MA5-14419
antibody from Invitrogen Antibodies
Targeting: RAD51
BRCC5, FANCR, HsRad51, HsT16930, RAD51A, RECA
Antibody data
- Antibody Data
- Antigen structure
- References [28]
- Comments [0]
- Validations
- Immunocytochemistry [5]
- Immunoprecipitation [1]
- Immunohistochemistry [1]
- Other assay [6]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA5-14419 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- RAD51 Monoclonal Antibody (51RAD01 (3C10))
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Description
- MA5-14419 targets RAD51 in IHC (P), IP, ICC/IF and WB applications and shows reactivity with Human, mouse, and Rat samples. The MA5-14419 immunogen is recombinant Rad51 protein.
- Reactivity
- Human, Mouse, Rat
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 51RAD01 (3C10)
- Vial size
- 500 μL
- Concentration
- 0.2 mg/mL
- Storage
- 4°C
Submitted references Local DNA synthesis is critical for DNA repair during oocyte maturation.
The association of Helicobacter pylori CagA EPIYA motifs and vacA genotypes with homologous recombination repair markers during the gastric precancerous cascade.
SPOP Deregulation Improves the Radiation Response of Prostate Cancer Models by Impairing DNA Damage Repair.
Proline-rich protein PRR19 functions with cyclin-like CNTD1 to promote meiotic crossing over in mouse.
The relevance of prelamin A and RAD51 as molecular biomarkers in cervical cancer.
Replication Stress Leading to Apoptosis within the S-phase Contributes to Synergism between Vorinostat and AZD1775 in HNSCC Harboring High-Risk TP53 Mutation.
A Zip3-like protein plays a role in crossover formation in the SC-less meiosis of the protist Tetrahymena.
Cataloging antineoplastic agents according to their effectiveness against platinum-resistant and platinum-sensitive ovarian carcinoma cell lines.
The DNA damage/repair cascade in glioblastoma cell lines after chemotherapeutic agent treatment.
Chiasmatic and achiasmatic inverted meiosis of plants with holocentric chromosomes.
Rad51 expression is a useful predictive factor for the efficacy of neoadjuvant chemoradiotherapy in squamous cell carcinoma of the esophagus.
A single cohesin complex performs mitotic and meiotic functions in the protist tetrahymena.
Human papillomavirus in oral atrophic lichen planus lesions.
Nucleostemin prevents telomere damage by promoting PML-IV recruitment to SUMOylated TRF1.
The Tetrahymena meiotic chromosome bouquet is organized by centromeres and promotes interhomolog recombination.
The recombinases Rad51 and Dmc1 play distinct roles in DNA break repair and recombination partner choice in the meiosis of Tetrahymena.
The role of meiotic cohesin REC8 in chromosome segregation in gamma irradiation-induced endopolyploid tumour cells.
p63 and p73 transcriptionally regulate genes involved in DNA repair.
DNA double-strand breaks, but not crossovers, are required for the reorganization of meiotic nuclei in Tetrahymena.
Selenium protection from DNA damage involves a Ref1/p53/Brca1 protein complex.
Meiotic recombination proteins localize to linear elements in Schizosaccharomyces pombe.
Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition.
Interaction of radiation- and bleomycin-induced lesions and influence of glutathione level on the interaction.
Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva).
Organization and pairing of meiotic chromosomes in the ciliate Tetrahymena thermophila.
Recruitment of cellular recombination and repair proteins to sites of herpes simplex virus type 1 DNA replication is dependent on the composition of viral proteins within prereplicative sites and correlates with the induction of the DNA damage response.
Endopolyploid cells produced after severe genotoxic damage have the potential to repair DNA double strand breaks.
Analysis of murine Brca2 reveals conservation of protein-protein interactions but differences in nuclear localization signals.
Singh AK, Kumar SL, Beniwal R, Mohanty A, Kushwaha B, Rao HBDP
Journal of cell science 2021 Oct 1;134(19)
Journal of cell science 2021 Oct 1;134(19)
The association of Helicobacter pylori CagA EPIYA motifs and vacA genotypes with homologous recombination repair markers during the gastric precancerous cascade.
Mi Y, Dong H, Sun X, Ren F, Tang Y, Zheng P
The International journal of biological markers 2020 Jun;35(2):49-55
The International journal of biological markers 2020 Jun;35(2):49-55
SPOP Deregulation Improves the Radiation Response of Prostate Cancer Models by Impairing DNA Damage Repair.
El Bezawy R, Tripari M, Percio S, Cicchetti A, Tortoreto M, Stucchi C, Tinelli S, Zuco V, Doldi V, Gandellini P, Valdagni R, Zaffaroni N
Cancers 2020 Jun 4;12(6)
Cancers 2020 Jun 4;12(6)
Proline-rich protein PRR19 functions with cyclin-like CNTD1 to promote meiotic crossing over in mouse.
Bondarieva A, Raveendran K, Telychko V, Rao HBDP, Ravindranathan R, Zorzompokou C, Finsterbusch F, Dereli I, Papanikos F, Tränkner D, Schleiffer A, Fei JF, Klimova A, Ito M, Kulkarni DS, Roeder I, Hunter N, Tóth A
Nature communications 2020 Jun 18;11(1):3101
Nature communications 2020 Jun 18;11(1):3101
The relevance of prelamin A and RAD51 as molecular biomarkers in cervical cancer.
Leonardi S, Buttarelli M, De Stefano I, Ferrandina G, Petrillo M, Babini G, Scambia G, Marino C, Mancuso M, Gallo D
Oncotarget 2017 Nov 7;8(55):94247-94258
Oncotarget 2017 Nov 7;8(55):94247-94258
Replication Stress Leading to Apoptosis within the S-phase Contributes to Synergism between Vorinostat and AZD1775 in HNSCC Harboring High-Risk TP53 Mutation.
Tanaka N, Patel AA, Tang L, Silver NL, Lindemann A, Takahashi H, Jaksik R, Rao X, Kalu NN, Chen TC, Wang J, Frederick MJ, Johnson F, Gleber-Netto FO, Fu S, Kimmel M, Wang J, Hittelman WN, Pickering CR, Myers JN, Osman AA
Clinical cancer research : an official journal of the American Association for Cancer Research 2017 Nov 1;23(21):6541-6554
Clinical cancer research : an official journal of the American Association for Cancer Research 2017 Nov 1;23(21):6541-6554
A Zip3-like protein plays a role in crossover formation in the SC-less meiosis of the protist Tetrahymena.
Shodhan A, Kataoka K, Mochizuki K, Novatchkova M, Loidl J
Molecular biology of the cell 2017 Mar 15;28(6):825-833
Molecular biology of the cell 2017 Mar 15;28(6):825-833
Cataloging antineoplastic agents according to their effectiveness against platinum-resistant and platinum-sensitive ovarian carcinoma cell lines.
Ishiguro K, Zhu YL, Lin ZP, Penketh PG, Shyam K, Zhu R, Baumann RP, Sartorelli AC, Rutherford TJ, Ratner ES
Journal of translational science 2016;2(2):117-124
Journal of translational science 2016;2(2):117-124
The DNA damage/repair cascade in glioblastoma cell lines after chemotherapeutic agent treatment.
Annovazzi L, Caldera V, Mellai M, Riganti C, Battaglia L, Chirio D, Melcarne A, Schiffer D
International journal of oncology 2015;46(6):2299-308
International journal of oncology 2015;46(6):2299-308
Chiasmatic and achiasmatic inverted meiosis of plants with holocentric chromosomes.
Cabral G, Marques A, Schubert V, Pedrosa-Harand A, Schlögelhofer P
Nature communications 2014 Oct 8;5:5070
Nature communications 2014 Oct 8;5:5070
Rad51 expression is a useful predictive factor for the efficacy of neoadjuvant chemoradiotherapy in squamous cell carcinoma of the esophagus.
Nakanoko T, Saeki H, Morita M, Nakashima Y, Ando K, Oki E, Ohga T, Kakeji Y, Toh Y, Maehara Y
Annals of surgical oncology 2014 Feb;21(2):597-604
Annals of surgical oncology 2014 Feb;21(2):597-604
A single cohesin complex performs mitotic and meiotic functions in the protist tetrahymena.
Howard-Till RA, Lukaszewicz A, Novatchkova M, Loidl J
PLoS genetics 2013 Mar;9(3):e1003418
PLoS genetics 2013 Mar;9(3):e1003418
Human papillomavirus in oral atrophic lichen planus lesions.
Mattila R, Rautava J, Syrjänen S
Oral oncology 2012 Oct;48(10):980-984
Oral oncology 2012 Oct;48(10):980-984
Nucleostemin prevents telomere damage by promoting PML-IV recruitment to SUMOylated TRF1.
Hsu JK, Lin T, Tsai RY
The Journal of cell biology 2012 May 28;197(5):613-24
The Journal of cell biology 2012 May 28;197(5):613-24
The Tetrahymena meiotic chromosome bouquet is organized by centromeres and promotes interhomolog recombination.
Loidl J, Lukaszewicz A, Howard-Till RA, Koestler T
Journal of cell science 2012 Dec 1;125(Pt 23):5873-80
Journal of cell science 2012 Dec 1;125(Pt 23):5873-80
The recombinases Rad51 and Dmc1 play distinct roles in DNA break repair and recombination partner choice in the meiosis of Tetrahymena.
Howard-Till RA, Lukaszewicz A, Loidl J
PLoS genetics 2011 Mar;7(3):e1001359
PLoS genetics 2011 Mar;7(3):e1001359
The role of meiotic cohesin REC8 in chromosome segregation in gamma irradiation-induced endopolyploid tumour cells.
Erenpreisa J, Cragg MS, Salmina K, Hausmann M, Scherthan H
Experimental cell research 2009 Sep 10;315(15):2593-603
Experimental cell research 2009 Sep 10;315(15):2593-603
p63 and p73 transcriptionally regulate genes involved in DNA repair.
Lin YL, Sengupta S, Gurdziel K, Bell GW, Jacks T, Flores ER
PLoS genetics 2009 Oct;5(10):e1000680
PLoS genetics 2009 Oct;5(10):e1000680
DNA double-strand breaks, but not crossovers, are required for the reorganization of meiotic nuclei in Tetrahymena.
Mochizuki K, Novatchkova M, Loidl J
Journal of cell science 2008 Jul 1;121(Pt 13):2148-58
Journal of cell science 2008 Jul 1;121(Pt 13):2148-58
Selenium protection from DNA damage involves a Ref1/p53/Brca1 protein complex.
Fischer JL, Lancia JK, Mathur A, Smith ML
Anticancer research 2006 Mar-Apr;26(2A):899-904
Anticancer research 2006 Mar-Apr;26(2A):899-904
Meiotic recombination proteins localize to linear elements in Schizosaccharomyces pombe.
Lorenz A, Estreicher A, Kohli J, Loidl J
Chromosoma 2006 Aug;115(4):330-40
Chromosoma 2006 Aug;115(4):330-40
Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition.
McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, Giavara S, O'Connor MJ, Tutt AN, Zdzienicka MZ, Smith GC, Ashworth A
Cancer research 2006 Aug 15;66(16):8109-15
Cancer research 2006 Aug 15;66(16):8109-15
Interaction of radiation- and bleomycin-induced lesions and influence of glutathione level on the interaction.
Dutta A, Chakraborty A, Saha A, Ray S, Chatterjee A
Mutagenesis 2005 Sep;20(5):329-35
Mutagenesis 2005 Sep;20(5):329-35
Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva).
Chinnaiyan P, Huang S, Vallabhaneni G, Armstrong E, Varambally S, Tomlins SA, Chinnaiyan AM, Harari PM
Cancer research 2005 Apr 15;65(8):3328-35
Cancer research 2005 Apr 15;65(8):3328-35
Organization and pairing of meiotic chromosomes in the ciliate Tetrahymena thermophila.
Loidl J, Scherthan H
Journal of cell science 2004 Nov 15;117(Pt 24):5791-801
Journal of cell science 2004 Nov 15;117(Pt 24):5791-801
Recruitment of cellular recombination and repair proteins to sites of herpes simplex virus type 1 DNA replication is dependent on the composition of viral proteins within prereplicative sites and correlates with the induction of the DNA damage response.
Wilkinson DE, Weller SK
Journal of virology 2004 May;78(9):4783-96
Journal of virology 2004 May;78(9):4783-96
Endopolyploid cells produced after severe genotoxic damage have the potential to repair DNA double strand breaks.
Ivanov A, Cragg MS, Erenpreisa J, Emzinsh D, Lukman H, Illidge TM
Journal of cell science 2003 Oct 15;116(Pt 20):4095-106
Journal of cell science 2003 Oct 15;116(Pt 20):4095-106
Analysis of murine Brca2 reveals conservation of protein-protein interactions but differences in nuclear localization signals.
Sarkisian CJ, Master SR, Huber LJ, Ha SI, Chodosh LA
The Journal of biological chemistry 2001 Oct 5;276(40):37640-8
The Journal of biological chemistry 2001 Oct 5;276(40):37640-8
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
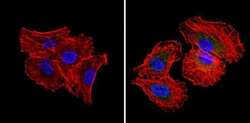
- Experimental details
- Immunofluorescent analysis of RAD51 (green) showing staining in the cytoplasm and nucleus of H1299 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a RAD51 monoclonal antibody (Product # MA5-14419) in 3% BSA-PBS at a dilution of 1:20 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. Actin was stained using Alexa Fluor 554 (red) and nuclei were stained with Hoechst or DAPI (blue). Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
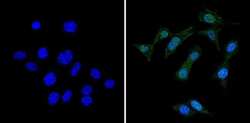
- Experimental details
- Immunofluorescent analysis of RAD51 (green) showing staining in the cytoplasm and nucleus of NIH-3T3 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a RAD51 monoclonal antibody (Product # MA5-14419) in 3% BSA-PBS at a dilution of 1:50 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. Nuclei were stained with Hoechst or DAPI (blue). Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
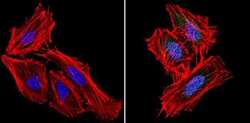
- Experimental details
- Immunofluorescent analysis of RAD51 (green) showing staining in the cytoplasm and nucleus of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a RAD51 monoclonal antibody (Product # MA5-14419) in 3% BSA-PBS at a dilution of 1:50 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. Actin was stained using Alexa Fluor 554 (red) and nuclei were stained with Hoechst or DAPI (blue). Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
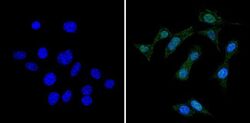
- Experimental details
- Immunofluorescent analysis of RAD51 (green) showing staining in the cytoplasm and nucleus of NIH-3T3 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a RAD51 monoclonal antibody (Product # MA5-14419) in 3% BSA-PBS at a dilution of 1:50 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. Nuclei were stained with Hoechst or DAPI (blue). Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
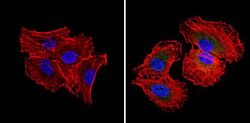
- Experimental details
- Immunofluorescent analysis of RAD51 (green) showing staining in the cytoplasm and nucleus of H1299 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a RAD51 monoclonal antibody (Product # MA5-14419) in 3% BSA-PBS at a dilution of 1:20 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. Actin was stained using Alexa Fluor 554 (red) and nuclei were stained with Hoechst or DAPI (blue). Images were taken at a magnification of 60x.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunoprecipitation of RAD51 using RAD51 Monoclonal Antibody (Product # MA5-14419) on Native Human LS174T Cells.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
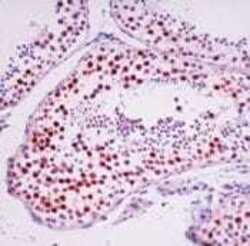
- Experimental details
- Formalin-fixed, paraffin-embedded human testis stained with Rad 51 antibody using peroxidase-conjugate and AEC chromogen. Note nuclear staining of germ cells.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
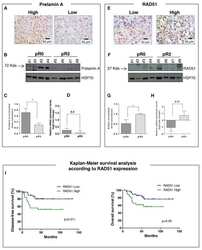
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
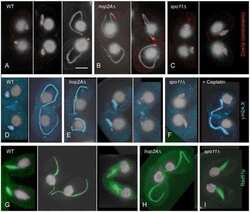
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunoprecipitation of RAD51 using RAD51 Monoclonal Antibody (Product # MA5-14419) on Native Human LS174T Cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
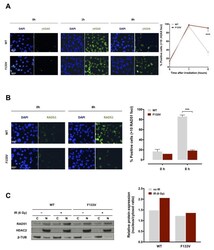
- Figure 4 F133V SPOP impairs cell ability to recover from radiation-induced DNA damage. ( A ) (Left panel) Representative immunofluorescence microphotographs of nuclear gammaH2AX foci in DU145 WT or F133V SPOP cells at 0, 1 and 8 h upon 6 Gy irradiation. (Right panel) Quantification of gammaH2AX foci in WT or F133V SPOP DU145 cells, expressed as mean percentage of cells containing >10 gammaH2AX foci at 0, 1 and 8 h upon irradiation. Data are reported as mean +- SD values. The level of significance was represented as *** p < 0.001, Student's t -test. ( B ) (Left panel) Representative immunofluorescence microphotographs of RAD51 nuclear foci in DU145 WT or F133V SPOP cells at 0 and 8 h upon irradiation (6 Gy). (Right panel) Quantification of RAD51 foci expressed as mean percentage of cells containing >10 RAD51 foci at 0 and 8 h upon irradiation (IR). Data are reported as mean +- SD. ( C ) Western blot analysis and relative quantification of RAD51 protein levels in nucleus/cytosol fractions of DU145 WT or F133V cells at 0 and 8 h upon irradiation (6 Gy). HDAC2 and beta-tubulin were used as equal protein loading controls for nuclear and cytosolic fractions, respectively. Relative protein expression was calculated as nuclear/cytosolic ratio of normalized RAD51 levels.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
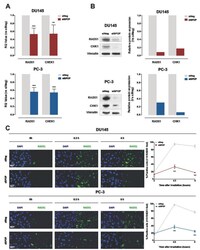
- Figure 6 SPOP knockdown impairs HR via RAD51 and CHK1 downregulation. ( A ) qRT-PCR detection of RAD51 and CHEK1 transcript levels in DU145 (upper panel) or PC-3 (lower panel) cells at 48 h upon siSPOP transfection, compared to controls, normalized to GAPDH. Data are reported as relative quantity (RQ) +- SD with respect to siNeg transfectants. ( B ) Western blot analysis and relative quantification of RAD51 and CHK1 protein levels in DU145 and PC-3 cells at 48 h upon siSPOP transfection. Vinculin was used as equal protein loading control. ( C ) Representative immunofluorescence microphotographs of nuclear RAD51 foci (cell nuclei: blue; RAD51 foci: green) in DU145 (upper panel) or PC-3 (lower panel) cells at 48 h upon transfection with siSPOP at 0, 0.5 and 6 h after exposure to 6 Gy irradiation and relative quantification, expressed as mean percentage of cells containing >10 RAD51 foci at 0, 0.5 and 6 h after exposure to 6 Gy irradiation. Data are reported as mean +- SD values from three independent experiments. The level of significance was represented as ** p < 0.01, *** p < 0.001, Student's t -test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
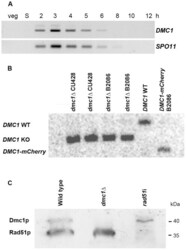
- Figure 1 Expression of Dmc1p and Rad51p. (A) Expression pattern of DMC1 as visualized by RT-PCR. While there is no transcription in vegetatively (veg) growing and starving (S) cells, DMC1 mRNA is maximally abundant in early meiotic cells from 2-4 h after meiosis induction. Timing of expression is the same as that of SPO11 . (B) Southern hybridization with a probe adjacent to the 3'end of the DMC1 locus. Digestion of genomic DNA from knockout, wild-type and DMC1-mCherry strains with EcoRI, which differentially cuts internally in the knockout and tagging constructs, produced the detected fragments of 2.1 kb, 2.8 kb, and 1.8 kb, respectively. The absence of the 2.8 kb fragment in knockout samples indicates that the replacement of the expressed wild-type sequences in the somatic nucleus by the knockout cassette is complete in several lines of both mating types. Also shown is the complete replacement of wild-type DMC1 by a version fused to a sequence encoding the mCherry fluorescent marker. (C) Western detection of Rad51p and Dmc1p with an antibody that recognizes both proteins. At t = 3.5 h after meiosis induction, both Rad51p and Dmc1p are expressed in wild type conjugating cells. In the dmc1 Delta strain, no detectable amount of Dmc1p was expressed 3.5 h after meiosis induction. In a crossing of two rad51 i strains, expression of Rad51p was strongly reduced. Positions of 35 kDa and 40 kDa marker bands are indicated.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry