 24 h. ( E ) The primary cultures of mouse hepatocytes were treated with 20 muM ACEA, or infected by adenovirus encoding GFP or ERRgamma. The hepatocytes were treated with 20 muM CS, 5 muM SR1078 or 20 muM JC1-40, or co-infected by Ad-RORalpha for 24 h as indicated. DNA fragments that contain flanking region of the ERRE on the CYP2E1 promoter were immunoprecipitated with indicated anti-histone antibodies (panel D ), anti-ERRgamma, anti-p300, anti-RORalpha or anti-NCoR1 antibodies (panel E ) and then amplified by PCR using primers shown in panel A . Representatives of at least three independent experiments with
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
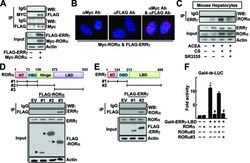
- Experimental details
- Figure 3. Physical protein interaction of RORa and ERRgamma. (A) Whole cell lysates obtained from the Chang liver cells transfected with FLAG-ERRgamma and MYC-RORa were immunoprecipitated (IP) and probed using the indicated antibodies by western blotting (WB). The level of proteins in the cell lysates was analyzed by WB as input. (B) Chang liver cells were transfected with FLAG-ERRgamma and MYC-RORa for 24 h. Interaction of ERRγ and RORa was visualized with red dots by in situ proximity ligation assay. As a negative control, a single staining with the anti-FLAG and anti-MYC antibodies was performed. DAPI was used to stain the nuclei. (C) The primary cultures of mouse hepatocytes were treated with 20 µM ACEA, 20 µM CS or 10 µM SR3335 for 24 h as indicated. Whole cell lysates obtained from the hepatocytes were IP and probed by the indicated antibodies by WB. The level of proteins in the cell lysates was analyzed by WB as input. (D and E) Chang liver cells were transfected with the FLAG- or MYC-tagged deleted constructs as indicated and whole cell lysates were IP and WB using specific antibodies. The level of proteins in the cell lysates was analyzed by WB as input. NT: N-terminus, DBD: DNA binding domain and LBD: ligand binding domain. (F) Chang liver cells were transfeced with Gal4-tk-Luc reporter and Gal4-ERRγ-LBD in the presence of the FLAG-tagged RORa deletion constructs for 24 h. Luciferase activity that normalized to the corresponding ß-galactosidase activity was converted to fold ac|
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 4. RORalpha recruits NcoR1 instead of p300 to ERRgamma in the CYP2E1 promoter. ( A ) The primary cultures of mouse hepatocytes were treated with 20 muM ACEA or 20 muM CS for 24 h as indicated. ( B ) The primary cultures of mouse hepatocytes were transfected with si-GFP or si-RORalpha, and treated with 20 muM ACEA and/or 20 muM CS as indicated. Whole cell lysates were IP and probed using the indicated antibodies by WB. The level of proteins in the cell lysates was analyzed by WB as input. ( C ) Chang liver cells were transfected with the ERRE-Luc with empty vector, the expression vectors encoding FLAG-ERRgamma, FLAG-RORalpha or increasing amount of MYC-p300 for 24 h as indicated (left). Or cells were transfected with the ERRE-Luc with the receptor expression vectors in the presence of si-NCoR1 for 48 h as indicated (right). Expression of NCoR1 protein after si-RNA-mediated knock-down was shown in Supplementary Figure S8. ( D ) Chang liver cells were transfected with the ERRE-Luc with the expression vectors encoding ERRgamma, RORalpha or NCoR1 for 24 h as indicated. Luciferase activity that normalized to the corresponding beta-galactosidase activity was converted to fold activity with no treatment as 1. The data represent mean +- SD. * P < 0.05 versus EV; # P < 0.05 versus ERRgamma; ≠ P < 0.05 versus ERRgamma with RORalpha (n = 3). ( E ) Whole cell lysates obtained from the Chang liver cells transfected with FLAG-ERRgamma, ERRgamma-DeltaAF2, MYC-RORalpha and/or RORalph
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
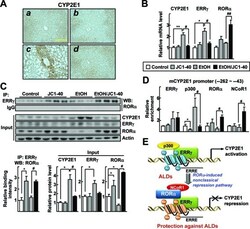
- Figure 6. JC1-40 decreases ERRgamma and CYP2E1 expression in the ethanol-induced mouse liver disease. Eight week-old C57BL/6N mice were fed with the isocaloric pair-fed diet ( A and B ) or 5% ethanol-containing Lieber-DeCarli liquid diet ( C and D ) for 5 weeks. After 3 weeks of diet feeding, vehicle ( A and C ) or JC1-40, 10 mg/kg/day ( B and D ), was administered daily at doses by oral gavage for 2 weeks. ( A ) Immunohistochemistry staining of CYP2E1 in liver sections. Magnification of X200. Yellow bars represent 200 mum. ( B ) mRNA levels of CYP2E1, ERRgamma and RORalpha in liver tissues were analyzed by qRT-PCR. * P < 0.05, and ** P < 0.01 versus pair-fed control group (n = 8); # P < 0.05, and ## P < 0.05 versus ethanol-fed with vehicle group (n = 12). ( C ) Whole liver tissue lysates were IP with anti-ERRgamma antibody and probed using anti-RORalpha antibody by WB. Protein expression levels of CYP2E1, ERRgamma and RORalpha in liver tissues were analyzed by WB (top) and the indicated band intensities were quantified (bottom). ( D ) DNA fragments obtained from liver tissues that contain flanking region of the ERRE on the CYP2E1 promoter were immunoprecipitated with the anti-ERRgamma, anti-p300, anti-RORalpha or anti-NCoR1 antibodies and then amplified by qPCR using primers shown in Figure 2E . * P < 0.05 versus pair-fed control group; # P < 0.05 versus ethanol-fed with vehicle group (n = 6). ( E ) Schematic model for repression of the ERRgamma-induced CYP2E1 gene expressio
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
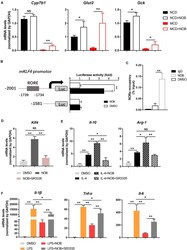
- FIGURE 7 NOB induces Klf4 expression and macrophage polarization through activiting RORalpha. (A) RT-qPCR analysis of RORalpha target genes Cyp7b1 , Glut2 , and Gck in the livers of MCD-fed mice. Data were expressed as the mean +- SD ( n = 5). (B) Schematic diagram of the mouse Klf4 promoter with the RORalpha binding element (RORE) shown as gray box. 293T cells were transfected with luciferase reporters driven by Klf4 promoter with or without RORE and treated with DMSO or NOB (100 muM) for 8 h. (C) RAW 264.7 cells were treated with DMSO or NOB (100 muM) for 16 h. The binding of RORalpha to Klf4 promotor was assessed by ChIP-qPCR. (D) RAW 264.7 cells were treated with DMSO, NOB (100 muM) or SR3335 (10 muM) for 16 h. The mRNA level of Klf4 determined by RT-qPCR. (E) RAW 264.7 cells were treated with DMSO, IL-4 (20 ng/mL), IL-4 (20 ng/mL) + NOB (100 muM) or IL-4 (20 ng/mL) + NOB (100 muM) + SR3335 (10 muM) for 16 h. The mRNA levels of Arg-1 and Il-10 determined by RT-qPCR. (F) RAW 264.7 cells were pretreated with DMSO, LPS (200 ng/mL), LPS (200 ng/mL) + NOB (100 muM) or LPS (200 ng/mL) + NOB (100 muM) + SR3335 (10 muM) for 16 h. Proinflammatory factors Il-6 , Il-1 beta, and Tnf- alpha determined by RT-qPCR. Values are expressed as mean +- SD ( n = 3). * P < 0.05, ** P < 0.01.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Flow cytometry
Flow cytometry