Antibody data
- Antibody Data
- Antigen structure
- References [214]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [3]
- Other assay [100]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA5-12960 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Cardiac Troponin T Monoclonal Antibody (13-11)
- Antibody type
- Monoclonal
- Antigen
- Purifed from natural sources
- Description
- MA5-12960 targets Troponin T Cardiac Isoform in IF/ICC, IHC (P), and IM applications and shows reactivity with Avian, Canine, Chicken, Fish, Guinea Pig, Human, mouse, Porcine, Rabbit, and Rat samples. The MA5-12960 immunogen is purified rabbit cardiac troponin T isoform (TnT4R).
- Reactivity
- Human, Mouse, Rat, Canine, Chicken/Avian, Guinea Pig, Porcine, Rabbit
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 13-11
- Vial size
- 200 μL
- Concentration
- 0.5 mg/mL
- Storage
- 4°C
Submitted references Ubiquitin Carboxyl-Terminal Hydrolase L1 of Cardiomyocytes Promotes Macroautophagy and Proteostasis and Protects Against Post-myocardial Infarction Cardiac Remodeling and Heart Failure.
FUCCI-Based Live Imaging Platform Reveals Cell Cycle Dynamics and Identifies Pro-proliferative Compounds in Human iPSC-Derived Cardiomyocytes.
CIEGAN: A Deep Learning Tool for Cell Image Enhancement.
Biomimetic cardiac tissue culture model (CTCM) to emulate cardiac physiology and pathophysiology ex vivo.
devCellPy is a machine learning-enabled pipeline for automated annotation of complex multilayered single-cell transcriptomic data.
Transient Receptor Potential Vanilloid Type 1 Protects Against Pressure Overload-Induced Cardiac Hypertrophy by Promoting Mitochondria-Associated Endoplasmic Reticulum Membranes.
Mettl3-mediated m(6)A modification of Fgf16 restricts cardiomyocyte proliferation during heart regeneration.
CD47 antibody protects mice from doxorubicin-induced myocardial damage by suppressing cardiomyocyte apoptosis.
Tamoxifen treatment ameliorates contractile dysfunction of Duchenne muscular dystrophy stem cell-derived cardiomyocytes on bioengineered substrates.
A medium-chain triglyceride containing ketogenic diet exacerbates cardiomyopathy in a CRISPR/Cas9 gene-edited rat model with Duchenne muscular dystrophy.
Cardiac ultrastructure inspired matrix induces advanced metabolic and functional maturation of differentiated human cardiomyocytes.
An automated do-it-yourself system for dynamic stem cell and organoid culture in standard multi-well plates.
Microgravity-induced stress mechanisms in human stem cell-derived cardiomyocytes.
Persistent fibrosis and decreased cardiac function following cardiac injury in the Ctenopharyngodon idella (grass carp).
A New Versatile Platform for Assessment of Improved Cardiac Performance in Human-Engineered Heart Tissues.
Inhibition of SARS-CoV-2 infection in human iPSC-derived cardiomyocytes by targeting the Sigma-1 receptor disrupts cytoarchitecture and beating.
Sall4 and Myocd Empower Direct Cardiac Reprogramming From Adult Cardiac Fibroblasts After Injury.
Free Feeding of CpG-Oligodeoxynucleotide Particles Prophylactically Attenuates Allergic Airway Inflammation and Hyperresponsiveness in Mice.
High-Throughput Drug Screening System Based on Human Induced Pluripotent Stem Cell-Derived Atrial Myocytes ∼ A Novel Platform to Detect Cardiac Toxicity for Atrial Arrhythmias.
Nrf1 promotes heart regeneration and repair by regulating proteostasis and redox balance.
Pharmacologic therapy for engraftment arrhythmia induced by transplantation of human cardiomyocytes.
Auto/paracrine factors and early Wnt inhibition promote cardiomyocyte differentiation from human induced pluripotent stem cells at initial low cell density.
AAV-mediated YAP expression in cardiac fibroblasts promotes inflammation and increases fibrosis.
Neonatal hyperoxia inhibits proliferation and survival of atrial cardiomyocytes by suppressing fatty acid synthesis.
Role of Heme-Oxygenase-1 in Biology of Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells.
Human heart-forming organoids recapitulate early heart and foregut development.
PMN-derived netrin-1 attenuates cardiac ischemia-reperfusion injury via myeloid ADORA2B signaling.
Fosl1 is vital to heart regeneration upon apex resection in adult Xenopus tropicalis.
Bioactive Lipid O-cyclic phytosphingosine-1-phosphate Promotes Differentiation of Human Embryonic Stem Cells into Cardiomyocytes via ALK3/BMPR Signaling.
m6A modification promotes miR-133a repression during cardiac development and hypertrophy via IGF2BP2.
ERRγ enhances cardiac maturation with T-tubule formation in human iPSC-derived cardiomyocytes.
Setd4 controlled quiescent c-Kit(+) cells contribute to cardiac neovascularization of capillaries beyond activation.
Non-permissive SARS-CoV-2 infection in human neurospheres.
Molecular-scale visualization of sarcomere contraction within native cardiomyocytes.
Microenvironmental determinants of organized iPSC-cardiomyocyte tissues on synthetic fibrous matrices.
Overcoming bioprocess bottlenecks in the large-scale expansion of high-quality hiPSC aggregates in vertical-wheel stirred suspension bioreactors.
Oxygen Is an Ambivalent Factor for the Differentiation of Human Pluripotent Stem Cells in Cardiac 2D Monolayer and 3D Cardiac Spheroids.
Pim1 maintains telomere length in mouse cardiomyocytes by inhibiting TGFβ signalling.
Capturing Cardiogenesis in Gastruloids.
3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels.
Gastrin exerts a protective effect against myocardial infarction via promoting angiogenesis.
Method for selective ablation of undifferentiated human pluripotent stem cell populations for cell-based therapies.
Engrafted Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Undergo Clonal Expansion In Vivo.
An Aurora Kinase B-Based Mouse System to Efficiently Identify and Analyze Proliferating Cardiomyocytes.
Liquid marble technology to create cost-effective 3D cardiospheres as a platform for in vitro drug testing and disease modelling.
Co-culture of induced pluripotent stem cells with cardiomyocytes is sufficient to promote their differentiation into cardiomyocytes.
Misoprostol attenuates neonatal cardiomyocyte proliferation through Bnip3, perinuclear calcium signaling, and inhibition of glycolysis.
Stirred suspension bioreactors maintain naïve pluripotency of human pluripotent stem cells.
NOTCH1 is critical for fibroblast-mediated induction of cardiomyocyte specialization into ventricular conduction system-like cells in vitro.
Differences in Mitochondrial Membrane Potential Identify Distinct Populations of Human Cardiac Mesenchymal Progenitor Cells.
Progesterone, via yes-associated protein, promotes cardiomyocyte proliferation and cardiac repair.
Heart slice culture system reliably demonstrates clinical drug-related cardiotoxicity.
Canonical Wnt signaling promotes pacemaker cell specification of cardiac mesodermal cells derived from mouse and human embryonic stem cells.
HIF1α-dependent mitophagy facilitates cardiomyoblast differentiation.
A human embryonic stem cell reporter line for monitoring chemical-induced cardiotoxicity.
Subtype-Directed Differentiation of Human iPSCs into Atrial and Ventricular Cardiomyocytes.
Human ARF Specifically Inhibits Epimorphic Regeneration in the Zebrafish Heart.
Variability in Cardiac miRNA-122 Level Determines Therapeutic Potential of miRNA-Regulated AAV Vectors.
Metabolic Maturation Media Improve Physiological Function of Human iPSC-Derived Cardiomyocytes.
Tissue-engineered human embryonic stem cell-containing cardiac patches: evaluating recellularization of decellularized matrix.
Heart-derived fibroblasts express LYPD-1 and negatively regulate angiogenesis in rat.
Cell-Type-Specific Gene Regulatory Networks Underlying Murine Neonatal Heart Regeneration at Single-Cell Resolution.
Comprehensive Biology and Genetics Compendium of Wilms Tumor Cell Lines with Different WT1 Mutations.
Generation of two human ISG15 knockout iPSC clones using CRISPR/Cas9 editing.
Dynamic Transcriptional Responses to Injury of Regenerative and Non-regenerative Cardiomyocytes Revealed by Single-Nucleus RNA Sequencing.
Bmi1 inhibitor PTC-209 promotes Chemically-induced Direct Cardiac Reprogramming of cardiac fibroblasts into cardiomyocytes.
miRNAs in Extracellular Vesicles from iPS-Derived Cardiac Progenitor Cells Effectively Reduce Fibrosis and Promote Angiogenesis in Infarcted Heart.
Long-Term Engraftment of Human Cardiomyocytes Combined with Biodegradable Microparticles Induces Heart Repair.
Chromatin compartment dynamics in a haploinsufficient model of cardiac laminopathy.
Irisin exerts a therapeutic effect against myocardial infarction via promoting angiogenesis.
Stoichiometric optimization of Gata4, Hand2, Mef2c, and Tbx5 expression for contractile cardiomyocyte reprogramming.
Generation of the human induced pluripotent stem cell (hiPSC) line PSMi004-A from a carrier of the KCNQ1-R594Q mutation.
Adaptation of Human iPSC-Derived Cardiomyocytes to Tyrosine Kinase Inhibitors Reduces Acute Cardiotoxicity via Metabolic Reprogramming.
Structural evidence for a new elaborate 3D-organization of the cardiomyocyte lateral membrane in adult mammalian cardiac tissues.
Treatment with apolipoprotein A1 protects mice against doxorubicin-induced cardiotoxicity in a scavenger receptor class B, type I-dependent manner.
Cardiac Reprogramming Factors Synergistically Activate Genome-wide Cardiogenic Stage-Specific Enhancers.
Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction.
Generation of human iPS cell line CBTCi001-A from dermal fibroblasts obtained from a healthy donor.
Adult human cardiac stem cell supplementation effectively increases contractile function and maturation in human engineered cardiac tissues.
Multifactorial Modeling Reveals a Dominant Role of Wnt Signaling in Lineage Commitment of Human Pluripotent Stem Cells.
A generally conserved response to hypoxia in iPSC-derived cardiomyocytes from humans and chimpanzees.
Transcriptome and DNA Methylome Dynamics during Triclosan-Induced Cardiomyocyte Differentiation Toxicity.
Phenotypic Screening Using Patient-Derived Induced Pluripotent Stem Cells Identified Pyr3 as a Candidate Compound for the Treatment of Infantile Hypertrophic Cardiomyopathy.
Single-Cell Transcriptomic Analysis of Cardiac Differentiation from Human PSCs Reveals HOPX-Dependent Cardiomyocyte Maturation.
Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration.
Low-temperature culturing improves survival rate of tissue-engineered cardiac cell sheets.
Atorvastatin Inhibits the HIF1α-PPAR Axis, Which Is Essential for Maintaining the Function of Human Induced Pluripotent Stem Cells.
The microRNA regulatory landscape of MSC-derived exosomes: a systems view.
Endocardial Hippo signaling regulates myocardial growth and cardiogenesis.
High-density lipoprotein protects cardiomyocytes against necrosis induced by oxygen and glucose deprivation through SR-B1, PI3K, and AKT1 and 2.
Culture in Glucose-Depleted Medium Supplemented with Fatty Acid and 3,3',5-Triiodo-l-Thyronine Facilitates Purification and Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes.
Cited4 is related to cardiogenic induction and maintenance of proliferation capacity of embryonic stem cell-derived cardiomyocytes during in vitro cardiogenesis.
ZNF281 enhances cardiac reprogramming by modulating cardiac and inflammatory gene expression.
A transcribed enhancer dictates mesendoderm specification in pluripotency.
Notch Inhibition Enhances Cardiac Reprogramming by Increasing MEF2C Transcriptional Activity.
Circadian networks in human embryonic stem cell-derived cardiomyocytes.
MED12 regulates a transcriptional network of calcium-handling genes in the heart.
Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker.
Role of FEN1 S187 phosphorylation in counteracting oxygen-induced stress and regulating postnatal heart development.
Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells.
Generation of PDGFRα(+) Cardioblasts from Pluripotent Stem Cells.
Differentiation of Human Pluripotent Stem Cells to Cardiomyocytes Under Defined Conditions.
Embryonic type Na(+) channel β-subunit, SCN3B masks the disease phenotype of Brugada syndrome.
Autonomous and Non-autonomous Defects Underlie Hypertrophic Cardiomyopathy in BRAF-Mutant hiPSC-Derived Cardiomyocytes.
Expression analysis of Baf60c during heart regeneration in axolotls and neonatal mice.
Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity.
Mesp1 controls the speed, polarity, and directionality of cardiovascular progenitor migration.
Essential role of the TFIID subunit TAF4 in murine embryogenesis and embryonic stem cell differentiation.
Postnatal Development of Right Ventricular Myofibrillar Biomechanics in Relation to the Sarcomeric Protein Phenotype in Pediatric Patients with Conotruncal Heart Defects.
Functional Coupling with Cardiac Muscle Promotes Maturation of hPSC-Derived Sympathetic Neurons.
Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes.
Desmin enters the nucleus of cardiac stem cells and modulates Nkx2.5 expression by participating in transcription factor complexes that interact with the nkx2.5 gene.
Atypical Protein Kinase C-Dependent Polarized Cell Division Is Required for Myocardial Trabeculation.
Simple Monolayer Differentiation of Murine Cardiomyocytes via Nutrient Deprivation-Mediated Activation of β-Catenin.
Bulk cell density and Wnt/TGFbeta signalling regulate mesendodermal patterning of human pluripotent stem cells.
Disease Model of GATA4 Mutation Reveals Transcription Factor Cooperativity in Human Cardiogenesis.
Poly(Limonene Thioether) Scaffold for Tissue Engineering.
Induction of Human iPSC-Derived Cardiomyocyte Proliferation Revealed by Combinatorial Screening in High Density Microbioreactor Arrays.
A Systemized Approach to Investigate Ca(2+) Synchronization in Clusters of Human Induced Pluripotent Stem-Cell Derived Cardiomyocytes.
Cardiac development in zebrafish and human embryonic stem cells is inhibited by exposure to tobacco cigarettes and e-cigarettes.
PKB-Mediated Thr649 Phosphorylation of AS160/TBC1D4 Regulates the R-Wave Amplitude in the Heart.
Activin-A and Bmp4 levels modulate cell type specification during CHIR-induced cardiomyogenesis.
An Orthologous Epigenetic Gene Expression Signature Derived from Differentiating Embryonic Stem Cells Identifies Regulators of Cardiogenesis.
Cyclosporin A induces cardiac differentiation but inhibits hemato-endothelial differentiation of P19 cells.
High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling.
A new system for profiling drug-induced calcium signal perturbation in human embryonic stem cell-derived cardiomyocytes.
Efficient Generation of Cardiac Purkinje Cells from ESCs by Activating cAMP Signaling.
Statistically based splicing detection reveals neural enrichment and tissue-specific induction of circular RNA during human fetal development.
Reprogramming fibroblasts toward cardiomyocytes, neural stem cells and hepatocytes by cell activation and signaling-directed lineage conversion.
Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart.
Alzheimer-associated Aβ oligomers impact the central nervous system to induce peripheral metabolic deregulation.
Non-genetic Purification of Ventricular Cardiomyocytes from Differentiating Embryonic Stem Cells through Molecular Beacons Targeting IRX-4.
An integrated statistical model for enhanced murine cardiomyocyte differentiation via optimized engagement of 3D extracellular matrices.
Brg1 modulates enhancer activation in mesoderm lineage commitment.
Identification and purification of human induced pluripotent stem cell-derived atrial-like cardiomyocytes based on sarcolipin expression.
Prohibitin 2 regulates the proliferation and lineage-specific differentiation of mouse embryonic stem cells in mitochondria.
Inhibition of TGFβ signaling increases direct conversion of fibroblasts to induced cardiomyocytes.
Complete restoration of multiple dystrophin isoforms in genetically corrected Duchenne muscular dystrophy patient-derived cardiomyocytes.
Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells.
Integrin-linked kinase mediates force transduction in cardiomyocytes by modulating SERCA2a/PLN function.
Molecular beacon-enabled purification of living cells by targeting cell type-specific mRNAs.
Combined biophysical and soluble factor modulation induces cardiomyocyte differentiation from human muscle derived stem cells.
Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors.
PCP4 regulates Purkinje cell excitability and cardiac rhythmicity.
Irx4 identifies a chamber-specific cell population that contributes to ventricular myocardium development.
Comparison of the molecular profiles of human embryonic and induced pluripotent stem cells of isogenic origin.
Mutations in Alström protein impair terminal differentiation of cardiomyocytes.
Dual modulation of the mitochondrial permeability transition pore and redox signaling synergistically promotes cardiomyocyte differentiation from pluripotent stem cells.
Insulin-like growth factor promotes cardiac lineage induction in vitro by selective expansion of early mesoderm.
Angiomodulin is required for cardiogenesis of embryonic stem cells and is maintained by a feedback loop network of p63 and Activin-A.
Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium.
Chemically defined generation of human cardiomyocytes.
The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response.
Evidence for a critical role of catecholamines for cardiomyocyte lineage commitment in murine embryonic stem cells.
Embryonic stem cells facilitate the isolation of persistent clonal cardiovascular progenitor cell lines and leukemia inhibitor factor maintains their self-renewal and myocardial differentiation potential in vitro.
A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells.
Wharton's jelly-derived mesenchymal stem cells promote myocardial regeneration and cardiac repair after miniswine acute myocardial infarction.
Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2.
Inactivation of Cdc42 in neural crest cells causes craniofacial and cardiovascular morphogenesis defects.
Specification of chondrocytes and cartilage tissues from embryonic stem cells.
Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success.
Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury.
The ROSA26-iPSC mouse: a conditional, inducible, and exchangeable resource for studying cellular (De)differentiation.
zebraflash transgenic lines for in vivo bioluminescence imaging of stem cells and regeneration in adult zebrafish.
Hippo signaling impedes adult heart regeneration.
Mouse embryonic stem cells irradiated with γ-rays differentiate into cardiomyocytes but with altered contractile properties.
HCN4 dynamically marks the first heart field and conduction system precursors.
Human embryonic stem cell-derived cardiomyocytes migrate in response to gradients of fibronectin and Wnt5a.
Efficient generation of human iPSCs by a synthetic self-replicative RNA.
ILK induces cardiomyogenesis in the human heart.
Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells.
Efficient and user-friendly pluripotin-based derivation of mouse embryonic stem cells.
Inhibition of cardiomyocytes differentiation of mouse embryonic stem cells by CD38/cADPR/Ca2+ signaling pathway.
Activation of Toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency.
Heart repair by reprogramming non-myocytes with cardiac transcription factors.
Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia.
Transgenic zebrafish illuminate the dynamics of thyroid morphogenesis and its relationship to cardiovascular development.
Eomesodermin induces Mesp1 expression and cardiac differentiation from embryonic stem cells in the absence of Activin.
Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression.
Expression of sumoylation deficient Nkx2.5 mutant in Nkx2.5 haploinsufficient mice leads to congenital heart defects.
Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell-derived cardiomyocytes.
β-myosin heavy chain is induced by pressure overload in a minor subpopulation of smaller mouse cardiac myocytes.
SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells.
The differentiation of cardiomyocytes from mouse embryonic stem cells is altered by dioxin.
Ouabain facilitates cardiac differentiation of mouse embryonic stem cells through ERK1/2 pathway.
Cardiac origin of smooth muscle cells in the inflow tract.
Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines.
Cobalt chloride pretreatment promotes cardiac differentiation of human embryonic stem cells under atmospheric oxygen level.
Isolation and functional characterization of alpha-smooth muscle actin expressing cardiomyocytes from embryonic stem cells.
Triiodothyronine promotes cardiac differentiation and maturation of embryonic stem cells via the classical genomic pathway.
Global transcriptional profiles of beating clusters derived from human induced pluripotent stem cells and embryonic stem cells are highly similar.
Timed inhibition of p38MAPK directs accelerated differentiation of human embryonic stem cells into cardiomyocytes.
Icariin-mediated differentiation of mouse adipose-derived stem cells into cardiomyocytes.
Mouse and human induced pluripotent stem cells as a source for multipotent Isl1+ cardiovascular progenitors.
Delayed enrichment of mesenchymal cells promotes cardiac lineage and calcium transient development.
Effects of iron oxide nanoparticles on cardiac differentiation of embryonic stem cells.
Enhanced proliferation of monolayer cultures of embryonic stem (ES) cell-derived cardiomyocytes following acute loss of retinoblastoma.
Negative effects of rofecoxib treatment on cardiac function after ischemia-reperfusion injury in APOE3Leiden mice are prevented by combined treatment with thromboxane prostanoid-receptor antagonist S18886 (terutroban).
A high-efficiency system for the generation and study of human induced pluripotent stem cells.
Electrostimulation induces cardiomyocyte predifferentiation of fibroblasts.
Gremlin enhances the determined path to cardiomyogenesis.
A myocardial lineage derives from Tbx18 epicardial cells.
Mesp1 acts as a master regulator of multipotent cardiovascular progenitor specification.
Generation of functional murine cardiac myocytes from induced pluripotent stem cells.
Coexpression of platelet-derived growth factor receptor alpha and fetal liver kinase 1 enhances cardiogenic potential in embryonic stem cell differentiation in vitro.
Clonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regeneration.
New cell lines from mouse epiblast share defining features with human embryonic stem cells.
ALCAM (CD166) is a surface marker for early murine cardiomyocytes.
G-CSF/SCF reduces inducible arrhythmias in the infarcted heart potentially via increased connexin43 expression and arteriogenesis.
Combined autologous cellular cardiomyoplasty with skeletal myoblasts and bone marrow cells in canine hearts for ischemic cardiomyopathy.
Single inner cell masses yield embryonic stem cell lines differing in lifr expression and their developmental potential.
The MRL mouse heart healing response shows donor dominance in allogeneic fetal liver chimeric mice.
Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells.
Epiblast cells that express MyoD recruit pluripotent cells to the skeletal muscle lineage.
Wu P, Li Y, Cai M, Ye B, Geng B, Li F, Zhu H, Liu J, Wang X
Frontiers in cardiovascular medicine 2022;9:866901
Frontiers in cardiovascular medicine 2022;9:866901
FUCCI-Based Live Imaging Platform Reveals Cell Cycle Dynamics and Identifies Pro-proliferative Compounds in Human iPSC-Derived Cardiomyocytes.
Murganti F, Derks W, Baniol M, Simonova I, Trus P, Neumann K, Khattak S, Guan K, Bergmann O
Frontiers in cardiovascular medicine 2022;9:840147
Frontiers in cardiovascular medicine 2022;9:840147
CIEGAN: A Deep Learning Tool for Cell Image Enhancement.
Sun Q, Yang X, Guo J, Zhao Y, Liu Y
Frontiers in genetics 2022;13:913372
Frontiers in genetics 2022;13:913372
Biomimetic cardiac tissue culture model (CTCM) to emulate cardiac physiology and pathophysiology ex vivo.
Miller JM, Meki MH, Elnakib A, Ou Q, Abouleisa RRE, Tang XL, Salama ABM, Gebreil A, Lin C, Abdeltawab H, Khalifa F, Hill BG, Abi-Gerges N, Bolli R, El-Baz AS, Giridharan GA, Mohamed TMA
Communications biology 2022 Sep 9;5(1):934
Communications biology 2022 Sep 9;5(1):934
devCellPy is a machine learning-enabled pipeline for automated annotation of complex multilayered single-cell transcriptomic data.
Galdos FX, Xu S, Goodyer WR, Duan L, Huang YV, Lee S, Zhu H, Lee C, Wei N, Lee D, Wu SM
Nature communications 2022 Sep 7;13(1):5271
Nature communications 2022 Sep 7;13(1):5271
Transient Receptor Potential Vanilloid Type 1 Protects Against Pressure Overload-Induced Cardiac Hypertrophy by Promoting Mitochondria-Associated Endoplasmic Reticulum Membranes.
Wang Y, Li X, Xu X, Qu X, Yang Y
Journal of cardiovascular pharmacology 2022 Sep 1;80(3):430-441
Journal of cardiovascular pharmacology 2022 Sep 1;80(3):430-441
Mettl3-mediated m(6)A modification of Fgf16 restricts cardiomyocyte proliferation during heart regeneration.
Jiang FQ, Liu K, Chen JX, Cao Y, Chen WY, Zhao WL, Song GH, Liang CQ, Zhou YM, Huang HL, Huang RJ, Zhao H, Park KS, Ju Z, Cai D, Qi XF
eLife 2022 Nov 18;11
eLife 2022 Nov 18;11
CD47 antibody protects mice from doxorubicin-induced myocardial damage by suppressing cardiomyocyte apoptosis.
Hao Y, Chen L, Jiang Z
Experimental and therapeutic medicine 2022 May;23(5):350
Experimental and therapeutic medicine 2022 May;23(5):350
Tamoxifen treatment ameliorates contractile dysfunction of Duchenne muscular dystrophy stem cell-derived cardiomyocytes on bioengineered substrates.
Birnbaum F, Eguchi A, Pardon G, Chang ACY, Blau HM
NPJ Regenerative medicine 2022 Mar 18;7(1):19
NPJ Regenerative medicine 2022 Mar 18;7(1):19
A medium-chain triglyceride containing ketogenic diet exacerbates cardiomyopathy in a CRISPR/Cas9 gene-edited rat model with Duchenne muscular dystrophy.
Fujikura Y, Kimura K, Yamanouchi K, Sugihara H, Hatakeyama M, Zhuang H, Abe T, Daimon M, Morita H, Komuro I, Oishi K
Scientific reports 2022 Jul 8;12(1):11580
Scientific reports 2022 Jul 8;12(1):11580
Cardiac ultrastructure inspired matrix induces advanced metabolic and functional maturation of differentiated human cardiomyocytes.
Afzal J, Liu Y, Du W, Suhail Y, Zong P, Feng J, Ajeti V, Sayyad WA, Nikolaus J, Yankova M, Deymier AC, Yue L, Kshitiz
Cell reports 2022 Jul 26;40(4):111146
Cell reports 2022 Jul 26;40(4):111146
An automated do-it-yourself system for dynamic stem cell and organoid culture in standard multi-well plates.
Tischler J, Swank Z, Hsiung HA, Vianello S, Lutolf MP, Maerkl SJ
Cell reports methods 2022 Jul 18;2(7):100244
Cell reports methods 2022 Jul 18;2(7):100244
Microgravity-induced stress mechanisms in human stem cell-derived cardiomyocytes.
Acharya A, Nemade H, Papadopoulos S, Hescheler J, Neumaier F, Schneider T, Rajendra Prasad K, Khan K, Hemmersbach R, Gusmao EG, Mizi A, Papantonis A, Sachinidis A
iScience 2022 Jul 15;25(7):104577
iScience 2022 Jul 15;25(7):104577
Persistent fibrosis and decreased cardiac function following cardiac injury in the Ctenopharyngodon idella (grass carp).
Long DW, Webb CH 4th, Wang Y
Anatomical record (Hoboken, N.J. : 2007) 2022 Jan;305(1):66-80
Anatomical record (Hoboken, N.J. : 2007) 2022 Jan;305(1):66-80
A New Versatile Platform for Assessment of Improved Cardiac Performance in Human-Engineered Heart Tissues.
Ribeiro MC, Rivera-Arbeláez JM, Cofiño-Fabres C, Schwach V, Slaats RH, Ten Den SA, Vermeul K, van den Berg A, Pérez-Pomares JM, Segerink LI, Guadix JA, Passier R
Journal of personalized medicine 2022 Feb 4;12(2)
Journal of personalized medicine 2022 Feb 4;12(2)
Inhibition of SARS-CoV-2 infection in human iPSC-derived cardiomyocytes by targeting the Sigma-1 receptor disrupts cytoarchitecture and beating.
Salerno JA, Torquato T, Temerozo JR, Goto-Silva L, Karmirian K, Mendes MA, Sacramento CQ, Fintelman-Rodrigues N, Souza LRQ, Ornelas IM, Veríssimo CP, Aragão LGHS, Vitória G, Pedrosa CSG, da Silva Gomes Dias S, Cardoso Soares V, Puig-Pijuan T, Salazar V, Dariolli R, Biagi D, Furtado DR, Barreto Chiarini L, Borges HL, Bozza PT, Zaluar P Guimarães M, Souza TML, Rehen SK
PeerJ 2021;9:e12595
PeerJ 2021;9:e12595
Sall4 and Myocd Empower Direct Cardiac Reprogramming From Adult Cardiac Fibroblasts After Injury.
Zhao H, Zhang Y, Xu X, Sun Q, Yang C, Wang H, Yang J, Yang Y, Yang X, Liu Y, Zhao Y
Frontiers in cell and developmental biology 2021;9:608367
Frontiers in cell and developmental biology 2021;9:608367
Free Feeding of CpG-Oligodeoxynucleotide Particles Prophylactically Attenuates Allergic Airway Inflammation and Hyperresponsiveness in Mice.
Okajima T, Shigemori S, Namai F, Ogita T, Sato T, Shimosato T
Frontiers in immunology 2021;12:738041
Frontiers in immunology 2021;12:738041
High-Throughput Drug Screening System Based on Human Induced Pluripotent Stem Cell-Derived Atrial Myocytes ∼ A Novel Platform to Detect Cardiac Toxicity for Atrial Arrhythmias.
Honda Y, Li J, Hino A, Tsujimoto S, Lee JK
Frontiers in pharmacology 2021;12:680618
Frontiers in pharmacology 2021;12:680618
Nrf1 promotes heart regeneration and repair by regulating proteostasis and redox balance.
Cui M, Atmanli A, Morales MG, Tan W, Chen K, Xiao X, Xu L, Liu N, Bassel-Duby R, Olson EN
Nature communications 2021 Sep 6;12(1):5270
Nature communications 2021 Sep 6;12(1):5270
Pharmacologic therapy for engraftment arrhythmia induced by transplantation of human cardiomyocytes.
Nakamura K, Neidig LE, Yang X, Weber GJ, El-Nachef D, Tsuchida H, Dupras S, Kalucki FA, Jayabalu A, Futakuchi-Tsuchida A, Nakamura DS, Marchianò S, Bertero A, Robinson MR, Cain K, Whittington D, Tian R, Reinecke H, Pabon L, Knollmann BC, Kattman S, Thies RS, MacLellan WR, Murry CE
Stem cell reports 2021 Oct 12;16(10):2473-2487
Stem cell reports 2021 Oct 12;16(10):2473-2487
Auto/paracrine factors and early Wnt inhibition promote cardiomyocyte differentiation from human induced pluripotent stem cells at initial low cell density.
Le MNT, Takahi M, Ohnuma K
Scientific reports 2021 Nov 2;11(1):21426
Scientific reports 2021 Nov 2;11(1):21426
AAV-mediated YAP expression in cardiac fibroblasts promotes inflammation and increases fibrosis.
Francisco J, Zhang Y, Nakada Y, Jeong JI, Huang CY, Ivessa A, Oka S, Babu GJ, Del Re DP
Scientific reports 2021 May 18;11(1):10553
Scientific reports 2021 May 18;11(1):10553
Neonatal hyperoxia inhibits proliferation and survival of atrial cardiomyocytes by suppressing fatty acid synthesis.
Cohen ED, Yee M, Porter GA Jr, Ritzer E, McDavid AN, Brookes PS, Pryhuber GS, O'Reilly MA
JCI insight 2021 Mar 8;6(5)
JCI insight 2021 Mar 8;6(5)
Role of Heme-Oxygenase-1 in Biology of Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells.
Jeż M, Martyniak A, Andrysiak K, Mucha O, Szade K, Kania A, Chrobok Ł, Palus-Chramiec K, Sanetra AM, Lewandowski MH, Pośpiech E, Stępniewski J, Dulak J
Cells 2021 Mar 1;10(3)
Cells 2021 Mar 1;10(3)
Human heart-forming organoids recapitulate early heart and foregut development.
Drakhlis L, Biswanath S, Farr CM, Lupanow V, Teske J, Ritzenhoff K, Franke A, Manstein F, Bolesani E, Kempf H, Liebscher S, Schenke-Layland K, Hegermann J, Nolte L, Meyer H, de la Roche J, Thiemann S, Wahl-Schott C, Martin U, Zweigerdt R
Nature biotechnology 2021 Jun;39(6):737-746
Nature biotechnology 2021 Jun;39(6):737-746
PMN-derived netrin-1 attenuates cardiac ischemia-reperfusion injury via myeloid ADORA2B signaling.
Li J, Conrad C, Mills TW, Berg NK, Kim B, Ruan W, Lee JW, Zhang X, Yuan X, Eltzschig HK
The Journal of experimental medicine 2021 Jun 7;218(6)
The Journal of experimental medicine 2021 Jun 7;218(6)
Fosl1 is vital to heart regeneration upon apex resection in adult Xenopus tropicalis.
Wu HY, Zhou YM, Liao ZQ, Zhong JW, Liu YB, Zhao H, Liang CQ, Huang RJ, Park KS, Feng SS, Zheng L, Cai DQ, Qi XF
NPJ Regenerative medicine 2021 Jun 29;6(1):36
NPJ Regenerative medicine 2021 Jun 29;6(1):36
Bioactive Lipid O-cyclic phytosphingosine-1-phosphate Promotes Differentiation of Human Embryonic Stem Cells into Cardiomyocytes via ALK3/BMPR Signaling.
Jang JH, Kim MS, Antao AM, Jo WJ, Kim HJ, Kim SJ, Choi MJ, Ramakrishna S, Kim KS
International journal of molecular sciences 2021 Jun 29;22(13)
International journal of molecular sciences 2021 Jun 29;22(13)
m6A modification promotes miR-133a repression during cardiac development and hypertrophy via IGF2BP2.
Qian B, Wang P, Zhang D, Wu L
Cell death discovery 2021 Jun 26;7(1):157
Cell death discovery 2021 Jun 26;7(1):157
ERRγ enhances cardiac maturation with T-tubule formation in human iPSC-derived cardiomyocytes.
Miki K, Deguchi K, Nakanishi-Koakutsu M, Lucena-Cacace A, Kondo S, Fujiwara Y, Hatani T, Sasaki M, Naka Y, Okubo C, Narita M, Takei I, Napier SC, Sugo T, Imaichi S, Monjo T, Ando T, Tamura N, Imahashi K, Nishimoto T, Yoshida Y
Nature communications 2021 Jun 21;12(1):3596
Nature communications 2021 Jun 21;12(1):3596
Setd4 controlled quiescent c-Kit(+) cells contribute to cardiac neovascularization of capillaries beyond activation.
Xing S, Tian JZ, Yang SH, Huang XT, Ding YF, Lu QY, Yang JS, Yang WJ
Scientific reports 2021 Jun 2;11(1):11603
Scientific reports 2021 Jun 2;11(1):11603
Non-permissive SARS-CoV-2 infection in human neurospheres.
Pedrosa CDSG, Goto-Silva L, Temerozo JR, Souza LRQ, Vitória G, Ornelas IM, Karmirian K, Mendes MA, Gomes IC, Sacramento CQ, Fintelman-Rodrigues N, Cardoso Soares V, Silva Gomes Dias SD, Salerno JA, Puig-Pijuan T, Oliveira JT, Aragão LGHS, Torquato TCQ, Veríssimo C, Biagi D, Cruvinel EM, Dariolli R, Furtado DR, Borges HL, Bozza PT, Rehen S, Moreno L Souza T, Guimarães MZP
Stem cell research 2021 Jul;54:102436
Stem cell research 2021 Jul;54:102436
Molecular-scale visualization of sarcomere contraction within native cardiomyocytes.
Burbaum L, Schneider J, Scholze S, Böttcher RT, Baumeister W, Schwille P, Plitzko JM, Jasnin M
Nature communications 2021 Jul 2;12(1):4086
Nature communications 2021 Jul 2;12(1):4086
Microenvironmental determinants of organized iPSC-cardiomyocyte tissues on synthetic fibrous matrices.
DePalma SJ, Davidson CD, Stis AE, Helms AS, Baker BM
Biomaterials science 2021 Jan 5;9(1):93-107
Biomaterials science 2021 Jan 5;9(1):93-107
Overcoming bioprocess bottlenecks in the large-scale expansion of high-quality hiPSC aggregates in vertical-wheel stirred suspension bioreactors.
Borys BS, Dang T, So T, Rohani L, Revay T, Walsh T, Thompson M, Argiropoulos B, Rancourt DE, Jung S, Hashimura Y, Lee B, Kallos MS
Stem cell research & therapy 2021 Jan 13;12(1):55
Stem cell research & therapy 2021 Jan 13;12(1):55
Oxygen Is an Ambivalent Factor for the Differentiation of Human Pluripotent Stem Cells in Cardiac 2D Monolayer and 3D Cardiac Spheroids.
Souidi M, Sleiman Y, Acimovic I, Pribyl J, Charrabi A, Baecker V, Scheuermann V, Pesl M, Jelinkova S, Skladal P, Dvorak P, Lacampagne A, Rotrekl V, Meli AC
International journal of molecular sciences 2021 Jan 11;22(2)
International journal of molecular sciences 2021 Jan 11;22(2)
Pim1 maintains telomere length in mouse cardiomyocytes by inhibiting TGFβ signalling.
Ebeid DE, Khalafalla FG, Broughton KM, Monsanto MM, Esquer CY, Sacchi V, Hariharan N, Korski KI, Moshref M, Emathinger J, Cottage CT, Quijada PJ, Nguyen JH, Alvarez R, Völkers M, Konstandin MH, Wang BJ, Firouzi F, Navarrete JM, Gude NA, Goumans MJ, Sussman MA
Cardiovascular research 2021 Jan 1;117(1):201-211
Cardiovascular research 2021 Jan 1;117(1):201-211
Capturing Cardiogenesis in Gastruloids.
Rossi G, Broguiere N, Miyamoto M, Boni A, Guiet R, Girgin M, Kelly RG, Kwon C, Lutolf MP
Cell stem cell 2021 Feb 4;28(2):230-240.e6
Cell stem cell 2021 Feb 4;28(2):230-240.e6
3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels.
Daly AC, Davidson MD, Burdick JA
Nature communications 2021 Feb 2;12(1):753
Nature communications 2021 Feb 2;12(1):753
Gastrin exerts a protective effect against myocardial infarction via promoting angiogenesis.
Fu J, Tang Y, Zhang Z, Tong L, Yue R, Cai L
Molecular medicine (Cambridge, Mass.) 2021 Aug 19;27(1):90
Molecular medicine (Cambridge, Mass.) 2021 Aug 19;27(1):90
Method for selective ablation of undifferentiated human pluripotent stem cell populations for cell-based therapies.
Chour T, Tian L, Lau E, Thomas D, Itzhaki I, Malak O, Zhang JZ, Qin X, Wardak M, Liu Y, Chandy M, Black KE, Lam MP, Neofytou E, Wu JC
JCI insight 2021 Apr 8;6(7)
JCI insight 2021 Apr 8;6(7)
Engrafted Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Undergo Clonal Expansion In Vivo.
El-Nachef D, Bugg D, Beussman KM, Steczina S, Martinson AM, Murry CE, Sniadecki NJ, Davis J
Circulation 2021 Apr 20;143(16):1635-1638
Circulation 2021 Apr 20;143(16):1635-1638
An Aurora Kinase B-Based Mouse System to Efficiently Identify and Analyze Proliferating Cardiomyocytes.
Fu W, Liao Q, Li L, Shi Y, Zeng A, Zeng C, Wang WE
Frontiers in cell and developmental biology 2020;8:570252
Frontiers in cell and developmental biology 2020;8:570252
Liquid marble technology to create cost-effective 3D cardiospheres as a platform for in vitro drug testing and disease modelling.
Aalders J, Léger L, Tuerlings T, Ledda S, van Hengel J
MethodsX 2020;7:101065
MethodsX 2020;7:101065
Co-culture of induced pluripotent stem cells with cardiomyocytes is sufficient to promote their differentiation into cardiomyocytes.
Chu AJ, Zhao EJ, Chiao M, Lim CJ
PloS one 2020;15(4):e0230966
PloS one 2020;15(4):e0230966
Misoprostol attenuates neonatal cardiomyocyte proliferation through Bnip3, perinuclear calcium signaling, and inhibition of glycolysis.
Martens MD, Field JT, Seshadri N, Day C, Chapman D, Keijzer R, Doucette CA, Hatch GM, West AR, Ivanco TL, Gordon JW
Journal of molecular and cellular cardiology 2020 Sep;146:19-31
Journal of molecular and cellular cardiology 2020 Sep;146:19-31
Stirred suspension bioreactors maintain naïve pluripotency of human pluripotent stem cells.
Rohani L, Borys BS, Razian G, Naghsh P, Liu S, Johnson AA, Machiraju P, Holland H, Lewis IA, Groves RA, Toms D, Gordon PMK, Li JW, So T, Dang T, Kallos MS, Rancourt DE
Communications biology 2020 Sep 7;3(1):492
Communications biology 2020 Sep 7;3(1):492
NOTCH1 is critical for fibroblast-mediated induction of cardiomyocyte specialization into ventricular conduction system-like cells in vitro.
Ribeiro da Silva A, Neri EA, Turaça LT, Dariolli R, Fonseca-Alaniz MH, Santos-Miranda A, Roman-Campos D, Venturini G, Krieger JE
Scientific reports 2020 Sep 30;10(1):16163
Scientific reports 2020 Sep 30;10(1):16163
Differences in Mitochondrial Membrane Potential Identify Distinct Populations of Human Cardiac Mesenchymal Progenitor Cells.
Gambini E, Martinelli I, Stadiotti I, Vinci MC, Scopece A, Eramo L, Sommariva E, Resta J, Benaouadi S, Cogliati E, Paolin A, Parini A, Pompilio G, Savagner F
International journal of molecular sciences 2020 Oct 10;21(20)
International journal of molecular sciences 2020 Oct 10;21(20)
Progesterone, via yes-associated protein, promotes cardiomyocyte proliferation and cardiac repair.
Lan C, Cao N, Chen C, Qu S, Fan C, Luo H, Zeng A, Yu C, Xue Y, Ren H, Li L, Wang H, Jose PA, Xu Z, Zeng C
Cell proliferation 2020 Nov;53(11):e12910
Cell proliferation 2020 Nov;53(11):e12910
Heart slice culture system reliably demonstrates clinical drug-related cardiotoxicity.
Miller JM, Meki MH, Ou Q, George SA, Gams A, Abouleisa RRE, Tang XL, Ahern BM, Giridharan GA, El-Baz A, Hill BG, Satin J, Conklin DJ, Moslehi J, Bolli R, Ribeiro AJS, Efimov IR, Mohamed TMA
Toxicology and applied pharmacology 2020 Nov 1;406:115213
Toxicology and applied pharmacology 2020 Nov 1;406:115213
Canonical Wnt signaling promotes pacemaker cell specification of cardiac mesodermal cells derived from mouse and human embryonic stem cells.
Liang W, Han P, Kim EH, Mak J, Zhang R, Torrente AG, Goldhaber JI, Marbán E, Cho HC
Stem cells (Dayton, Ohio) 2020 Mar;38(3):352-368
Stem cells (Dayton, Ohio) 2020 Mar;38(3):352-368
HIF1α-dependent mitophagy facilitates cardiomyoblast differentiation.
Zhao JF, Rodger CE, Allen GFG, Weidlich S, Ganley IG
Cell stress 2020 Mar 4;4(5):99-113
Cell stress 2020 Mar 4;4(5):99-113
A human embryonic stem cell reporter line for monitoring chemical-induced cardiotoxicity.
Tsai SY, Ghazizadeh Z, Wang HJ, Amin S, Ortega FA, Badieyan ZS, Hsu ZT, Gordillo M, Kumar R, Christini DJ, Evans T, Chen S
Cardiovascular research 2020 Mar 1;116(3):658-670
Cardiovascular research 2020 Mar 1;116(3):658-670
Subtype-Directed Differentiation of Human iPSCs into Atrial and Ventricular Cardiomyocytes.
Kleinsorge M, Cyganek L
STAR protocols 2020 Jun 19;1(1):100026
STAR protocols 2020 Jun 19;1(1):100026
Human ARF Specifically Inhibits Epimorphic Regeneration in the Zebrafish Heart.
Lee S, Hesse R, Tamaki S, Garland C, Pomerantz JH
Genes 2020 Jun 18;11(6)
Genes 2020 Jun 18;11(6)
Variability in Cardiac miRNA-122 Level Determines Therapeutic Potential of miRNA-Regulated AAV Vectors.
Kraszewska I, Tomczyk M, Andrysiak K, Biniecka M, Geisler A, Fechner H, Zembala M, Stępniewski J, Dulak J, Jaźwa-Kusior A
Molecular therapy. Methods & clinical development 2020 Jun 12;17:1190-1201
Molecular therapy. Methods & clinical development 2020 Jun 12;17:1190-1201
Metabolic Maturation Media Improve Physiological Function of Human iPSC-Derived Cardiomyocytes.
Feyen DAM, McKeithan WL, Bruyneel AAN, Spiering S, Hörmann L, Ulmer B, Zhang H, Briganti F, Schweizer M, Hegyi B, Liao Z, Pölönen RP, Ginsburg KS, Lam CK, Serrano R, Wahlquist C, Kreymerman A, Vu M, Amatya PL, Behrens CS, Ranjbarvaziri S, Maas RGC, Greenhaw M, Bernstein D, Wu JC, Bers DM, Eschenhagen T, Metallo CM, Mercola M
Cell reports 2020 Jul 21;32(3):107925
Cell reports 2020 Jul 21;32(3):107925
Tissue-engineered human embryonic stem cell-containing cardiac patches: evaluating recellularization of decellularized matrix.
Hochman-Mendez C, Pereira de Campos DB, Pinto RS, Mendes BJDS, Rocha GM, Monnerat G, Weissmuller G, Sampaio LC, Carvalho AB, Taylor DA, de Carvalho ACC
Journal of tissue engineering 2020 Jan-Dec;11:2041731420921482
Journal of tissue engineering 2020 Jan-Dec;11:2041731420921482
Heart-derived fibroblasts express LYPD-1 and negatively regulate angiogenesis in rat.
Sakamoto S, Matsuura K, Masuda S, Hagiwara N, Shimizu T
Regenerative therapy 2020 Dec;15:27-33
Regenerative therapy 2020 Dec;15:27-33
Cell-Type-Specific Gene Regulatory Networks Underlying Murine Neonatal Heart Regeneration at Single-Cell Resolution.
Wang Z, Cui M, Shah AM, Tan W, Liu N, Bassel-Duby R, Olson EN
Cell reports 2020 Dec 8;33(10):108472
Cell reports 2020 Dec 8;33(10):108472
Comprehensive Biology and Genetics Compendium of Wilms Tumor Cell Lines with Different WT1 Mutations.
Royer-Pokora B, Busch MA, Tenbusch S, Schmidt M, Beier M, Woods AD, Thiele H, Mora J
Cancers 2020 Dec 28;13(1)
Cancers 2020 Dec 28;13(1)
Generation of two human ISG15 knockout iPSC clones using CRISPR/Cas9 editing.
Merkert S, Jaboreck MC, Engels L, Malik MNH, Göhring G, Pessler F, Martin U, Olmer R
Stem cell research 2020 Dec 22;50:102135
Stem cell research 2020 Dec 22;50:102135
Dynamic Transcriptional Responses to Injury of Regenerative and Non-regenerative Cardiomyocytes Revealed by Single-Nucleus RNA Sequencing.
Cui M, Wang Z, Chen K, Shah AM, Tan W, Duan L, Sanchez-Ortiz E, Li H, Xu L, Liu N, Bassel-Duby R, Olson EN
Developmental cell 2020 Apr 6;53(1):102-116.e8
Developmental cell 2020 Apr 6;53(1):102-116.e8
Bmi1 inhibitor PTC-209 promotes Chemically-induced Direct Cardiac Reprogramming of cardiac fibroblasts into cardiomyocytes.
Testa G, Russo M, Di Benedetto G, Barbato M, Parisi S, Pirozzi F, Tocchetti CG, Abete P, Bonaduce D, Russo T, Passaro F
Scientific reports 2020 Apr 28;10(1):7129
Scientific reports 2020 Apr 28;10(1):7129
miRNAs in Extracellular Vesicles from iPS-Derived Cardiac Progenitor Cells Effectively Reduce Fibrosis and Promote Angiogenesis in Infarcted Heart.
Xuan W, Wang L, Xu M, Weintraub NL, Ashraf M
Stem cells international 2019;2019:3726392
Stem cells international 2019;2019:3726392
Long-Term Engraftment of Human Cardiomyocytes Combined with Biodegradable Microparticles Induces Heart Repair.
Saludas L, Garbayo E, Mazo M, Pelacho B, Abizanda G, Iglesias-Garcia O, Raya A, Prósper F, Blanco-Prieto MJ
The Journal of pharmacology and experimental therapeutics 2019 Sep;370(3):761-771
The Journal of pharmacology and experimental therapeutics 2019 Sep;370(3):761-771
Chromatin compartment dynamics in a haploinsufficient model of cardiac laminopathy.
Bertero A, Fields PA, Smith AST, Leonard A, Beussman K, Sniadecki NJ, Kim DH, Tse HF, Pabon L, Shendure J, Noble WS, Murry CE
The Journal of cell biology 2019 Sep 2;218(9):2919-2944
The Journal of cell biology 2019 Sep 2;218(9):2919-2944
Irisin exerts a therapeutic effect against myocardial infarction via promoting angiogenesis.
Liao Q, Qu S, Tang LX, Li LP, He DF, Zeng CY, Wang WE
Acta pharmacologica Sinica 2019 Oct;40(10):1314-1321
Acta pharmacologica Sinica 2019 Oct;40(10):1314-1321
Stoichiometric optimization of Gata4, Hand2, Mef2c, and Tbx5 expression for contractile cardiomyocyte reprogramming.
Zhang Z, Zhang W, Nam YJ
Scientific reports 2019 Oct 18;9(1):14970
Scientific reports 2019 Oct 18;9(1):14970
Generation of the human induced pluripotent stem cell (hiPSC) line PSMi004-A from a carrier of the KCNQ1-R594Q mutation.
Mura M, Lee YK, Pisano F, Ginevrino M, Boni M, Calabrò F, Crotti L, Valente EM, Schwartz PJ, Tse HF, Gnecchi M
Stem cell research 2019 May;37:101431
Stem cell research 2019 May;37:101431
Adaptation of Human iPSC-Derived Cardiomyocytes to Tyrosine Kinase Inhibitors Reduces Acute Cardiotoxicity via Metabolic Reprogramming.
Wang H, Sheehan RP, Palmer AC, Everley RA, Boswell SA, Ron-Harel N, Ringel AE, Holton KM, Jacobson CA, Erickson AR, Maliszewski L, Haigis MC, Sorger PK
Cell systems 2019 May 22;8(5):412-426.e7
Cell systems 2019 May 22;8(5):412-426.e7
Structural evidence for a new elaborate 3D-organization of the cardiomyocyte lateral membrane in adult mammalian cardiac tissues.
Guilbeau-Frugier C, Cauquil M, Karsenty C, Lairez O, Dambrin C, Payré B, Cassard H, Josse C, Seguelas MH, Allart S, Branchereau M, Heymes C, Mandel F, Delisle MB, Pathak A, Dague E, Sénard JM, Galés C
Cardiovascular research 2019 May 1;115(6):1078-1091
Cardiovascular research 2019 May 1;115(6):1078-1091
Treatment with apolipoprotein A1 protects mice against doxorubicin-induced cardiotoxicity in a scavenger receptor class B, type I-dependent manner.
Durham KK, Kluck G, Mak KC, Deng YD, Trigatti BL
American journal of physiology. Heart and circulatory physiology 2019 Jun 1;316(6):H1447-H1457
American journal of physiology. Heart and circulatory physiology 2019 Jun 1;316(6):H1447-H1457
Cardiac Reprogramming Factors Synergistically Activate Genome-wide Cardiogenic Stage-Specific Enhancers.
Hashimoto H, Wang Z, Garry GA, Malladi VS, Botten GA, Ye W, Zhou H, Osterwalder M, Dickel DE, Visel A, Liu N, Bassel-Duby R, Olson EN
Cell stem cell 2019 Jul 3;25(1):69-86.e5
Cell stem cell 2019 Jul 3;25(1):69-86.e5
Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction.
Park SJ, Kim RY, Park BW, Lee S, Choi SW, Park JH, Choi JJ, Kim SW, Jang J, Cho DW, Chung HM, Moon SH, Ban K, Park HJ
Nature communications 2019 Jul 16;10(1):3123
Nature communications 2019 Jul 16;10(1):3123
Generation of human iPS cell line CBTCi001-A from dermal fibroblasts obtained from a healthy donor.
Martins GLS, Paredes BD, Sampaio GLA, Nonaka CKV, da Silva KN, Allahdadi KJ, França LSA, Soares MBP, Dos Santos RR, Souza BSF
Stem cell research 2019 Dec;41:101630
Stem cell research 2019 Dec;41:101630
Adult human cardiac stem cell supplementation effectively increases contractile function and maturation in human engineered cardiac tissues.
Murphy JF, Mayourian J, Stillitano F, Munawar S, Broughton KM, Agullo-Pascual E, Sussman MA, Hajjar RJ, Costa KD, Turnbull IC
Stem cell research & therapy 2019 Dec 4;10(1):373
Stem cell research & therapy 2019 Dec 4;10(1):373
Multifactorial Modeling Reveals a Dominant Role of Wnt Signaling in Lineage Commitment of Human Pluripotent Stem Cells.
Dias TP, Fernandes TG, Diogo MM, Cabral JMS
Bioengineering (Basel, Switzerland) 2019 Aug 15;6(3)
Bioengineering (Basel, Switzerland) 2019 Aug 15;6(3)
A generally conserved response to hypoxia in iPSC-derived cardiomyocytes from humans and chimpanzees.
Ward MC, Gilad Y
eLife 2019 Apr 8;8
eLife 2019 Apr 8;8
Transcriptome and DNA Methylome Dynamics during Triclosan-Induced Cardiomyocyte Differentiation Toxicity.
Du G, Yu M, Wang L, Hu W, Song L, Lu C, Wang X
Stem cells international 2018;2018:8608327
Stem cells international 2018;2018:8608327
Phenotypic Screening Using Patient-Derived Induced Pluripotent Stem Cells Identified Pyr3 as a Candidate Compound for the Treatment of Infantile Hypertrophic Cardiomyopathy.
Sakai T, Naito AT, Kuramoto Y, Ito M, Okada K, Higo T, Nakagawa A, Shibamoto M, Yamaguchi T, Sumida T, Nomura S, Umezawa A, Miyagawa S, Sawa Y, Morita H, Lee JK, Shiojima I, Sakata Y, Komuro I
International heart journal 2018 Sep 26;59(5):1096-1105
International heart journal 2018 Sep 26;59(5):1096-1105
Single-Cell Transcriptomic Analysis of Cardiac Differentiation from Human PSCs Reveals HOPX-Dependent Cardiomyocyte Maturation.
Friedman CE, Nguyen Q, Lukowski SW, Helfer A, Chiu HS, Miklas J, Levy S, Suo S, Han JJ, Osteil P, Peng G, Jing N, Baillie GJ, Senabouth A, Christ AN, Bruxner TJ, Murry CE, Wong ES, Ding J, Wang Y, Hudson J, Ruohola-Baker H, Bar-Joseph Z, Tam PPL, Powell JE, Palpant NJ
Cell stem cell 2018 Oct 4;23(4):586-598.e8
Cell stem cell 2018 Oct 4;23(4):586-598.e8
Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration.
Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D
Cell 2018 Mar 22;173(1):104-116.e12
Cell 2018 Mar 22;173(1):104-116.e12
Low-temperature culturing improves survival rate of tissue-engineered cardiac cell sheets.
Sakaguchi K, Hinata Y, Kagawa Y, Iwasaki K, Tsuneda S, Shimizu T, Umezu M
Biochemistry and biophysics reports 2018 Jul;14:89-97
Biochemistry and biophysics reports 2018 Jul;14:89-97
Atorvastatin Inhibits the HIF1α-PPAR Axis, Which Is Essential for Maintaining the Function of Human Induced Pluripotent Stem Cells.
Nakashima Y, Miyagi-Shiohira C, Noguchi H, Omasa T
Molecular therapy : the journal of the American Society of Gene Therapy 2018 Jul 5;26(7):1715-1734
Molecular therapy : the journal of the American Society of Gene Therapy 2018 Jul 5;26(7):1715-1734
The microRNA regulatory landscape of MSC-derived exosomes: a systems view.
Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, Nguyen J
Scientific reports 2018 Jan 23;8(1):1419
Scientific reports 2018 Jan 23;8(1):1419
Endocardial Hippo signaling regulates myocardial growth and cardiogenesis.
Artap S, Manderfield LJ, Smith CL, Poleshko A, Aghajanian H, See K, Li L, Jain R, Epstein JA
Developmental biology 2018 Aug 1;440(1):22-30
Developmental biology 2018 Aug 1;440(1):22-30
High-density lipoprotein protects cardiomyocytes against necrosis induced by oxygen and glucose deprivation through SR-B1, PI3K, and AKT1 and 2.
Durham KK, Chathely KM, Trigatti BL
The Biochemical journal 2018 Apr 5;475(7):1253-1265
The Biochemical journal 2018 Apr 5;475(7):1253-1265
Culture in Glucose-Depleted Medium Supplemented with Fatty Acid and 3,3',5-Triiodo-l-Thyronine Facilitates Purification and Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes.
Lin B, Lin X, Stachel M, Wang E, Luo Y, Lader J, Sun X, Delmar M, Bu L
Frontiers in endocrinology 2017;8:253
Frontiers in endocrinology 2017;8:253
Cited4 is related to cardiogenic induction and maintenance of proliferation capacity of embryonic stem cell-derived cardiomyocytes during in vitro cardiogenesis.
Miake J, Notsu T, Higaki K, Hidaka K, Morisaki T, Yamamoto K, Hisatome I
PloS one 2017;12(8):e0183225
PloS one 2017;12(8):e0183225
ZNF281 enhances cardiac reprogramming by modulating cardiac and inflammatory gene expression.
Zhou H, Morales MG, Hashimoto H, Dickson ME, Song K, Ye W, Kim MS, Niederstrasser H, Wang Z, Chen B, Posner BA, Bassel-Duby R, Olson EN
Genes & development 2017 Sep 1;31(17):1770-1783
Genes & development 2017 Sep 1;31(17):1770-1783
A transcribed enhancer dictates mesendoderm specification in pluripotency.
Alexanian M, Maric D, Jenkinson SP, Mina M, Friedman CE, Ting CC, Micheletti R, Plaisance I, Nemir M, Maison D, Kernen J, Pezzuto I, Villeneuve D, Burdet F, Ibberson M, Leib SL, Palpant NJ, Hernandez N, Ounzain S, Pedrazzini T
Nature communications 2017 Nov 27;8(1):1806
Nature communications 2017 Nov 27;8(1):1806
Notch Inhibition Enhances Cardiac Reprogramming by Increasing MEF2C Transcriptional Activity.
Abad M, Hashimoto H, Zhou H, Morales MG, Chen B, Bassel-Duby R, Olson EN
Stem cell reports 2017 Mar 14;8(3):548-560
Stem cell reports 2017 Mar 14;8(3):548-560
Circadian networks in human embryonic stem cell-derived cardiomyocytes.
Dierickx P, Vermunt MW, Muraro MJ, Creyghton MP, Doevendans PA, van Oudenaarden A, Geijsen N, Van Laake LW
EMBO reports 2017 Jul;18(7):1199-1212
EMBO reports 2017 Jul;18(7):1199-1212
MED12 regulates a transcriptional network of calcium-handling genes in the heart.
Baskin KK, Makarewich CA, DeLeon SM, Ye W, Chen B, Beetz N, Schrewe H, Bassel-Duby R, Olson EN
JCI insight 2017 Jul 20;2(14)
JCI insight 2017 Jul 20;2(14)
Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker.
Protze SI, Liu J, Nussinovitch U, Ohana L, Backx PH, Gepstein L, Keller GM
Nature biotechnology 2017 Jan;35(1):56-68
Nature biotechnology 2017 Jan;35(1):56-68
Role of FEN1 S187 phosphorylation in counteracting oxygen-induced stress and regulating postnatal heart development.
Zhou L, Dai H, Wu J, Zhou M, Yuan H, Du J, Yang L, Wu X, Xu H, Hua Y, Xu J, Zheng L, Shen B
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2017 Jan;31(1):132-147
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2017 Jan;31(1):132-147
Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells.
Palpant NJ, Pabon L, Friedman CE, Roberts M, Hadland B, Zaunbrecher RJ, Bernstein I, Zheng Y, Murry CE
Nature protocols 2017 Jan;12(1):15-31
Nature protocols 2017 Jan;12(1):15-31
Generation of PDGFRα(+) Cardioblasts from Pluripotent Stem Cells.
Hong SP, Song S, Cho SW, Lee S, Koh BI, Bae H, Kim KH, Park JS, Do HS, Im I, Heo HJ, Ko TH, Park JH, Youm JB, Kim SJ, Kim I, Han J, Han YM, Koh GY
Scientific reports 2017 Feb 6;7:41840
Scientific reports 2017 Feb 6;7:41840
Differentiation of Human Pluripotent Stem Cells to Cardiomyocytes Under Defined Conditions.
van den Berg CW, Elliott DA, Braam SR, Mummery CL, Davis RP
Methods in molecular biology (Clifton, N.J.) 2016;1353:163-80
Methods in molecular biology (Clifton, N.J.) 2016;1353:163-80
Embryonic type Na(+) channel β-subunit, SCN3B masks the disease phenotype of Brugada syndrome.
Okata S, Yuasa S, Suzuki T, Ito S, Makita N, Yoshida T, Li M, Kurokawa J, Seki T, Egashira T, Aizawa Y, Kodaira M, Motoda C, Yozu G, Shimojima M, Hayashiji N, Hashimoto H, Kuroda Y, Tanaka A, Murata M, Aiba T, Shimizu W, Horie M, Kamiya K, Furukawa T, Fukuda K
Scientific reports 2016 Sep 28;6:34198
Scientific reports 2016 Sep 28;6:34198
Autonomous and Non-autonomous Defects Underlie Hypertrophic Cardiomyopathy in BRAF-Mutant hiPSC-Derived Cardiomyocytes.
Josowitz R, Mulero-Navarro S, Rodriguez NA, Falce C, Cohen N, Ullian EM, Weiss LA, Rauen KA, Sobie EA, Gelb BD
Stem cell reports 2016 Sep 13;7(3):355-369
Stem cell reports 2016 Sep 13;7(3):355-369
Expression analysis of Baf60c during heart regeneration in axolotls and neonatal mice.
Nakamura R, Koshiba-Takeuchi K, Tsuchiya M, Kojima M, Miyazawa A, Ito K, Ogawa H, Takeuchi JK
Development, growth & differentiation 2016 May;58(4):367-82
Development, growth & differentiation 2016 May;58(4):367-82
Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity.
Burridge PW, Li YF, Matsa E, Wu H, Ong SG, Sharma A, Holmström A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC
Nature medicine 2016 May;22(5):547-56
Nature medicine 2016 May;22(5):547-56
Mesp1 controls the speed, polarity, and directionality of cardiovascular progenitor migration.
Chiapparo G, Lin X, Lescroart F, Chabab S, Paulissen C, Pitisci L, Bondue A, Blanpain C
The Journal of cell biology 2016 May 23;213(4):463-77
The Journal of cell biology 2016 May 23;213(4):463-77
Essential role of the TFIID subunit TAF4 in murine embryogenesis and embryonic stem cell differentiation.
Langer D, Martianov I, Alpern D, Rhinn M, Keime C, Dollé P, Mengus G, Davidson I
Nature communications 2016 Mar 30;7:11063
Nature communications 2016 Mar 30;7:11063
Postnatal Development of Right Ventricular Myofibrillar Biomechanics in Relation to the Sarcomeric Protein Phenotype in Pediatric Patients with Conotruncal Heart Defects.
Elhamine F, Iorga B, Krüger M, Hunger M, Eckhardt J, Sreeram N, Bennink G, Brockmeier K, Pfitzer G, Stehle R
Journal of the American Heart Association 2016 Jun 27;5(6)
Journal of the American Heart Association 2016 Jun 27;5(6)
Functional Coupling with Cardiac Muscle Promotes Maturation of hPSC-Derived Sympathetic Neurons.
Oh Y, Cho GS, Li Z, Hong I, Zhu R, Kim MJ, Kim YJ, Tampakakis E, Tung L, Huganir R, Dong X, Kwon C, Lee G
Cell stem cell 2016 Jul 7;19(1):95-106
Cell stem cell 2016 Jul 7;19(1):95-106
Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes.
Eng G, Lee BW, Protas L, Gagliardi M, Brown K, Kass RS, Keller G, Robinson RB, Vunjak-Novakovic G
Nature communications 2016 Jan 19;7:10312
Nature communications 2016 Jan 19;7:10312
Desmin enters the nucleus of cardiac stem cells and modulates Nkx2.5 expression by participating in transcription factor complexes that interact with the nkx2.5 gene.
Fuchs C, Gawlas S, Heher P, Nikouli S, Paar H, Ivankovic M, Schultheis M, Klammer J, Gottschamel T, Capetanaki Y, Weitzer G
Biology open 2016 Jan 19;5(2):140-53
Biology open 2016 Jan 19;5(2):140-53
Atypical Protein Kinase C-Dependent Polarized Cell Division Is Required for Myocardial Trabeculation.
Passer D, van de Vrugt A, Atmanli A, Domian IJ
Cell reports 2016 Feb 23;14(7):1662-1672
Cell reports 2016 Feb 23;14(7):1662-1672
Simple Monolayer Differentiation of Murine Cardiomyocytes via Nutrient Deprivation-Mediated Activation of β-Catenin.
Hofbauer P, Jung JP, McArdle TJ, Ogle BM
Stem cell reviews and reports 2016 Dec;12(6):731-743
Stem cell reviews and reports 2016 Dec;12(6):731-743
Bulk cell density and Wnt/TGFbeta signalling regulate mesendodermal patterning of human pluripotent stem cells.
Kempf H, Olmer R, Haase A, Franke A, Bolesani E, Schwanke K, Robles-Diaz D, Coffee M, Göhring G, Dräger G, Pötz O, Joos T, Martinez-Hackert E, Haverich A, Buettner FFR, Martin U, Zweigerdt R
Nature communications 2016 Dec 9;7:13602
Nature communications 2016 Dec 9;7:13602
Disease Model of GATA4 Mutation Reveals Transcription Factor Cooperativity in Human Cardiogenesis.
Ang YS, Rivas RN, Ribeiro AJS, Srivas R, Rivera J, Stone NR, Pratt K, Mohamed TMA, Fu JD, Spencer CI, Tippens ND, Li M, Narasimha A, Radzinsky E, Moon-Grady AJ, Yu H, Pruitt BL, Snyder MP, Srivastava D
Cell 2016 Dec 15;167(7):1734-1749.e22
Cell 2016 Dec 15;167(7):1734-1749.e22
Poly(Limonene Thioether) Scaffold for Tissue Engineering.
Fischer KM, Morgan KY, Hearon K, Sklaviadis D, Tochka ZL, Fenton OS, Anderson DG, Langer R, Freed LE
Advanced healthcare materials 2016 Apr 6;5(7):813-21
Advanced healthcare materials 2016 Apr 6;5(7):813-21
Induction of Human iPSC-Derived Cardiomyocyte Proliferation Revealed by Combinatorial Screening in High Density Microbioreactor Arrays.
Titmarsh DM, Glass NR, Mills RJ, Hidalgo A, Wolvetang EJ, Porrello ER, Hudson JE, Cooper-White JJ
Scientific reports 2016 Apr 21;6:24637
Scientific reports 2016 Apr 21;6:24637
A Systemized Approach to Investigate Ca(2+) Synchronization in Clusters of Human Induced Pluripotent Stem-Cell Derived Cardiomyocytes.
Jones AR, Edwards DH, Cummins MJ, Williams AJ, George CH
Frontiers in cell and developmental biology 2015;3:89
Frontiers in cell and developmental biology 2015;3:89
Cardiac development in zebrafish and human embryonic stem cells is inhibited by exposure to tobacco cigarettes and e-cigarettes.
Palpant NJ, Hofsteen P, Pabon L, Reinecke H, Murry CE
PloS one 2015;10(5):e0126259
PloS one 2015;10(5):e0126259
PKB-Mediated Thr649 Phosphorylation of AS160/TBC1D4 Regulates the R-Wave Amplitude in the Heart.
Quan C, Xie B, Wang HY, Chen S
PloS one 2015;10(4):e0124491
PloS one 2015;10(4):e0124491
Activin-A and Bmp4 levels modulate cell type specification during CHIR-induced cardiomyogenesis.
Kim MS, Horst A, Blinka S, Stamm K, Mahnke D, Schuman J, Gundry R, Tomita-Mitchell A, Lough J
PloS one 2015;10(2):e0118670
PloS one 2015;10(2):e0118670
An Orthologous Epigenetic Gene Expression Signature Derived from Differentiating Embryonic Stem Cells Identifies Regulators of Cardiogenesis.
Busser BW, Lin Y, Yang Y, Zhu J, Chen G, Michelson AM
PloS one 2015;10(10):e0141066
PloS one 2015;10(10):e0141066
Cyclosporin A induces cardiac differentiation but inhibits hemato-endothelial differentiation of P19 cells.
Choi SC, Lee H, Choi JH, Kim JH, Park CY, Joo HJ, Park JH, Hong SJ, Yu CW, Lim DS
PloS one 2015;10(1):e0117410
PloS one 2015;10(1):e0117410
High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling.
Zhao Y, Londono P, Cao Y, Sharpe EJ, Proenza C, O'Rourke R, Jones KL, Jeong MY, Walker LA, Buttrick PM, McKinsey TA, Song K
Nature communications 2015 Sep 10;6:8243
Nature communications 2015 Sep 10;6:8243
A new system for profiling drug-induced calcium signal perturbation in human embryonic stem cell-derived cardiomyocytes.
Lewis KJ, Silvester NC, Barberini-Jammaers S, Mason SA, Marsh SA, Lipka M, George CH
Journal of biomolecular screening 2015 Mar;20(3):330-40
Journal of biomolecular screening 2015 Mar;20(3):330-40
Efficient Generation of Cardiac Purkinje Cells from ESCs by Activating cAMP Signaling.
Tsai SY, Maass K, Lu J, Fishman GI, Chen S, Evans T
Stem cell reports 2015 Jun 9;4(6):1089-102
Stem cell reports 2015 Jun 9;4(6):1089-102
Statistically based splicing detection reveals neural enrichment and tissue-specific induction of circular RNA during human fetal development.
Szabo L, Morey R, Palpant NJ, Wang PL, Afari N, Jiang C, Parast MM, Murry CE, Laurent LC, Salzman J
Genome biology 2015 Jun 16;16(1):126
Genome biology 2015 Jun 16;16(1):126
Reprogramming fibroblasts toward cardiomyocytes, neural stem cells and hepatocytes by cell activation and signaling-directed lineage conversion.
Zhu S, Wang H, Ding S
Nature protocols 2015 Jul;10(7):959-73
Nature protocols 2015 Jul;10(7):959-73
Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart.
Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abderrahman Y, Chen R, Garcia JA, Shelton JM, Richardson JA, Ashour AM, Asaithamby A, Liang H, Xing C, Lu Z, Zhang CC, Sadek HA
Nature 2015 Jul 9;523(7559):226-30
Nature 2015 Jul 9;523(7559):226-30
Alzheimer-associated Aβ oligomers impact the central nervous system to induce peripheral metabolic deregulation.
Clarke JR, Lyra E Silva NM, Figueiredo CP, Frozza RL, Ledo JH, Beckman D, Katashima CK, Razolli D, Carvalho BM, Frazão R, Silveira MA, Ribeiro FC, Bomfim TR, Neves FS, Klein WL, Medeiros R, LaFerla FM, Carvalheira JB, Saad MJ, Munoz DP, Velloso LA, Ferreira ST, De Felice FG
EMBO molecular medicine 2015 Feb;7(2):190-210
EMBO molecular medicine 2015 Feb;7(2):190-210
Non-genetic Purification of Ventricular Cardiomyocytes from Differentiating Embryonic Stem Cells through Molecular Beacons Targeting IRX-4.
Ban K, Wile B, Cho KW, Kim S, Song MK, Kim SY, Singer J, Syed A, Yu SP, Wagner M, Bao G, Yoon YS
Stem cell reports 2015 Dec 8;5(6):1239-1249
Stem cell reports 2015 Dec 8;5(6):1239-1249
An integrated statistical model for enhanced murine cardiomyocyte differentiation via optimized engagement of 3D extracellular matrices.
Jung JP, Hu D, Domian IJ, Ogle BM
Scientific reports 2015 Dec 21;5:18705
Scientific reports 2015 Dec 21;5:18705
Brg1 modulates enhancer activation in mesoderm lineage commitment.
Alexander JM, Hota SK, He D, Thomas S, Ho L, Pennacchio LA, Bruneau BG
Development (Cambridge, England) 2015 Apr 15;142(8):1418-30
Development (Cambridge, England) 2015 Apr 15;142(8):1418-30
Identification and purification of human induced pluripotent stem cell-derived atrial-like cardiomyocytes based on sarcolipin expression.
Josowitz R, Lu J, Falce C, D'Souza SL, Wu M, Cohen N, Dubois NC, Zhao Y, Sobie EA, Fishman GI, Gelb BD
PloS one 2014;9(7):e101316
PloS one 2014;9(7):e101316
Prohibitin 2 regulates the proliferation and lineage-specific differentiation of mouse embryonic stem cells in mitochondria.
Kowno M, Watanabe-Susaki K, Ishimine H, Komazaki S, Enomoto K, Seki Y, Wang YY, Ishigaki Y, Ninomiya N, Noguchi TA, Kokubu Y, Ohnishi K, Nakajima Y, Kato K, Intoh A, Takada H, Yamakawa N, Wang PC, Asashima M, Kurisaki A
PloS one 2014;9(4):e81552
PloS one 2014;9(4):e81552
Inhibition of TGFβ signaling increases direct conversion of fibroblasts to induced cardiomyocytes.
Ifkovits JL, Addis RC, Epstein JA, Gearhart JD
PloS one 2014;9(2):e89678
PloS one 2014;9(2):e89678
Complete restoration of multiple dystrophin isoforms in genetically corrected Duchenne muscular dystrophy patient-derived cardiomyocytes.
Zatti S, Martewicz S, Serena E, Uno N, Giobbe G, Kazuki Y, Oshimura M, Elvassore N
Molecular therapy. Methods & clinical development 2014;1:1
Molecular therapy. Methods & clinical development 2014;1:1
Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells.
Xie Y, Ibrahim A, Cheng K, Wu Z, Liang W, Malliaras K, Sun B, Liu W, Shen D, Cheol Cho H, Li T, Lu L, Lu G, Marbán E
Stem cells (Dayton, Ohio) 2014 Sep;32(9):2397-406
Stem cells (Dayton, Ohio) 2014 Sep;32(9):2397-406
Integrin-linked kinase mediates force transduction in cardiomyocytes by modulating SERCA2a/PLN function.
Traister A, Li M, Aafaqi S, Lu M, Arab S, Radisic M, Gross G, Guido F, Sherret J, Verma S, Slorach C, Mertens L, Hui W, Roy A, Delgado-Olguín P, Hannigan G, Maynes JT, Coles JG
Nature communications 2014 Sep 11;5:4533
Nature communications 2014 Sep 11;5:4533
Molecular beacon-enabled purification of living cells by targeting cell type-specific mRNAs.
Wile BM, Ban K, Yoon YS, Bao G
Nature protocols 2014 Oct;9(10):2411-24
Nature protocols 2014 Oct;9(10):2411-24
Combined biophysical and soluble factor modulation induces cardiomyocyte differentiation from human muscle derived stem cells.
Tchao J, Han L, Lin B, Yang L, Tobita K
Scientific reports 2014 Oct 14;4:6614
Scientific reports 2014 Oct 14;4:6614
Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors.
Nam YJ, Lubczyk C, Bhakta M, Zang T, Fernandez-Perez A, McAnally J, Bassel-Duby R, Olson EN, Munshi NV
Development (Cambridge, England) 2014 Nov;141(22):4267-78
Development (Cambridge, England) 2014 Nov;141(22):4267-78
PCP4 regulates Purkinje cell excitability and cardiac rhythmicity.
Kim EE, Shekhar A, Lu J, Lin X, Liu FY, Zhang J, Delmar M, Fishman GI
The Journal of clinical investigation 2014 Nov;124(11):5027-36
The Journal of clinical investigation 2014 Nov;124(11):5027-36
Irx4 identifies a chamber-specific cell population that contributes to ventricular myocardium development.
Nelson DO, Jin DX, Downs KM, Kamp TJ, Lyons GE
Developmental dynamics : an official publication of the American Association of Anatomists 2014 Mar;243(3):381-92
Developmental dynamics : an official publication of the American Association of Anatomists 2014 Mar;243(3):381-92
Comparison of the molecular profiles of human embryonic and induced pluripotent stem cells of isogenic origin.
Mallon BS, Hamilton RS, Kozhich OA, Johnson KR, Fann YC, Rao MS, Robey PG
Stem cell research 2014 Mar;12(2):376-86
Stem cell research 2014 Mar;12(2):376-86
Mutations in Alström protein impair terminal differentiation of cardiomyocytes.
Shenje LT, Andersen P, Halushka MK, Lui C, Fernandez L, Collin GB, Amat-Alarcon N, Meschino W, Cutz E, Chang K, Yonescu R, Batista DA, Chen Y, Chelko S, Crosson JE, Scheel J, Vricella L, Craig BD, Marosy BA, Mohr DW, Hetrick KN, Romm JM, Scott AF, Valle D, Naggert JK, Kwon C, Doheny KF, Judge DP
Nature communications 2014 Mar 4;5:3416
Nature communications 2014 Mar 4;5:3416
Dual modulation of the mitochondrial permeability transition pore and redox signaling synergistically promotes cardiomyocyte differentiation from pluripotent stem cells.
Cho SW, Park JS, Heo HJ, Park SW, Song S, Kim I, Han YM, Yamashita JK, Youm JB, Han J, Koh GY
Journal of the American Heart Association 2014 Mar 13;3(2):e000693
Journal of the American Heart Association 2014 Mar 13;3(2):e000693
Insulin-like growth factor promotes cardiac lineage induction in vitro by selective expansion of early mesoderm.
Engels MC, Rajarajan K, Feistritzer R, Sharma A, Nielsen UB, Schalij MJ, de Vries AA, Pijnappels DA, Wu SM
Stem cells (Dayton, Ohio) 2014 Jun;32(6):1493-502
Stem cells (Dayton, Ohio) 2014 Jun;32(6):1493-502
Angiomodulin is required for cardiogenesis of embryonic stem cells and is maintained by a feedback loop network of p63 and Activin-A.
Wolchinsky Z, Shivtiel S, Kouwenhoven EN, Putin D, Sprecher E, Zhou H, Rouleau M, Aberdam D
Stem cell research 2014 Jan;12(1):49-59
Stem cell research 2014 Jan;12(1):49-59
Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium.
Turnbull IC, Karakikes I, Serrao GW, Backeris P, Lee JJ, Xie C, Senyei G, Gordon RE, Li RA, Akar FG, Hajjar RJ, Hulot JS, Costa KD
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2014 Feb;28(2):644-54
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2014 Feb;28(2):644-54
Chemically defined generation of human cardiomyocytes.
Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC
Nature methods 2014 Aug;11(8):855-60
Nature methods 2014 Aug;11(8):855-60
The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response.
Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA
Cell 2014 Apr 24;157(3):565-79
Cell 2014 Apr 24;157(3):565-79
Evidence for a critical role of catecholamines for cardiomyocyte lineage commitment in murine embryonic stem cells.
Lehmann M, Nguemo F, Wagh V, Pfannkuche K, Hescheler J, Reppel M
PloS one 2013;8(8):e70913
PloS one 2013;8(8):e70913
Embryonic stem cells facilitate the isolation of persistent clonal cardiovascular progenitor cell lines and leukemia inhibitor factor maintains their self-renewal and myocardial differentiation potential in vitro.
Hoebaus J, Heher P, Gottschamel T, Scheinast M, Auner H, Walder D, Wiedner M, Taubenschmid J, Miksch M, Sauer T, Schultheis M, Kuzmenkin A, Seiser C, Hescheler J, Weitzer G
Cells, tissues, organs 2013;197(4):249-68
Cells, tissues, organs 2013;197(4):249-68
A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells.
Später D, Abramczuk MK, Buac K, Zangi L, Stachel MW, Clarke J, Sahara M, Ludwig A, Chien KR
Nature cell biology 2013 Sep;15(9):1098-106
Nature cell biology 2013 Sep;15(9):1098-106
Wharton's jelly-derived mesenchymal stem cells promote myocardial regeneration and cardiac repair after miniswine acute myocardial infarction.
Zhang W, Liu XC, Yang L, Zhu DL, Zhang YD, Chen Y, Zhang HY
Coronary artery disease 2013 Nov;24(7):549-58
Coronary artery disease 2013 Nov;24(7):549-58
Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2.
Maejima Y, Kyoi S, Zhai P, Liu T, Li H, Ivessa A, Sciarretta S, Del Re DP, Zablocki DK, Hsu CP, Lim DS, Isobe M, Sadoshima J
Nature medicine 2013 Nov;19(11):1478-88
Nature medicine 2013 Nov;19(11):1478-88
Inactivation of Cdc42 in neural crest cells causes craniofacial and cardiovascular morphogenesis defects.
Liu Y, Jin Y, Li J, Seto E, Kuo E, Yu W, Schwartz RJ, Blazo M, Zhang SL, Peng X
Developmental biology 2013 Nov 15;383(2):239-52
Developmental biology 2013 Nov 15;383(2):239-52
Specification of chondrocytes and cartilage tissues from embryonic stem cells.
Craft AM, Ahmed N, Rockel JS, Baht GS, Alman BA, Kandel RA, Grigoriadis AE, Keller GM
Development (Cambridge, England) 2013 Jun;140(12):2597-610
Development (Cambridge, England) 2013 Jun;140(12):2597-610
Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success.
Addis RC, Ifkovits JL, Pinto F, Kellam LD, Esteso P, Rentschler S, Christoforou N, Epstein JA, Gearhart JD
Journal of molecular and cellular cardiology 2013 Jul;60:97-106
Journal of molecular and cellular cardiology 2013 Jul;60:97-106
Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury.
Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, Yamamoto T, Zhang N, Yabuta N, Nojima H, Pan D, Sadoshima J
The Journal of biological chemistry 2013 Feb 8;288(6):3977-88
The Journal of biological chemistry 2013 Feb 8;288(6):3977-88
The ROSA26-iPSC mouse: a conditional, inducible, and exchangeable resource for studying cellular (De)differentiation.
Haenebalcke L, Goossens S, Dierickx P, Bartunkova S, D'Hont J, Haigh K, Hochepied T, Wirth D, Nagy A, Haigh JJ
Cell reports 2013 Feb 21;3(2):335-41
Cell reports 2013 Feb 21;3(2):335-41
zebraflash transgenic lines for in vivo bioluminescence imaging of stem cells and regeneration in adult zebrafish.
Chen CH, Durand E, Wang J, Zon LI, Poss KD
Development (Cambridge, England) 2013 Dec;140(24):4988-97
Development (Cambridge, England) 2013 Dec;140(24):4988-97
Hippo signaling impedes adult heart regeneration.
Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF
Development (Cambridge, England) 2013 Dec;140(23):4683-90
Development (Cambridge, England) 2013 Dec;140(23):4683-90
Mouse embryonic stem cells irradiated with γ-rays differentiate into cardiomyocytes but with altered contractile properties.
Rebuzzini P, Fassina L, Mulas F, Bellazzi R, Redi CA, Di Liberto R, Magenes G, Adjaye J, Zuccotti M, Garagna S
Mutation research 2013 Aug 30;756(1-2):37-45
Mutation research 2013 Aug 30;756(1-2):37-45
HCN4 dynamically marks the first heart field and conduction system precursors.
Liang X, Wang G, Lin L, Lowe J, Zhang Q, Bu L, Chen Y, Chen J, Sun Y, Evans SM
Circulation research 2013 Aug 2;113(4):399-407
Circulation research 2013 Aug 2;113(4):399-407
Human embryonic stem cell-derived cardiomyocytes migrate in response to gradients of fibronectin and Wnt5a.
Moyes KW, Sip CG, Obenza W, Yang E, Horst C, Welikson RE, Hauschka SD, Folch A, Laflamme MA
Stem cells and development 2013 Aug 15;22(16):2315-25
Stem cells and development 2013 Aug 15;22(16):2315-25
Efficient generation of human iPSCs by a synthetic self-replicative RNA.
Yoshioka N, Gros E, Li HR, Kumar S, Deacon DC, Maron C, Muotri AR, Chi NC, Fu XD, Yu BD, Dowdy SF
Cell stem cell 2013 Aug 1;13(2):246-54
Cell stem cell 2013 Aug 1;13(2):246-54
ILK induces cardiomyogenesis in the human heart.
Traister A, Aafaqi S, Masse S, Dai X, Li M, Hinek A, Nanthakumar K, Hannigan G, Coles JG
PloS one 2012;7(5):e37802
PloS one 2012;7(5):e37802
Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells.
Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, Delgado SM, Wakatsuki T, Crone WC, Pruitt BL, Palecek SP
International journal of cell biology 2012;2012:508294
International journal of cell biology 2012;2012:508294
Efficient and user-friendly pluripotin-based derivation of mouse embryonic stem cells.
Pieters T, Haenebalcke L, Hochepied T, D'Hont J, Haigh JJ, van Roy F, van Hengel J
Stem cell reviews and reports 2012 Sep;8(3):768-78
Stem cell reviews and reports 2012 Sep;8(3):768-78
Inhibition of cardiomyocytes differentiation of mouse embryonic stem cells by CD38/cADPR/Ca2+ signaling pathway.
Wei WJ, Sun HY, Ting KY, Zhang LH, Lee HC, Li GR, Yue J
The Journal of biological chemistry 2012 Oct 12;287(42):35599-35611
The Journal of biological chemistry 2012 Oct 12;287(42):35599-35611
Activation of Toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency.
Mastri M, Shah Z, McLaughlin T, Greene CJ, Baum L, Suzuki G, Lee T
American journal of physiology. Cell physiology 2012 Nov 15;303(10):C1021-33
American journal of physiology. Cell physiology 2012 Nov 15;303(10):C1021-33
Heart repair by reprogramming non-myocytes with cardiac transcription factors.
Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN
Nature 2012 May 13;485(7400):599-604
Nature 2012 May 13;485(7400):599-604
Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia.
Jung CB, Moretti A, Mederos y Schnitzler M, Iop L, Storch U, Bellin M, Dorn T, Ruppenthal S, Pfeiffer S, Goedel A, Dirschinger RJ, Seyfarth M, Lam JT, Sinnecker D, Gudermann T, Lipp P, Laugwitz KL
EMBO molecular medicine 2012 Mar;4(3):180-91
EMBO molecular medicine 2012 Mar;4(3):180-91
Transgenic zebrafish illuminate the dynamics of thyroid morphogenesis and its relationship to cardiovascular development.
Opitz R, Maquet E, Huisken J, Antonica F, Trubiroha A, Pottier G, Janssens V, Costagliola S
Developmental biology 2012 Dec 15;372(2):203-16
Developmental biology 2012 Dec 15;372(2):203-16
Eomesodermin induces Mesp1 expression and cardiac differentiation from embryonic stem cells in the absence of Activin.
van den Ameele J, Tiberi L, Bondue A, Paulissen C, Herpoel A, Iacovino M, Kyba M, Blanpain C, Vanderhaeghen P
EMBO reports 2012 Apr;13(4):355-62
EMBO reports 2012 Apr;13(4):355-62
Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression.
Uosaki H, Fukushima H, Takeuchi A, Matsuoka S, Nakatsuji N, Yamanaka S, Yamashita JK
PloS one 2011;6(8):e23657
PloS one 2011;6(8):e23657
Expression of sumoylation deficient Nkx2.5 mutant in Nkx2.5 haploinsufficient mice leads to congenital heart defects.
Kim EY, Chen L, Ma Y, Yu W, Chang J, Moskowitz IP, Wang J
PloS one 2011;6(6):e20803
PloS one 2011;6(6):e20803
Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell-derived cardiomyocytes.
Guo L, Abrams RM, Babiarz JE, Cohen JD, Kameoka S, Sanders MJ, Chiao E, Kolaja KL
Toxicological sciences : an official journal of the Society of Toxicology 2011 Sep;123(1):281-9
Toxicological sciences : an official journal of the Society of Toxicology 2011 Sep;123(1):281-9
β-myosin heavy chain is induced by pressure overload in a minor subpopulation of smaller mouse cardiac myocytes.
López JE, Myagmar BE, Swigart PM, Montgomery MD, Haynam S, Bigos M, Rodrigo MC, Simpson PC
Circulation research 2011 Sep 2;109(6):629-38
Circulation research 2011 Sep 2;109(6):629-38
SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells.
Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, Gramolini A, Keller G
Nature biotechnology 2011 Oct 23;29(11):1011-8
Nature biotechnology 2011 Oct 23;29(11):1011-8
The differentiation of cardiomyocytes from mouse embryonic stem cells is altered by dioxin.
Neri T, Merico V, Fiordaliso F, Salio M, Rebuzzini P, Sacchi L, Bellazzi R, Redi CA, Zuccotti M, Garagna S
Toxicology letters 2011 May 10;202(3):226-36
Toxicology letters 2011 May 10;202(3):226-36
Ouabain facilitates cardiac differentiation of mouse embryonic stem cells through ERK1/2 pathway.
Lee YK, Ng KM, Lai WH, Man C, Lieu DK, Lau CP, Tse HF, Siu CW
Acta pharmacologica Sinica 2011 Jan;32(1):52-61
Acta pharmacologica Sinica 2011 Jan;32(1):52-61
Cardiac origin of smooth muscle cells in the inflow tract.
Nakano H, Williams E, Hoshijima M, Sasaki M, Minamisawa S, Chien KR, Nakano A
Journal of molecular and cellular cardiology 2011 Feb;50(2):337-45
Journal of molecular and cellular cardiology 2011 Feb;50(2):337-45
Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines.
Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G
Cell stem cell 2011 Feb 4;8(2):228-40
Cell stem cell 2011 Feb 4;8(2):228-40
Cobalt chloride pretreatment promotes cardiac differentiation of human embryonic stem cells under atmospheric oxygen level.
Ng KM, Chan YC, Lee YK, Lai WH, Au KW, Fung ML, Siu CW, Li RA, Tse HF
Cellular reprogramming 2011 Dec;13(6):527-37
Cellular reprogramming 2011 Dec;13(6):527-37
Isolation and functional characterization of alpha-smooth muscle actin expressing cardiomyocytes from embryonic stem cells.
Potta SP, Liang H, Winkler J, Doss MX, Chen S, Wagh V, Pfannkuche K, Hescheler J, Sachinidis A
Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 2010;25(6):595-604
Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 2010;25(6):595-604
Triiodothyronine promotes cardiac differentiation and maturation of embryonic stem cells via the classical genomic pathway.
Lee YK, Ng KM, Chan YC, Lai WH, Au KW, Ho CY, Wong LY, Lau CP, Tse HF, Siu CW
Molecular endocrinology (Baltimore, Md.) 2010 Sep;24(9):1728-36
Molecular endocrinology (Baltimore, Md.) 2010 Sep;24(9):1728-36
Global transcriptional profiles of beating clusters derived from human induced pluripotent stem cells and embryonic stem cells are highly similar.
Gupta MK, Illich DJ, Gaarz A, Matzkies M, Nguemo F, Pfannkuche K, Liang H, Classen S, Reppel M, Schultze JL, Hescheler J, Sarić T
BMC developmental biology 2010 Sep 15;10:98
BMC developmental biology 2010 Sep 15;10:98
Timed inhibition of p38MAPK directs accelerated differentiation of human embryonic stem cells into cardiomyocytes.
Gaur M, Ritner C, Sievers R, Pedersen A, Prasad M, Bernstein HS, Yeghiazarians Y
Cytotherapy 2010 Oct;12(6):807-17
Cytotherapy 2010 Oct;12(6):807-17
Icariin-mediated differentiation of mouse adipose-derived stem cells into cardiomyocytes.
Jin MS, Shi S, Zhang Y, Yan Y, Sun XD, Liu W, Liu HW
Molecular and cellular biochemistry 2010 Nov;344(1-2):1-9
Molecular and cellular biochemistry 2010 Nov;344(1-2):1-9
Mouse and human induced pluripotent stem cells as a source for multipotent Isl1+ cardiovascular progenitors.
Moretti A, Bellin M, Jung CB, Thies TM, Takashima Y, Bernshausen A, Schiemann M, Fischer S, Moosmang S, Smith AG, Lam JT, Laugwitz KL
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2010 Mar;24(3):700-11
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2010 Mar;24(3):700-11
Delayed enrichment of mesenchymal cells promotes cardiac lineage and calcium transient development.
Grajales L, García J, Banach K, Geenen DL
Journal of molecular and cellular cardiology 2010 Apr;48(4):735-45
Journal of molecular and cellular cardiology 2010 Apr;48(4):735-45
Effects of iron oxide nanoparticles on cardiac differentiation of embryonic stem cells.
Au KW, Liao SY, Lee YK, Lai WH, Ng KM, Chan YC, Yip MC, Ho CY, Wu EX, Li RA, Siu CW, Tse HF
Biochemical and biophysical research communications 2009 Feb 20;379(4):898-903
Biochemical and biophysical research communications 2009 Feb 20;379(4):898-903
Enhanced proliferation of monolayer cultures of embryonic stem (ES) cell-derived cardiomyocytes following acute loss of retinoblastoma.
Yamanaka S, Zahanich I, Wersto RP, Boheler KR
PloS one 2008;3(12):e3896
PloS one 2008;3(12):e3896
Negative effects of rofecoxib treatment on cardiac function after ischemia-reperfusion injury in APOE3Leiden mice are prevented by combined treatment with thromboxane prostanoid-receptor antagonist S18886 (terutroban).
van der Hoorn JW, Jukema JW, Bekkers ME, Princen HM, Corda S, Emeis JJ, Steendijk P
Critical care medicine 2008 Sep;36(9):2576-82
Critical care medicine 2008 Sep;36(9):2576-82
A high-efficiency system for the generation and study of human induced pluripotent stem cells.
Maherali N, Ahfeldt T, Rigamonti A, Utikal J, Cowan C, Hochedlinger K
Cell stem cell 2008 Sep 11;3(3):340-5
Cell stem cell 2008 Sep 11;3(3):340-5
Electrostimulation induces cardiomyocyte predifferentiation of fibroblasts.
Genovese JA, Spadaccio C, Langer J, Habe J, Jackson J, Patel AN
Biochemical and biophysical research communications 2008 Jun 6;370(3):450-5
Biochemical and biophysical research communications 2008 Jun 6;370(3):450-5
Gremlin enhances the determined path to cardiomyogenesis.
Kami D, Shiojima I, Makino H, Matsumoto K, Takahashi Y, Ishii R, Naito AT, Toyoda M, Saito H, Watanabe M, Komuro I, Umezawa A
PloS one 2008 Jun 11;3(6):e2407
PloS one 2008 Jun 11;3(6):e2407
A myocardial lineage derives from Tbx18 epicardial cells.
Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM
Nature 2008 Jul 3;454(7200):104-8
Nature 2008 Jul 3;454(7200):104-8
Mesp1 acts as a master regulator of multipotent cardiovascular progenitor specification.
Bondue A, Lapouge G, Paulissen C, Semeraro C, Iacovino M, Kyba M, Blanpain C
Cell stem cell 2008 Jul 3;3(1):69-84
Cell stem cell 2008 Jul 3;3(1):69-84
Generation of functional murine cardiac myocytes from induced pluripotent stem cells.
Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U
Circulation 2008 Jul 29;118(5):507-17
Circulation 2008 Jul 29;118(5):507-17
Coexpression of platelet-derived growth factor receptor alpha and fetal liver kinase 1 enhances cardiogenic potential in embryonic stem cell differentiation in vitro.
Hirata H, Kawamata S, Murakami Y, Inoue K, Nagahashi A, Tosaka M, Yoshimura N, Miyamoto Y, Iwasaki H, Asahara T, Sawa Y
Journal of bioscience and bioengineering 2007 May;103(5):412-9
Journal of bioscience and bioengineering 2007 May;103(5):412-9
Clonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regeneration.
Tateishi K, Ashihara E, Takehara N, Nomura T, Honsho S, Nakagami T, Morikawa S, Takahashi T, Ueyama T, Matsubara H, Oh H
Journal of cell science 2007 May 15;120(Pt 10):1791-800
Journal of cell science 2007 May 15;120(Pt 10):1791-800
New cell lines from mouse epiblast share defining features with human embryonic stem cells.
Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD
Nature 2007 Jul 12;448(7150):196-9
Nature 2007 Jul 12;448(7150):196-9
ALCAM (CD166) is a surface marker for early murine cardiomyocytes.
Hirata H, Murakami Y, Miyamoto Y, Tosaka M, Inoue K, Nagahashi A, Jakt LM, Asahara T, Iwata H, Sawa Y, Kawamata S
Cells, tissues, organs 2006;184(3-4):172-80
Cells, tissues, organs 2006;184(3-4):172-80
G-CSF/SCF reduces inducible arrhythmias in the infarcted heart potentially via increased connexin43 expression and arteriogenesis.
Kuhlmann MT, Kirchhof P, Klocke R, Hasib L, Stypmann J, Fabritz L, Stelljes M, Tian W, Zwiener M, Mueller M, Kienast J, Breithardt G, Nikol S
The Journal of experimental medicine 2006 Jan 23;203(1):87-97
The Journal of experimental medicine 2006 Jan 23;203(1):87-97
Combined autologous cellular cardiomyoplasty with skeletal myoblasts and bone marrow cells in canine hearts for ischemic cardiomyopathy.
Memon IA, Sawa Y, Miyagawa S, Taketani S, Matsuda H
The Journal of thoracic and cardiovascular surgery 2005 Sep;130(3):646-53
The Journal of thoracic and cardiovascular surgery 2005 Sep;130(3):646-53
Single inner cell masses yield embryonic stem cell lines differing in lifr expression and their developmental potential.
Lauss M, Stary M, Tischler J, Egger G, Puz S, Bader-Allmer A, Seiser C, Weitzer G
Biochemical and biophysical research communications 2005 Jun 17;331(4):1577-86
Biochemical and biophysical research communications 2005 Jun 17;331(4):1577-86
The MRL mouse heart healing response shows donor dominance in allogeneic fetal liver chimeric mice.
Bedelbaeva K, Gourevitch D, Clark L, Chen P, Leferovich JM, Heber-Katz E
Cloning and stem cells 2004;6(4):352-63
Cloning and stem cells 2004;6(4):352-63
Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells.
Shim WS, Jiang S, Wong P, Tan J, Chua YL, Tan YS, Sin YK, Lim CH, Chua T, Teh M, Liu TC, Sim E
Biochemical and biophysical research communications 2004 Nov 12;324(2):481-8
Biochemical and biophysical research communications 2004 Nov 12;324(2):481-8
Epiblast cells that express MyoD recruit pluripotent cells to the skeletal muscle lineage.
Gerhart J, Neely C, Stewart B, Perlman J, Beckmann D, Wallon M, Knudsen K, George-Weinstein M
The Journal of cell biology 2004 Mar 1;164(5):739-46
The Journal of cell biology 2004 Mar 1;164(5):739-46
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
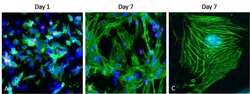
- Experimental details
- Immunofluorescent analysis of Troponin T Cardiac Isoform (green) in cultured primary cardiomyocytes. Primary cardiomyocytes were isolated and cultured using the Primary Cardiomyocyte Isolation Kit (Product # 88281). Cells were grown in 24-well plates (seeded at 5x10^5 cells/well) or in 35mm glass bottom plates (at a density of 2.5 x 106 cells/well). At day 1 and day 7, cells were fixed with 4% paraformaldehyde, permeablilized with 0.1% Triton X-100 in HBSS for 10 minutes at room temperature, and blocked with 3% BSA in PBS (Product # 37525) for 30 minutes at room temperature. Cells were probed with a Troponin T Cardiac Isoform monoclonal antibody (Product # MA5-12960) at dilution of 1:300 for 2 hours at room temperature or overnight at 4 °C, washed with HBSS, and incubated with a DyLight 488-conjugated goat anti-mouse IgG secondary antibody (Product # 35502) at dilution of 1:500 for 1 hour at room temperature. Nuclei (blue) were visualized using Hoechst 33342 (Product # 62249) and dead cells were visualized using Propidium iodide (red). Images were taken at 20X (Panels A and B) or 60X (Panel C) magnifications on a Carl Zeiss microscope (AxioVision Rel. 4.7).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
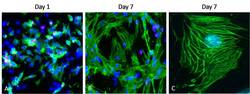
- Experimental details
- Immunofluorescent analysis of Troponin T Cardiac Isoform (green) in cultured primary cardiomyocytes. Primary cardiomyocytes were isolated and cultured using the Primary Cardiomyocyte Isolation Kit (Product # 88281). Cells were grown in 24-well plates (seeded at 5x10^5 cells/well) or in 35mm glass bottom plates (at a density of 2.5 x 106 cells/well). At day 1 and day 7, cells were fixed with 4% paraformaldehyde, permeablilized with 0.1% Triton X-100 in HBSS for 10 minutes at room temperature, and blocked with 3% BSA in PBS (Product # 37525) for 30 minutes at room temperature. Cells were probed with a Troponin T Cardiac Isoform monoclonal antibody (Product # MA5-12960) at dilution of 1:300 for 2 hours at room temperature or overnight at 4 °C, washed with HBSS, and incubated with a DyLight 488-conjugated goat anti-mouse IgG secondary antibody (Product # 35502) at dilution of 1:500 for 1 hour at room temperature. Nuclei (blue) were visualized using Hoechst 33342 (Product # 62249) and dead cells were visualized using Propidium iodide (red). Images were taken at 20X (Panels A and B) or 60X (Panel C) magnifications on a Carl Zeiss microscope (AxioVision Rel. 4.7).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
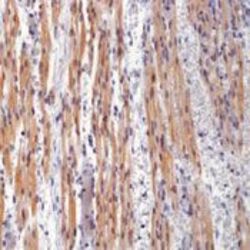
- Experimental details
- Formalin-fixed, paraffin-embedded human heart stained with Troponin antibody using peroxidase-conjugate and DAB chromogen. Note cytoplasmic staining of cardiac muscle cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
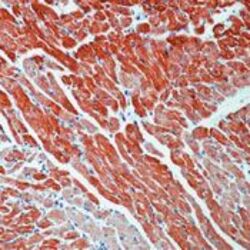
- Experimental details
- Formalin-fixed, paraffin-embedded rat heart stained with Troponin T antibody using peroxidase-conjugate and AEC chromogen. Note cytoplasmic staining of cardiac muscles.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
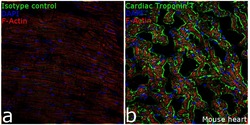
- Experimental details
- Immunofluorescence analysis of Cardiac Troponin T in mouse heart tissue: Frozen sections were fixed with 4% paraformaldehyde for 20 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 10% Normal goat serum for 1 hour at room temperature. Whole heart longitudinal sections were then incubated with Cardiac Troponin T Mouse Monoclonal Antibody (Product# MA5-12960, 5µg/mL) overnight at 4°C, followed by Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175, 1:2000,45 mins). Nuclei (blue) were stained using SlowFade® Gold Antifade Mountant with DAPI (Product # S36938), and cytoskeletal F-actin (red) was stained using Rhodamine Phalloidin (Product # R415, 1:300). Panel a) represents staining with the matched isotype control. Panel b) shows representative sections stained for Cardiac Troponin T (green). The images were captured at 20X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
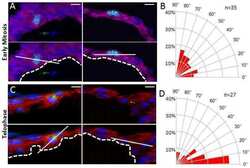
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
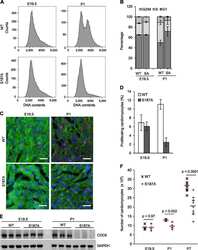
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
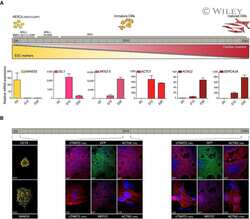
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
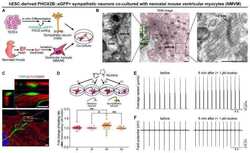
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
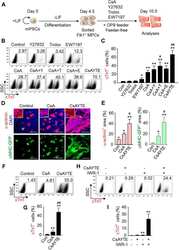
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
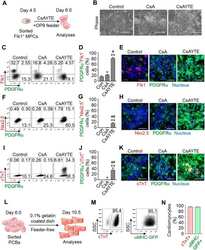
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
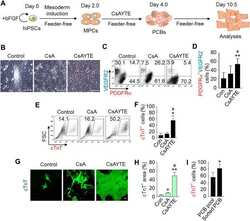
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
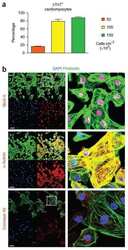
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
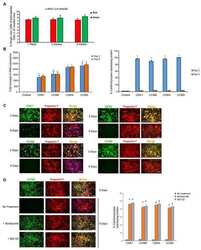
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
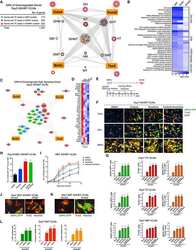
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
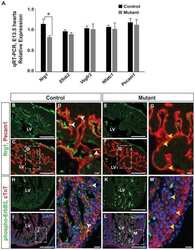
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
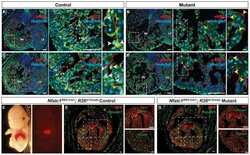
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
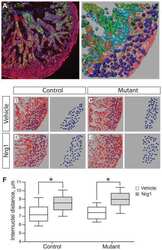
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
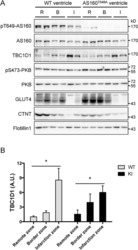
- Experimental details
- Fig 4 Expression and phosphorylation of AS160 and TBC1D1 in the infarct heart. Immunoblotting analyses of phosphorylation of AS160-Thr 649 and PKB-Ser 473 , and total AS160, TBC1D1, PKB, GLUT4 and cTnT in the lysates (40 mug) of the infarct zone (I), border zone (B) and remote zone (R) of mouse heart 4 weeks after myocardial infarction. Flotillin-1 was used as a loading control. A, representative blots. B, quantitation of TBC1D1 signals, n = 4. Asterisk indicates p < 0.05 (infarct zone versus remote zone).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 4 hPSC-derived Cardiomyocyte Proliferation Screening in the HDMA Platform. ( A ) Experimental timeline used in the screen. ( B ) Representative flow cytometric analyses of cell population used in HDMA screening, with percentages marked. Dot plots of CD90 + stromal cell content from 15 d digestion of differentiated hPSCs; dot plots of Ki67 and cTnT expression in both 15 d digestion of differentiated hPSCs and cells replated for 2 d prior to assay startpoint. ( C ) Representative phase contrast micrographs of CMs before and after seeding, and cultured for up to 3 d with drug treatments, as indicated. Scale bars for indicated objective magnification: 4x, 200 mum; 10x, 200 mum; 20x, 100 mum. ( D ) Matrix of design culture conditions in each of the 81 column pairs in the array, for the screened factors: CHIR99021 (CHIR), muM; Purmorphamine (Pm), muM; IGF-1, ng/mL; FGF-2, ng/mL. DMSO was included so as to be at a uniform total concentration of 0.05% v/v in the CHIR channels, acting as a vehicle control. ( E ) Tile-scan confocal image of the entire HDMA (~91 x 28 mm) treated for 24 h and used in further analysis, after fixation and in situ immunostaining for Ki67, cTnT and DNA. ( F ) Higher-magnification confocal image of subsection of replicate HDMA treated for 3 d, showing cell population arrangement in arrayed culture chambers. ( G ) Confocal image showing detail of individual HDMA chambers from replicate HDMA treated for 3 d, demonstrating presence of various proliferatin
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
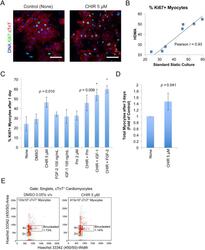
- Experimental details
- Figure 6 Confirmation of HDMA Screen Results in Standard Static Cultures. ( A ) Confocal fluorescence images of Ki67 expression in myocyte population after 24 h treatment with Control medium or medium containing 5 muM CHIR. Scale bar: 100 mum. ( B ) Scatterplot of results from HDMA individual column-pairs against conditions tested in standard static culture, with linear regression and correlation coefficient marked. ( C ) Quantification of percentage Ki67 + myocytes after 24 h treatment in static cultures. Bars represent mean +- S.D. of 2-3 independent experiments including separate cardiomyocyte inductions. *indicates p < 0.05 by one-way ANOVA with Tukey post-hoc tests vs. None condition. No other differences were significant. p -values (paired two-tailed t -test) against None condition are indicated for treatments with large effect magnitudes. ( D ) Quantification of total myocytes after treatment for 3 days with Control (None) medium or medium containing 5 muM CHIR. Bars represent mean +- S.D. of 4 independent experiments including inductions, normalised to control (None) condition. p -value (paired two-tailed t -test) indicated. ( E ) Quantification of percentage of binucleated cardiomyocytes after treatment for 3 days with Control (DMSO 0.05% v/v) medium or medium containing 5 muM CHIR. Dotplot from flow cytometric analysis of nuclear staining (Hoechst 33342) pulsewidth versus pulse area. Gating is on singlets by FSC and SSC area, height and width, and cTnT + cardiomyocy
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
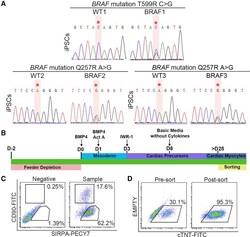
- Experimental details
- Figure 1 Differentiation and Purification of hiPSC-derived CMs (A) Sequencing confirmed the heterozygous BRAF T599R (BRAF1) and the Q257R mutations (BRAF2, BRAF3) in hiPSCs. Red asterisks note the positions of the BRAF mutations. (B) Cardiac differentiation protocol. hiPSCs were exposed to a series of cytokines to induce cardiogenesis. CMs were sorted for purification after day 25. (C) Cell-sorting strategy to purify SIRPalpha + /CD90 - cells and SIRPalpha - /CD90 + cells. (D) Staining for cTNT demonstrated purification of >95% CMs after sorting for SIRPalpha + /CD90 - cells. See also Figures S1 and S2 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
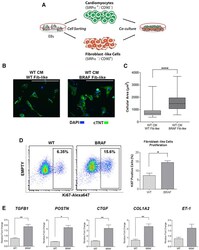
- Experimental details
- Figure 5 BRAF-Mutant FLCs Influence the Hypertrophic Phenotype (A) Schematic of co-culture experiment. WT CMs and WT and BRAF-mutant FLCs (Fib-like) were sorted from EBs and re-cultured together. (B) Representative images of co-culture treatment groups stained with cTNT. Scale bars, 200 mum. (C) Quantification of cellular area as depicted in (B). WT CMs became significantly larger upon co-culture with BRAF-mutant Fib-like cells (n = 67) compared with co-culture with WT Fib-like cells (n = 62) ( **** p < 0.0001). Box-and-whisker plots show the median to the first and third quartiles and the minimum and maximum values. Data represent two biological (WT2, 3; BRAF1, 2) and three technical replicates. (D) Increased proliferation rate of purified BRAF-mutant Fib-like cells (n = 3) compared with WT (n = 2) as demonstrated by increased staining for Ki67 by flow cytometry ( * p = 0.03). Data represent two (WT2, 3) or three (BRAF1, 2, 3) biological and two technical replicates. Data are presented as means +- SEM. (E) Purified BRAF-mutant Fib-like cells expressed increased levels of fibrosis-associated genes compared with WT; TGFbeta1 (BRAF n = 24, WT n = 18, ** p = 0.002), POSTN (BRAF n = 15, WT n = 3, * p = 0.01), CTGF (BRAF n = 18, WT n = 12, ** p = 0.002), COL1A2 (BRAF n = 24, WT n = 15, ** p = 0.003), and ET-1 (BRAF n = 15, WT n = 8, p = 0.07, not significant). Data represent three biological (WT1, 2, 3; BRAF1, 2, 3) and three technical replicates. Data are presented as means +- SE
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
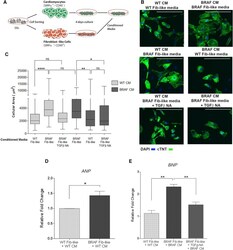
- Experimental details
- Figure 6 BRAF-Mutant FLCs Influence CM Hypertrophy through Paracrine TGFbeta1 Secretion (A) Schematic of conditioned media experiment. WT and BRAF-mutant CMs and FLCs (Fib-like) were sorted from EBs and re-cultured separately. After 4 days, CMs were exposed to Fib-like conditioned media. (B) Representative images of treatment groups stained with cTNT. TGFbeta-NA indicates pre-incubation of Fib-like conditioned media with a TGFbeta neutralizing antibody prior to CM exposure. Scale bars, 200 mum. (C) Quantification of cellular area as depicted in (B). WT CMs exposed to BRAF-mutant Fib-like conditioned media (n = 97) became significantly enlarged compared with exposure to WT Fib-like conditioned media (n = 91) ( **** p < 0.0001). This effect was prevented by pre-incubation with TGFbeta-NA (n = 92). Cellular hypertrophy in BRAF-mutant CMs (n = 86) was significantly reduced upon incubation with WT Fib-like conditioned media (n = 88) ( ** p = 0.008), or pre-incubation of BRAF-mutant Fib-like conditioned media with TGFbeta-NA (n = 134) ( * p = 0.01). Box-and-whisker plots show the median to the first and third quartiles and the minimum and maximum values. ns, not significant. (D) WT CMs exposed to BRAF-mutant Fib-like conditioned media upregulated ANP (n = 3) compared with WT Fib-like conditioned media (n = 3) ( * p = 0.03). Data are presented as means +- SEM. (E) BRAF-mutant CMs exposed to BRAF-mutant Fib-like conditioned media (n = 3) upregulated BNP expression ( ** p = 0.001), wh
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
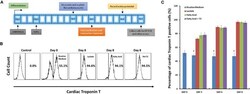
- Experimental details
- Figure 1 High purity of cardiomyocytes can be obtained under glucose-depleted and fatty acid (with or without T 3 )-supplemented conditions. (A) The schematic for purification and maturation using metabolic selection. Glycogen synthase kinase 3 inhibitor CHIR99021 and Wnt pathway inhibitor IWP2 (gray boxes) were added to the differentiation medium on differentiation days 1 and 4, respectively. On differentiation day 10, cells were dissociated and plated on glass cover slips. After 3 days recovery, cells were cultured with metabolic selection medium (No-glucose DMEM medium supplemented with lactate, fatty acid, and fatty acid + T 3 ). On differentiation day 18, cells were collected for patch clamping experiments. On differentiation day 21, cells were collected for other experiments. (B) Representative fluorescence-activated cell sorting (FACS) analyses of cardiac Troponin T expression in the human pluripotent stem cell (hPSC)-derived cells on day 0 with routine culture medium, and on day 8 with metabolic selection medium. In the control group, the isotype control (mouse IgG) was used instead of the primary antibody. (C) Time courses of selection efficiency using the lactate-supplemented condition (red), the fatty acid-supplemented condition (green), and the fatty acid with T 3 -supplemented condition (purple); cells cultured with routine medium (blue) were set up as the control groups ( n = 3). The asterisk means each test group is significantly different from controls, P < 0.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
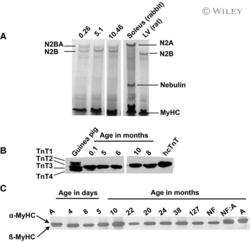
- Experimental details
- Development of titin, myosin heavy chain (MyHC) and troponin T (TnT) isoform expression. A, Representative Coomassie-stained 1% SDS-agarose gel of titin isoforms in RV samples obtained at the indicated ages; rabbit soleus and rat left ventricle (LV) were used as markers. B, Western blot probing for TnT, guinea pig myocardium expressing 4 isoforms, and human recombinant (hcTnT) are shown for comparison; in all human samples the predominant isoform is TnT3. C, Representative SDS-PAGE of separation of MyHC isoforms, markers for alpha- and beta-MyHC isoforms: atrium (A), nonfailing adult heart (NF), and comigration (NF:A). Pediatric patients express only the beta-MyHC.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
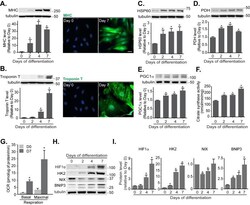
- Experimental details
- Figure 3 FIGURE 3: Mitochondrial functions increase during cardiomyoblast differentiation. Protein levels and immunofluorescence staining of myosin heavy chain (MHC) (A) and cardiac Troponin T (B) were performed in H9c2 cells following differentiation for 0, 2, 4 and 7 days. HSP60 (C) , PDH (D) , PGC1alpha (E) protein levels and citrate synthase activity (F) were determined during this period. (G) Oxygen consumption rate (OCR) was measured in H9c2 cells following 7 days differentiation. (H-I) HIF1alpha, Hexokinase 2 (HK2), NIX and BNIP3 protein levels were examined and quantified in H9c2 cells during the indicated days of differentiation. alpha-tubulin was used as a loading control. All quantitative data are mean +- SEM from 3 independent experiments. Scale bar, 20 mum. * P < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
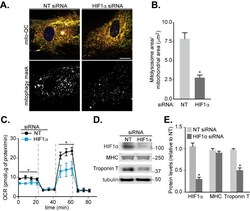
- Experimental details
- Figure 5 FIGURE 5: Involvement of HIF1alpha in mitophagy and cardiomyocyte differentiation. H9c2 cells were cultured in differentiation medium for 7 days. (A) Representative images of mito -QC H9c2 cells transfected with non-targeting (NT) siRNA or HIF1alpha siRNA at day 4 of differentiation. Mitophagy mask represented as the mCherry/GFP ratio intensity above the mean of mCherry intensity. (B) Quantitation from (A) of total mitolysosome area per mitochondrial content as indicated. (C) OCR was measured following 7 days H9c2 differentiation with cells transfected with NT siRNA or HIF1alpha siRNA at day 4. 1 muM oligomycin A, 1 muM FCCP and 1/2 muM rotenone/antimycin A were injected at the indicated times to determine the proportion of oxygen consumption due to ATP turnover, maximal rate of respiration and amount of proton leak respectively. (D-E) HIF1alpha, MHC and cardiac Troponin T protein levels were examined in 7 days-differentiated H9c2 cells transfected with NT or HIF1alpha siRNA at day 4. alpha-tubulin was used as a loading control. Scale bar, 20 mum. All quantitative data are mean +- SEM from 3 independent experiments. * P < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
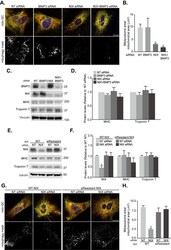
- Experimental details
- Figure 6 FIGURE 6: NIX-mediated mitophagy is not essential for cardiomyocyte differentiation. H9c2 cells were cultured in differentiation medium for 7 days. (A) Representative images of mito -QC H9c2 cells transfected with non-targeting (NT), BNIP3, or NIX siRNA as well as NIX and BNIP3 (NIX/BNIP3) siRNA in combination at day 4 differentiation. Mitophagy mask represented as the mCherry/GFP ratio intensity above the mean of mCherry intensity. (B) Quantitation from (A) of total mitolysosome area per mitochondrial content as indicated. (C-D) BNIP3, NIX, MHC and cardiac Troponin T protein levels were examined after H9c2 cells transfected with NT, BNIP3, NIX or NIX/BNIP3 siRNAs at day 4 differentiation. Vinculin was used as a loading control. (E) H9c2 cells stably expressing WT or siRNA resistant NIX were cultured in differentiation medium for 7 days. NT siRNA or NIX siRNA was applied into medium at day 4 differentiation. NIX, MHC and cardiac Troponin T protein levels were examined after 7 days differentiation. Quantitation from (E) of indicated proteins was shown in (F) . alpha-tubulin was used as a loading control. (G) Representative images of NIX WT or siRNA-resistant cells transfected with NT siRNA or NIX siRNA at day 4. (H) Quantitation from (G) of total mitolysosome area per mitochondrial content as indicated. Scale bar, 20 mum. All quantitative data are mean +- SEM from 3 independent experiments. * P < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
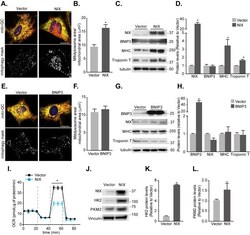
- Experimental details
- Figure 7 FIGURE 7: Increasing NIX-dependent mitophagy promotes cardiomyocyte differentiation. (A) Representative images from mito -QC H9c2 vector control cells or cells overexpressing NIX cultured following 7 days differentiation. Mitophagy mask represented as the mCherry/GFP ratio intensity above the mean of mCherry intensity. (B) Quantitation from (A) of total mitolysosome area per mitochondrial content as indicated. (C) Representative immunoblot of NIX, BNIP3, MHC and cardiac Troponin T protein levels from 7 days differentiated H9c2 cells stably overexpressing NIX. (D) Quantitation of data from (C). (E) Representative images from mito -QC H9c2 cells overexpressing vector or BNIP3 cultured in differentiation medium for 7 days. (F) Quantitation from (E) of total mitolysosome area per mitochondrial content as indicated. (G) Representative immunoblot of NIX, BNIP3, MHC and cardiac Troponin T protein levels from 7 days differentiated H9c2 cells stably overexpressing BNIP3. (H) Quantitation of data from (G). (I) Oxygen consumption rate (OCR) was measured after H9c2 cells stably overexpressing vector or NIX following 7 days differentiation. (J) Immunoblot of HK2 and Pyruvate kinase (PKM2) from H9c2 cells stably overexpressing vector or NIX cultured in differentiation medium for 7 days. Quanitation of HK2 (K) and PKM2 (L) is shown. Scale bar, 20 mum. All quantitative data are mean +- SEM from 3 independent experiments. * P < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
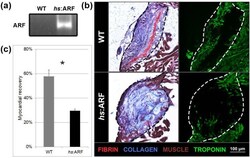
- Experimental details
- Figure 1 Induced Alternative Reading Frame (ARF) expression in hs :ARF fish suppresses cardiac regeneration. ( a ) Representative polymerase chain reaction (PCR) product of ARF expression from three experimental replicates of wild type (WT) and hs :ARF heart RNA on 15 dpi. The product run on gel electrophoresis shows ARF expression present in hs :ARF fish while absent in WT fish. ( b ) Acid Fuchsin Orange G (AFOG) and troponin staining of WT and hs :ARF heart cryosections on 15 days post-injury (dpi). The dotted lines demarcate the injury site. Red stains are for fibrin. Blue stains are for collagen. Brown stains are for muscle. Green stains are for troponin. ( c ) Myocardial recovery measured by troponin infiltration into the injury site. Infiltration quantified by imaging software shows decreased troponin in the injury site of hs :ARF fish compared to WT fish. N = 8 hearts. Results are shown as mean +- standard error. The * represents a statistically significant difference.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
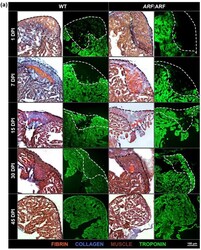
- Experimental details
- Figure 2 ARF expressed under control of the endogenous human ARF promoter in ARF :ARF fish suppresses cardiac regeneration over time. ( a ) AFOG and troponin staining of WT and ARF :ARF heart cryosections at 1, 7, 15, and 30 dpi. The dotted lines demarcate the injury site. Red stains are for fibrin. Blue stains are for collagen. Brown stains are for muscle. Green stains are for troponin. ( b ) Myocardial recovery measured by troponin infiltration into the injury site. Infiltration quantified by imaging software shows decreased troponin in the injury site of ARF :ARF fish compared to WT fish from 7 dpi onward. N = 49 hearts. The grey and black lines are logarithmic approximations of the WT and ARF :ARF heart recovery trends respectively. Results are shown as mean +- standard error. The * represents a statistically significant difference.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
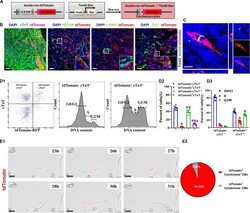
- Experimental details
- FIGURE 2 The proliferating ventricular cardiomyocytes were efficiently labeled in Tnnt2-Dre x Aurkb-rox-tdTomato neonates. (A) Tnnt2-Dre mice were used to cross with Aurkb-rox-tdTomato mice to label proliferating cardiomyocytes. (B) Immunostaining showed the coexpression of tdTomato with cTnT, PECAM, a-SMA, or Col1 on P1 Tnnt2-Dre x Aurkb-rox-tdTomato heart sections. Scale bar = 40 mum. (C) Immunostaining for tdTomato with Aurkb on P56 Tnnt2-Dre x Aurkb-rox-tdTomato heart sections. Scale bar = 20 mum. (D) Representative images of flow cytometry analysis (D1) and quantification of tdTomato +- cells (D2) and numbers in G1, S, G2/M phases (D3) from ventricles of P1 Tnnt2-Dre x Aurkb-rox-tdTomato mice. (D2) n = 6, * p < 0.05 vs. tdTomato - cTnT - ; # p < 0.05 vs. tdTomato + cTnT - ; & p < 0.05 vs. tdTomato - cTnT + . (D3) n = 5, * p < 0.05 vs. tdTomato - cTnT + . (E) Representative images (E1) and quantification (E2) of time-lapse imaging analysis showed cell proliferation in vitro of tdTomato + cardiomyocytes from the ventricles of P1 Tnnt2-Dre x Aurkb-rox-tdTomato mice. Scale bar = 20 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
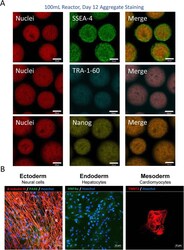
- Experimental details
- Fig. 7 a Representative confocal microscope images of hiPSC aggregates taken from the final day of the vertical-wheel bioreactor serial passage (day 12) and stained for pluripotency markers SSEA-4, TRA-1-60, and Nanog (scale bar = 100 mum) and b differentiated into neuronal cells (immuno-stained for beta-tubulin and PAX6), hepatocytes (immuno-stained for HNF4alpha), and cardiomyocytes (immuno-stained for TNNT2) (scale bar = 50 mum)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
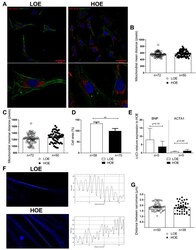
- Experimental details
- Figure 3 The mitochondrial distribution is similar in both 3D cardiac spheroids differentiated under LOE and HOE conditions. Mitochondrial localization and cell area in 25-day-old hPSC-CMs (using hiPSC UEFhfiPS1.4 and hESC CCTL12 lines) differentiated in LOE and HOE conditions were evaluated using MitoTracker dye. ( A ) Immunostaining showing the MitoTracker signal (red) and the cTnT staining (green) in 25-day-old hPSC-CMs obtained from 3D cardiac spheroids differentiated under LOE and HOE conditions. Nuclei are stained with DAPI (blue). Scale bars: 40 um. ( B ) Mitochondrial mean distance in 25-day-old hPSC-CMs obtained from 3D cardiac spheroids differentiated under LOE (white dot plots) and HOE (black dot plots) conditions. ( C ) Mitochondrial maximal distance in 25-day-old hPSC-CMs obtained from 3D cardiac spheroids differentiated in LOE and HOE conditions. ( D ) Cell area of 25-day-old hPSC-CMs (using hiPSC UEFhfiPS1.4 and UB47 lines) obtained from 3D cardiac spheroids differentiated in LOE and HOE conditions. ( E ) DeltaDeltaCt relative gene expression analysis by qRT-PCR in 25-day-old hPSC-CMs obtained from 3D cardiac spheroids differentiated in LOE (white bars) and HOE (black bars) conditions for BNP= natriuretic peptide B and ACTA1= actin alpha skeletal muscle 1. ( F ) Measurement of the sarcomere length of hPSC-CMs (using hiPSC UEFhfiPS1.4 and hESC CCTL12 lines) in the two differentiation protocols by tracing a line of 17 uM across the sarcomeres using an in-house de
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
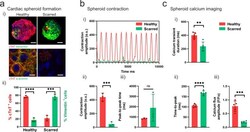
- Experimental details
- Fig. 4 Fabrication of cardiac spheroids for disease modeling applications. a (i) Development of healthy and scarred spheroids through mixing iPSC-derived cardiomyocytes (iPSC-CMs) with primary adult cardiac fibroblasts (CFs) at defined ratios of cell numbers (4:1 for healthy; 1:4 for scarred). Top: Images (cardiac troponin-T (cTnT) (red; iPSC-CMs); vimentin (green; CFs)) taken 3 days after cell seeding. Scalebar 50 um. Bottom: Immunofluorescence staining for alpha-actinin (green; sarcomeres) and cTnT (red; iPSC-CMs) in healthy and scarred spheroids at 3 days. Scalebar 10 um. (ii) Quantification of cellular composition through staining for cTnT (iPSC-CMs) and vimentin (CFs) ( n = 3 biologically independent samples, mean +- s.d, two-sided student t test, healthy - cTnT + vs. Vimentin + p = 5.6 x 10 -5 , scarred - cTnT + vs. Vimentin + p = 6.7 x 10 -4 ). b (i) Contraction profiles, (ii) contraction amplitude (a.u. absolute units), and (iii) peak-to-peak time (ms) of healthy and scarred cardiac spheroids at 3 days ( n = 3 biologically independent samples, mean +- s.d, two-sided student t test, (ii) p = 5.0 x 10 -4 ). c Quantification of calcium activation parameters from calcium mapping experiments in healthy and scarred spheroids at 3 days, including (i) calcium transient duration (ms), (ii) time-to-peak (ms), and (iii) calcium flux amplitude (F/Fo) ( n = 5 biologically independent samples, mean +- s.d, two-sided student t test, (i) p = 0.0014, (ii) p = 5.0 x 10 -6 , (iii) p = 2
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
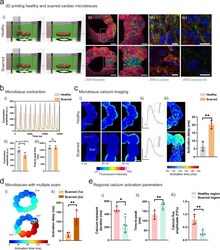
- Experimental details
- Fig. 5 3D bioprinting cardiac microtissues for disease modeling applications. a (i) Schematic of 3D bioprinting of healthy and scarred cardiac microtissue rings and (ii) immunofluorescence staining for cTnT and vimentin in healthy and scarred cardiac microtissues after 5 days of fusion within the support hydrogel. Scalebar 100 um (insets 50 um). (iii) Immunofluorescence staining for alpha-actinin (green; sarcomeres) and cTnT (red; iPSC-CMs) and (iv) connexin-43 (green; gap junctions) and cTnT (red; iPSC-CMs), in healthy and scarred regions of microtissues after 5 days of fusion within the support hydrogel. Scalebar 10 um. b (i) Contraction profiles of healthy and scarred cardiac microtissues following removal from the support hydrogel after 5 days of culture. (ii) Contraction amplitude (a.u. absolute units) and (iii) Peak-to-peak time (ms) at 5 days ( n = 3 biologically independent samples, mean +- s.d, two-sided student t test, (ii) p = 0.061). c (i) Calcium mapping in healthy and scarred cardiac microtissues after 5 days culture, each image represents a 20 ms frame. Scalebar 100 um. (ii) Representative calcium traces from regions 1 and 2 (see methods) in healthy and scarred cardiac microtissues. Scalebars 0.5 DeltaF/F o (y), 500 ms (x). (iii) Activation maps of healthy and scarred cardiac microtissues, and activation delay (ms) (difference in activation time (ms) between regions 1 and 2) in healthy and scarred cardiac microtissues ( n = 3 biologically independent samples, m
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
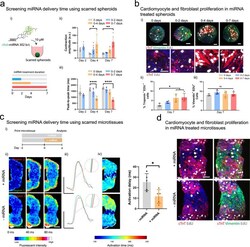
- Experimental details
- Fig. 6 Evaluating miRNA on the behavior of cardiac microtissues. a (i) Schematic of cholesterol modified miR302 (chol-miRNA 302 b/c) delivery to scarred cardiac spheroids for 0, 0-2, 0-4, and 0-7 days. (ii) Contraction amplitude (a.u) and (iii) peak-to-peak time (ms) within scarred spheroids measured after 2, 4, and 7 days for each treatment period ( n = 4, 6, 5, 5, 7, 5, 5, 5, 5 biologically independent samples (from left to right), mean +- s.d, one-way ANOVA, (ii) day 4: 0 vs. 0-4 days treatment p = 0.014, (ii) day 7: 0 vs. 0-7 days treatment p = 0.0045, (iii) day 4: 0 vs. 0-4 days treatment p = 1.6 x 10 -7 , (iii) day 7: 0 vs. 0-7 days treatment p = 4.1 x 10 -9 ). b (i) Immunofluorescence staining for cTnT (red; iPSC-CMs), vimentin (green; cardiac fibroblasts), and EdU (proliferation marker) in scarred spheroids at day 7 for each treatment condition. Top panel scalebar 50 um, and bottom panel scalebar 40 um. (ii) Quantification of cardiomyocyte proliferation (EdU + and cTnT + ) and (iii) fibroblast proliferation (EdU + and Vimentin + ) at day 7 for each treatment condition. ( n = 3, 4, 4, 4 biologically independent samples (from left to right), mean +- s.d, one-way ANOVA, 0 vs. 0-4 days treatment p = 0.044, 0 vs. 0-7 days treatment p = 0.026). Scalebar 50 um. c (i) Experimental outline where scarred microtissues are bioprinted in the support hydrogel as previously described, followed by 4 days treatment with miR302, and analysis compared to non-treated controls. (ii) Calci
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
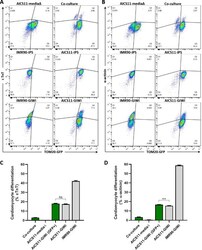
- Experimental details
- 966.g005 Fig 5 Cardiomyocyte differentiation efficiency of AICS11 co-cultured with iCMs evaluated by flow cytometry. AICS11 were either co-cultured with IMR90-iCMs, or subjected to media change only as a control. Additional controls include non-differentiated iPS or GiWi-differentiated AICS11 or IMR90 cells. Total cells were harvested and immuno-stained for the cardiomyocyte markers (A) cTnT and (B) a-actinin, and analyzed by flow cytometry in conjunction with TOM20-GFP to distinguish AICS11 cells. As shown are representative flow plots for an experiment performed in triplicates. Calculation of cardiomyocyte differentiation efficiency for (C) cTnT and (D) a-actinin. Green bars indicate cells gated for GFP only, where % = Q2/(Q2+Q3)*100, while grey bars indicate total cells, where % = (Q1+Q2)/(Q1+Q2+Q3+Q4)*100. P-value for all pairwise comparison is
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
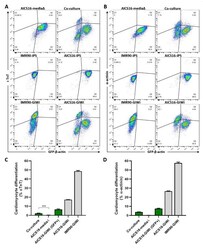
- Experimental details
- 966.g006 Fig 6 Cardiomyocyte differentiation efficiency of AICS16 co-cultured with iCMs evaluated by flow cytometry. Description for this experiment is identical to that for Fig 5 , but with AICS16 cells (expressing GFP-b-actin) in place of AICS11. P-value for all pairwise comparison is
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
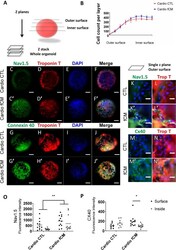
- Experimental details
- Figure 6 Fibroblast-secreted mediators induce 3-dimensional patterning of VCS markers. Evaluation of the influence of fibroblast-secreted factors on the expression of VCS markers in neonatal rat cardiomyocyte organoids using automated high content screening analysis. Phenotypic parameters were retrieved for each single-cell identified in 12 Z-planes and only cardiomyocytes were included in the analysis. ( A ) Illustration of cardiomyocyte organoid analysis ( B ) Total number of cells per z plane. N = 6-8. ( C - J '') Control and fCM-treated organoids representative z-stack immunofluorescence images from summed intensities. Cell nuclei (DAPI), cardiomyocytes (cardiac Troponin T positive cells, Red), connexin 40 and NAV1.5 (Green). Scale bars = 100 um. ( K - N '') Single z plane view of representative outer surface regions of control and fCM-treated organoids. ( O - P ) Mean expression of connexin 40 and NAV1.5 per cardiomyocyte given by the average of fluorescence intensity in the cardiomyocyte population. The mean fluorescence intensity was measured in three different z-positions for the organoid outer (z4-z6) and inner surfaces (z8-z10). Results are disposed as a mean +- SEM. Data were subjected to a two-way ANOVA followed by a post test comparing each group to every other group; p < 0.05 was considered significant. N = 9-12.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
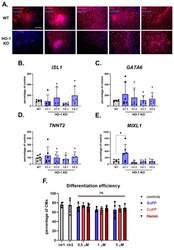
- Experimental details
- Figure 3 HO-1 does not influence the efficiency of hiPSCs.2 differentiation to cardiomyocytes. ( A ) Immunofluorescence analysis of markers of three germ-layers (Vimentin, alpha-SMA, GATA4, AFP, and NFH) in spontaneously differentiated WT (upper panel) and HO-1 KO (bottom panel) hiPSC.3 via embryoid bodies. Bar indicates 100 um. qRT-PCR analysis of expression of cardiac mesoderm markers: ( B ) ISL1 , ( C ) GATA6 , ( D ) TNNT2 and ( E ) MIXL1. Expression was normalized to EEF-2 levels. ( F ) Flow cytometric analysis of direct cardiomyocyte differentiation efficiency (based on TNNT2 expression) of WT hiPSC.2 treated with tin protoporphyrin IX (SnPP), cobalt protoporphyrin IX (CoPP) and hemin. Ctr1-DMSO control for SnPP and CoPP, ctr2-2 uM NaOH control for hemin. Bars represent mean +- SD of N = 3 experiments. Dots, squares and triangles represent each replicate for corresponding groups. * p < 0.05, one-way ANOVA test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
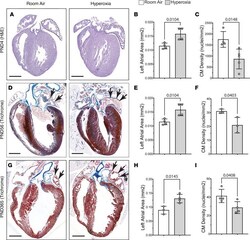
- Experimental details
- Figure 1 Neonatal hyperoxia enlarges the left atria of mice but reduces the density of cardiomyocyte (CM) nuclei in the left atrial myocardia. ( A ) H&E-stained sections of hearts from P4 mice that were exposed to room air (left) or hyperoxia (right). Dotted lines outline the left atria. ( B ) Mean area of the left atria in sections of room air- and hyperoxia-exposed mice on P4. n = 4 mice per condition. ( C ) Numbers of DAPI-stained nuclei per mm 2 of TNNT2 + myocardium. Room air, n = 4; hyperoxia, n = 5 mice per condition. ( D and G ) Sectioned hearts of P56 ( D ) and P365 ( G ) mice exposed to room air (right) or hyperoxia (right) from P0-P4 stained with Masson's trichrome. ( E and H ) Graphs show mean area of the left atria in sections of room air- and hyperoxia-exposed mice on P56 ( E ) and P365 ( F ). ( E ) n = 4 per condition. ( H ) Room air, n = 3; hyperoxia, n = 4. ( F and I ) Graphs show numbers of DAPI-stained nuclei per mm 2 of TNNT2 + myocardium in room air- and hyperoxia-exposed mice on P56 ( F ) and P365 ( I ). ( F ) n = 3 mice per condition. ( I ) n = 4 mice per condition. Bars in graphs show mean values, circles show individual data points, and error bars show SDs. The P values between data sets are from unpaired 2-tailed t tests. Scale bars: 400 um ( A ) or 100 uM ( D and G ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
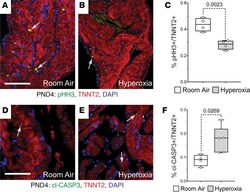
- Experimental details
- Figure 2 Hyperoxia reduces postnatal proliferation and survival of left atrial cardiomyocytes in neonatal mice. ( A and B ) Sections of left atrial appendages from P4 control ( A ) and hyperoxia-exposed ( B ) mice stained with phosphorylated Histone H3 (pHH3, green), cardiac troponin T (TNNT2, red), and DAPI (blue). Arrows point to pHH3 + cardiomyocytes. ( C ) Percentage of pHH3 + cardiomyocytes in P4 mice exposed to room air or hyperoxia. n = 4 mice per condition. ( D and E ) Sectioned left atrial appendages of P4 control ( D ) and hyperoxia-exposed ( E ) mice stained for the active, cleaved form of Caspase 3 (cl-Casp3), TNNT2, and DAPI. Arrows point to cl-Casp3 + cardiomyocytes. ( F ) Percentage of cl-Casp3-labeled CMs in the left atria of P4 hyperoxia-exposed and control mice. Room air, n = 4; hyperoxia, n = 5 mice per condition. Box plots show median, second quartiles, and third quartiles; whiskers indicate range, and circles represent individual data points. P values are results of unpaired 2-tailed t tests. Scale bars: 200 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
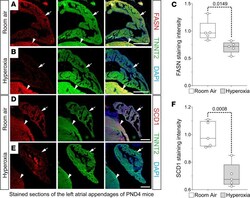
- Experimental details
- Figure 5 Neonatal hyperoxia represses fatty acid synthesis enzymes in murine atrial cardiomyocytes. ( A , B , D , and E ). Sections of left atrial appendages from P4 neonates exposed to room air ( A and B ) or hyperoxia ( D and E ) costained for FASN (red, A and B ) or SCD1 (red, D and E ) and TNNT2 (green, A , B , D , and E ). Sections were also stained with DAPI to label nuclei (blue, A , B , D , and E ). Arrows and arrowheads show TNNT2 + cardiomyocytes in left atrial appendage and LV, respectively. Scale bars: 200 mum. ( C and F ) Graphs show relative staining intensities for Fasn ( C ) and Scd1 ( F ) measured using NIH ImageJ 2.0/Fiji. ( C and F ) Room air, n = 5; hyperoxia, n = 5. Box plots show median values and inner quartiles. Whiskers show the range of values. Circles show values for individual room air- and hyperoxia-exposed mice, respectively. F tests were used to determine if samples had equal or unequal variances. P values are the results of unpaired 2-tailed t tests.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
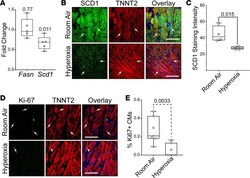
- Experimental details
- Figure 8 Hyperoxia suppresses fatty acid synthesis genes in left atrial tissue explanted from human infants. ( A ) Results of qPCR for Fasn and Scd1 in left atrial tissue explanted from human infants who died at birth due to anencephaly and exposed to room air or hyperoxia for 24 hours. n = 5 donors. ( B ) Sectioned explants exposed to room air (top) or hyperoxia (bottom) were stained for SCD1 (green), TNNT2 (red), and DAPI (blue). ( C ) Graph shows staining intensities for SCD1 in sections of control and hyperoxia-exposed mice determined using NIH ImageJ 2.0/Fiji. n = 4 donors. ( D ) Sections of explants exposed to room air (top) and hyperoxia (bottom) stained for the proliferation marker Ki67 (green), TNNT2 (red), and DAPI (blue). ( E ) Graph shows percentages of TNNT2-expressing cells with Ki67 + nuclei in explants exposed to room air or hyperoxia. n = 7 donors. ( A , C , and E ) Circles indicate individual values for explants of each donor. Boxes show medians and inner quartiles; whiskers represent the range. P values are the results of either single-sample 1-tailed t test and Wilcox test ( A ) or unpaired 2-tailed t tests ( C and E ). ( B and D ) Scale bars = 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure EV2 Cardiomyocyte quantification and characterization by FACS and qRT-PCR FACS staining for cTNNT2 in D10 and D15 human ES cell-derived cardiac cells. IgG 1 isotype was used as a control. POU5F1 mRNA expression levels in human ES cells and D15 as well as D30 cardiac cells, as measured by qRT-PCR. Expression levels were normalized to a non-oscillatory housekeeping gene ( PPIA ). Data are represented as mean +- s.e.m. of three independent replicates.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
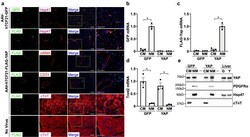
- Experimental details
- Figure 2 Characterization of AAV-hTCF21-GFP and AAV-hTCF21-FLAG-YAP expression in mouse hearts. Wild type C57BL/6J mice were administered AAV-hTCF21-GFP, AAV-hTCF21-FLAG-YAP, or no virus control. Hearts were excised and processed for immunostaining or cell isolation and fractionation. ( a ) Heart sections were stained to detect GFP or FLAG (green), and counterstained to visualize Hsp47, alphaSMA, CD31, or cardiac troponin T (red) and nuclei (DAPI). Right column panels are higher magnification merged images. Scale bar, 100 mum. ( b-e ) Heart cells were isolated from mice administered AAV-hTCF21-GFP or AAV-hTCF21-FLAG-YAP and separated into cardiomyocyte-enriched (CM) and non-myocyte-enriched (NM) fractions. RNA and protein were isolated for qPCR analysis and immunoblotting, respectively. ( b-d ) Expression of GFP, FLAG-Yap, and cardiac troponin T (Tnnt2) mRNA was determined by qPCR. ( e ) Protein fractions (20 mug per sample) were analyzed by western blot to detect YAP, PDGFRalpha, Hsp47, and cTnT protein levels. Whole liver homogenates from AAV-hTCF21-GFP and AAV-hTCF21-FLAG-YAP transduced mice (G and Y, respectively) were also analyzed (5 mug per sample). N = 3 mice/group. Representative blots are shown. *p < 0.05. Full-length blots are presented in Supplementary Fig. S3 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
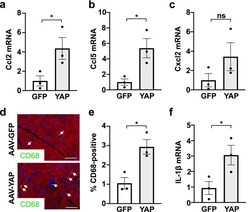
- Experimental details
- Figure 6 Transduction with AAV-hTCF21-FLAG-YAP upregulates inflammatory markers in the heart. Wild type C57BL/6J mice were administered AAV-hTCF21-GFP or AAV-hTCF21-FLAG-YAP. ( a-c ) RNA was isolated from LV tissue for qPCR analysis. ( d , e ) Hearts were sectioned for immunostaining to detect CD68 + macrophages (green), cardiac troponin T (red), and nuclei (DAPI). White arrows identify CD68-positive cells. Quantification of CD68-positive cells in panel ( e ). Scale bar, 100 mum. ( f ) IL-1b mRNA was measured in LV tissue by qPCR. N = 3 mice/group. *p < 0.05. ns not significant.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
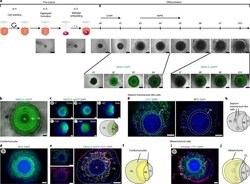
- Experimental details
- Fig. 1 HFOs recapitulate patterns of early cardiomyogenesis. a , The protocol for HFO formation. hPSC aggregates were individually embedded in Matrigel and differentiated using CHIR and IWP2. The figure shows the development of HFOs from d-4 until d10 of differentiation (upper row) as well as MIXL1-GFP and NKX2.5-eGFP expression patterns (bottom row). b , A typical HES3 NKX2.5-eGFP-derived HFO forming three layers: IC, ML and OL. c , An HFO rotated around its virtual axis and respective schemes. d , Whole-mount IF staining for the myocardial marker MHC. e , A paraffin section stained for cTnT and an anti-GFP antibody. f , A scheme of an HFO with highlighted cardiomyocytes. g , Sequential paraffin sections stained for cTnT and the ST marker WT1. h , A scheme of an HFO with highlighted ST-like cells. i , Whole-mount IF staining for the mesenchymal cell marker vimentin. j , A scheme of an HFO with highlighted mesenchymal cells. Scale bars, 500 um ( a ) and 200 um ( b - e , g , i ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
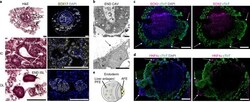
- Experimental details
- Fig. 3 Differential expression of SOX17, SOX2 and HNF4alpha reveals formation of distinct foregut endoderm tissues. a , H&E staining (left column) and staining for the endoderm marker SOX17 (right column) with enlargements showing endodermal cavities (END CAV) in the IC and endodermal islands (END ISL) in the OL. b , TEM images of endodermal cavities in the IC with microvilli (arrows). c , d , Cryosections of HFOs stained for cTnT and the AFE marker SOX2 ( c ) or the hepatocyte marker HNF4alpha ( d ). The arrows point at endodermal islands in the OL. e , A scheme of an HFO with highlighted distinct endoderm tissues. Scale bars, 100 um ( a ), 5 um ( b ) and 200 um ( c , d ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
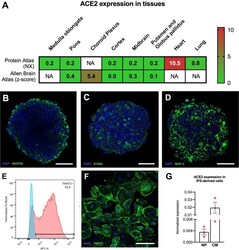
- Experimental details
- Fig. 1 ACE2 expression in adult human tissues and iPS-derived cells. (A) ACE2 expression in adult human tissues based on Protein Atlas and Allen Brain Atlas databases. Consensus normalized expression (NX) and z-score are used to represent the relative abundances within the respective databases (Citations in the main text). NA = not analyzed in the database. Characterization of NP and CM. NP were cultivated for 7 days and CM were differentiated for 35 days and characterized by IF (B-D and F) and flow cytometry (E). NP were immunostained for (B) Nestin (neural progenitors), (C) S100b, (astrocytes); and (D) MAP-2 (neurons). (E) Representative flow cytometry graph showing a quantification of 83.2% TNNT2 + cells from one of the cell batches used in this study; (F) CM immunostained for cardiac troponin T (TNNT2). Scale bars: (B-D) 40 um and (F) 250 um. (G) Relative mRNA expression levels of ACE2 in iPSC-derived cultures; NP cultivated for 7 days and CM differentiated for 35 days. (n = 3 cell culture batches for both), normalized to reference genes, GAPDH and HPRT-1. The data correspond to 3 independent cell lines for NP.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
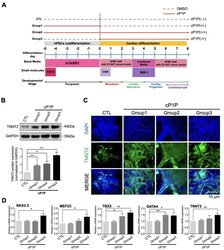
- Experimental details
- Figure 1 cP1P enhances cardiac differentiation in human embryonic stem cells. ( A ) Illustration of the chemically defined cardiac differentiation protocol utilized in this study. cP1P was administered during different stages of cardiac differentiation. Group 1 represents treatment with cP1P from days -5 to 0; Group 2, from days 5 to 8; and Group 3, from days 0 to 8 during differentiation. ( B ) Immunoblot analysis of the cardiomyocyte marker TNNT2 in Groups 1, 2, and 3 were analyzed at day 8 of cardiac differentiation. Graph represents the relative protein expression of TNNT2 as normalized to GAPDH ( n = 3, ** p < 0.01, *** p < 0.001). ( C ) Immunofluorescence analysis of the cardiomyocyte marker TNNT2 in Groups 1, 2, and 3 were analyzed at day 8 of cardiac differentiation, Scale bar = 10 mum. ( D ) Gene expression analysis of cP1P-treated cardiomyocytes by qRT-PCR at day 7 of cardiac differentiation showed higher expression levels of NKX2.5 , MEF2C , TBX5 , GATA4 , and TNNT2 in Group 3 when compared to their respective controls. Data are presented as the means +- SDs of three independent experiments ( n = 3, * p < 0.05, ** p < 0.01).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
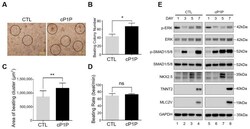
- Experimental details
- Figure 2 Bioactive lipid cP1P augments beating colony number and contracting area by activating SMAD signaling in human embryonic stem cells (hESC)-derived cardiomyocytes. ( A ) Representative image of the number of beating cardiomyocytes in the control (DMSO-treated) and cP1P-treated groups at day 6. Scale bar = 500 mum. ( B ) Cell number measurements of beating cardiomyocytes. hESCs were seeded into 12-well plates (7.5 x 10 4 cells/well), treated with DMSO (control) or cP1P as indicated, and induced to differentiate for 8 days. Data are presented as the means +- SDs of three independent experiments ( n = 3, * p < 0.05). ( C ) Areas of contraction of the beating clusters obtained from the control or cP1P groups at day 8 were calculated by ImageJ software using the captured images. Contracting areas were individually marked using ImageJ tools and a calibration of 0.46 pixels/mum was used to measure the area. Data are presented as the means +- SDs ( n = 11, ** p < 0.01). ( D ) Beating rates for the control and cP1P groups were measured as beats/min. Data are presented as the means +- SDs ( n = 3, ns = non-significant). ( E ) The expression levels of p-ERK, p-SMAD 1/5/8, total ERK, and SMAD 1/5/8 were analyzed along with the expression levels of cardiogenic transcriptional factors NKX2.5, TNNT2, and MLC2V by Western blot during CM differentiation in DMSO-treated control and cP1P-treated CMs at the indicated time points.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
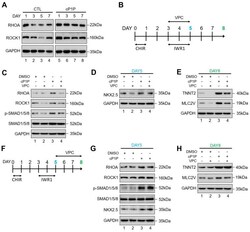
- Experimental details
- Figure 3 cP1P promotes cardiac differentiation in hESCs by partially regulating S1PR-mediated SMAD1/5/8 signaling. ( A ) The activity of RHOA/ROCK1/SMAD signaling with or without cP1P was tested by evaluating the expression of RHOA and ROCK1 by Western blot at the indicated time points along with GAPDH expression as a reference. ( B ) Schematic representation of the treatment with S1PR inhibitor VPC during days 3 to 5 of CM differentiation coinciding with IWR1 inhibitor treatment. ( C ) The activity of RHOA/ROCK1/SMAD signaling was inhibited by the administration of VPC, as shown by the downregulation of RHOA, ROCK1, and p-SMAD 1/5/8, but cP1P treatment could partly rescue the inhibitory effect of VPC, as shown by the rescue of p-SMAD 1/5/8, demonstrated by Western blot. The effects of S1PR inhibition by VPC on cardiomyocyte differentiation, shown by ( D ) the downregulation of NKX2.5 at day 5. ( E ) Effect of VPC treatment between days 3 to 5 on the expression of TNNT2 and MLC2V at day 8 of CM differentiation showed that cP1P treatment could partially rescue the inhibitory effects of VPC treatment, as demonstrated by Western blot. ( F ) Schematic representation of the treatment with VPC during days 5 to 8 of CM differentiation. ( G ) Immunoblots of RHOA, ROCK1, p-SMAD1/5/8, SMAD1/5/8, and NKX2.5 at day 5 of VPC treatment in DMSO-treated controls and cP1P-treated CMs. ( H ) Immunoblots of TNNT2 and MLC2V at day 8 of VPC treatment in DMSO-treated controls and cP1P-treated CMs.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
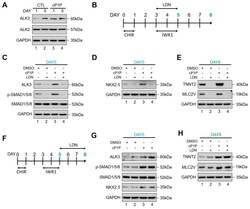
- Experimental details
- Figure 4 cP1P-augmented cardiac differentiation in hESCs is dependent on BMP receptor-mediated SMAD1/5/8 signaling. ( A ) The activity of BMPR/SMAD signaling with or without cP1P treatment was evaluated by the expression levels of ALK2 and ALK3 by Western blot at the indicated time points along with GAPDH expression as the loading control. ( B ) Schematic representation of the treatment with BMPR inhibitor LDN during days 3 to 5 of CM differentiation coinciding with IWR1 inhibitor treatment. ( C ) The activity of BMPR/SMAD signaling was inhibited by the administration of LDN, as shown by the downregulation of ALK3 and p-SMAD 1/5/8, while cP1P treatment could not reverse the inhibitory effect of LDN. The effects of BMPR inhibition by LDN on cardiomyocyte differentiation, shown by ( D ) the downregulation of NKX2.5 at day 5. ( E ) Treatment of LDN between day 3 to 5 also downregulated the expression of TNNT2 and MLC2V at day 8 of CM differentiation even upon cP1P treatment, as demonstrated by Western blot. ( F ) Schematic representation of the treatment with LDN during days 5 to 8 of CM differentiation. ( G ) Immunoblots of ALK3, p-SMAD1/5/8, SMAD1/5/8, and NKX2.5 at day 5 of LDN treatment in DMSO-treated controls and cP1P-treated CMs. ( H ) Immunoblots of TNNT2 and MLC2V at day 8 of LDN treatment in DMSO-treated controls and cP1P-treated CMs.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
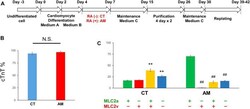
- Experimental details
- FIGURE 1 Cardiac differentiation from hiPSCs (A) Schematic of the protocol for the differentiation of cardiomyocytes from hiPS cells with (RA (+)) or without RA (RA (-)) treatment (B) The ratio of cardiomyocytes quantified by immunostaining with cardiac troponin-T (cTnT). The proportion of cTnT positive cells was 93.91 +- 2.51% in CT (control hiPS-CMs), or 96.79 +- 1.81% in AM (RA-treated hiPS-CMs), respectively (P = N.S.). Error bars represent SD of the mean from the values of independent experiments. n = 3. N.S., not significant (C) Immunofluorescence analysis of the percentage of MLC2a (atrial isoform) and MLC2v (ventricular isoform) positive expression in the CT and AM. Error bars represent SD of the mean from the values of independent experiments. ** p < 0.01 for comparison with MLC 2a+/MLC 2v-cardiomyocyte in CT, ## p < 0.01 for comparison with MLC 2a+/MLC 2v-cardiomyocyte in AM, n = 3.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
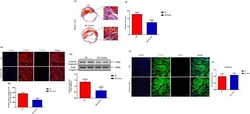
- Experimental details
- Fig. 3 Gastrin decreases cardiomyocyte apoptosis and fibrosis after myocardial infarction. a Representative images ( a1 ) and quantification ( a2 ) of the fibrotic area in infarct border zone by Masson trichrome staining at 28 days post-MI. b Representative images ( b1 ) and quantification ( b2 ) of terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive (TUNEL + ) cardiomyocytes in infarct border zone at 2 days after MI. TUNEL + nuclei are stained green, cardiac troponin T antibody (cTnT) is red, and 4'-6-diamidino-2-phenylindole (DAPI) is blue. b3 Western blot was used to measure the protein levels of Caspase 3 in the infarct border zone at 2 days after MI. Results are expressed as the ratio of cleaved caspased 3 to total caspase 3. c , Representative images ( c1 ) and quantification ( c2 ) of cardiomyocyte proliferation 28 days after myocardial infarction, through Ki67 immunostaining. Cardiac troponin T antibody (cTnT) is green, Ki67 is red, and 4'-6-diamidino-2-phenylindole (DAPI) is blue. a and c Scale bar = 50 um. b Scale bar = 20 um. n = 8. * P < 0.05, vs. MI mice. P = NS means no significant difference
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
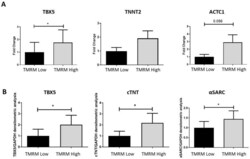
- Experimental details
- Figure 7 In vitro cardiac differentiation of TMRM-low and high cells. Expression of early cardiac gene/protein: T-Box transcription factor 5 ( TBX5 ) and late cardiac genes cTNT ( TNNT2 ) and alpha-sarcomeric actin ( ACTC1 ) in TMRM-low and high cells after 1 week of culture in cardiomyogenic differentiation media. The expression of these genes was analyzed by qRT-PCR ( n = 5) ( A ), and Western blot ( n = 4) ( B ) Data are represented as mean +- SD of the fold change. Statistical differences were calculated significant as * p < 0.05, determined by paired Student's t -test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
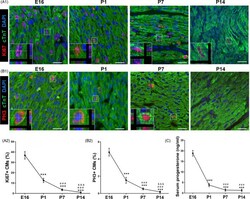
- Experimental details
- Figure 1 Serum progesterone concentration declines after birth and is associated with decrease in CM proliferation. A,B, Evaluation of CM proliferation by Ki67 and PH3 immunostaining at E16, P1, P7 and P14 hearts. Representative images with z-stacking are shown in A1 and B1, and quantification of percentages of Ki67 + and PH3 + CMs is shown in A2 and B2, respectively (n = 5). Scale bars are 20 um. cTnT indicates cardiac troponin T. C, Serum concentrations of progesterone in mice at E16, P1, P7 and P14 detected using ELISA kit (n = 5-8). *** P < .001 vs E16; ### P < .001 vs P1; &&& P < .001 vs P7. CM, cardiomyocyte
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
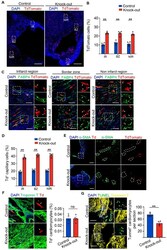
- Experimental details
- Figure 7 Knock-out of Setd4 inhibits cardiomyocyte apoptosis in MI-injured mice. ( A , B ) Representative immunofluorescence ( A ) and quantification ( B ) for recombinant cells. Scale bars = 500 mum. In the three areas of the MI-injured left ventricle after four weeks of Setd4 knock-out. Scale bars = 500 mum. ( C , D ) Immunostaining ( C ) and quantification ( D ) for recombinant cells of capillary (Td + FABP4 + ) in the three areas of the MI-injured left ventricle. Scale bars = 50 mum. ( E , F ) Immunostaining for alpha-SMA (Scale bars = 200 mum) ( E ) and Troponin T (Scale bars = 50 mum) ( F ) after four weeks of Setd4 knock-out. ( G ) Representative immunofluorescence and quantification for apoptotic cardiomyocytes by performing TUNEL assay after two weeks of Setd4 knock-out. Scale bars = 50 mum. Nuclei were stained with DAPI. All data are represented as mean +- SEM. n = 4 mice. Unpaired t test for ( F , G ), two-way ANOVA with Bonferroi's correction for multiple comparisons test for ( B , D ). ** p < 0.01. ns not significant, IR infarction region, BZ border zone, NIR non-infarction region.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
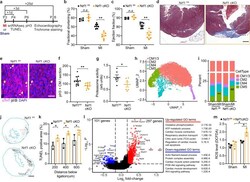
- Experimental details
- Fig. 2 Nrf1 deletion impairs neonatal heart regeneration. a Illustration showing timeline of MI and Sham surgeries performed at P3 and subsequent days of sample collection. b , c Fractional shortening ( b ) and ejection fraction ( c ) of Nrf1 fl/fl and Nrf1 cKO mouse hearts at 25 days after MI or Sham surgery; n = 4 animals for Sham groups and n = 7-8 animals for MI groups; ** p = 0.0022 ( b ), ** p = 0.0009 ( c ) by Student's t -test two-tailed. d Masson's trichrome staining showing fibrotic scarring in Nrf1 fl/fl and Nrf1 cKO mouse hearts at 25-day after MI; similar results were observed in six animals; Scale bar, 200 mum. e Immunohistochemistry showing pH3 + cardiomyocytes (cTnT + ), marked by arrows, in heart sections collected from Nrf1 cKO and Nrf1 fl/fl mice 3-day post-MI; Scale bar, 50 mum. f Quantification of pH3 + cardiomyocytes (cTnT + ) in heart sections from ( e ); n = 12 animals for WT and n = 11 animals for Nrf1 cKO, ** p = 0.0034 by Student's t -test two-tailed. g Chymotrypsin-like activity of the proteasome in hearts from Nrf1 cKO and Nrf1 fl/fl mice at P4. arb. units, arbitrary units; n = 6 animals for each group; * p = 0.0373 by Student's t -test one-tailed. h UMAP visualization of cardiomyocyte clusters colored by identity. CM1 and CM3 were merged as a single cluster due to their transcriptome similarity. i Fraction of cardiomyocyte populations in Nrf1 fl/fl and Nrf1 cKO hearts in MI or Sham conditions; n = 4-6 animals for each group examined over 1 indepe
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
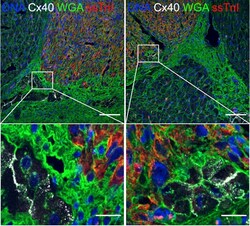
- Experimental details
- Figure 6 Transplanted hESC-CM grafts interact with a diffuse Purkinje conduction system in the porcine myocardium An hPSC-cardiomyocyte graft 2 weeks post-transplantation, marked by human-specific slow skeletal cardiac troponin I (ssTnI) (red) (), interacts with Cx40-positive (white) Purkinje fibers. Wheat germ agglutinin (WGA) (green) delineates cardiac sarcolemma. Purkinje-transitional cell-graft (left panel) and direct Purkinje-graft (right panel) interactions are observed. Sale bars, 100 or 20 mum (magnified).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
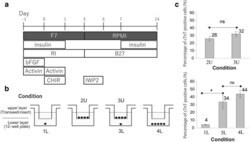
- Experimental details
- Figure 1 Co-culture of cells plated at an initial high cell density enhanced the cardiomyocyte differentiation efficiency of cells plated at an initial low cell density. ( a ) Schematic of the previously established protocol of cardiomyocyte differentiation . ( b ) Schematic of co-culture experiments. The cells were plated as follows. Cells were plated at a low cell density of 5 x 10 3 cells/cm 2 and high cell density of 2 x 10 5 cells/cm 2 . Condition 1: Only low cell density cells in the lower layer (L indicates lower). Condition 2: Only high-density cells in the upper layer (U indicates upper). Conditions 1 and 2 were used as controls. Condition 3: Co-culture of high-density cells in the upper layer and low cell density cells in the lower layer to assess cell-secreted signals. Condition 4: The same total number of cells at an initial low and high density, as specified in condition 3, was plated on the same surface to assess both cell-secreted signals and cell-cell contact signals. ( c ) The percentage of cTnT-positive cells measured by flow cytometry on day 14. Mean +- standard error (SE), * P < 0.05, t-tests with Holm's correction, ns: non-significance, n = 3.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 3 Higher concentrations of Wnt inhibitor did not increase cardiac gene expression at an initial low cell density. ( a ) Expression of anti-cardiac and cardiac progenitor genes on day 5, treated with 5 and 10 muM IWP2 during days 3-5. ( b ) Flow cytometry for cTnT on day 14, in cells treated with 5 and 10 muM IWP2 during day 3-5.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
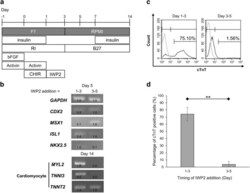
- Experimental details
- Figure 4 Early addition of Wnt inhibitor during days 1-3 was sufficient to improve cardiomyocyte differentiation efficiency at a low initial cell density. ( a ) Schematic of the new protocol of cardiomyocyte differentiation at an initial low cell density, with early addition of IWP2 from day 1-3. ( b ) Expression of anti-cardiac mesoderm genes CDX2 and MSX1 and cardiac progenitor genes ISL1 and NKX2.5 on day 5. The expression levels of cardiomyocyte genes ( MYL2 , TNNI3 , and TNNT2 ) on day 14. IWP2 was added during days 1-3 or days 3-5. ( c ) Flow cytometry of cTnT-positive cells induced by IWP2 treatment during days 1-3 and days 3-5. ( d ) The percentage of cTnT-positive cells under the same conditions as in ( c ). Mean +- SE, ** P < 0.01, t-tests with Dunnett's correction, n = 4.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
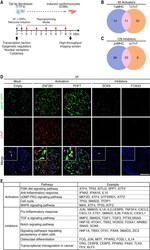
- Experimental details
- Figure 1. Identification of activators and inhibitors of 5F-mediated cardiac reprogramming from a human ORF cDNA screen. ( A ) Schematic diagram of the human ORF cDNA library screen strategy for cardiac reprogramming in adult TTFs. ( B ) Venn diagram showing the number of activators identified from the screen. Genes with Z -scores of alphaMHC-GFP or cTnT expression >=2 were defined as activators. Twenty-five genes induced alphaMHC-GFP expression only, 35 genes induced cTnT expression only, and 11 genes induced expression of both markers. ( C ) Venn diagram showing the number of inhibitors identified from the screen. Genes with Z -scores of alphaMHC-GFP or cTnT expression -2 or lower were defined as inhibitors. One-hundred-twenty-one genes repressed alphaMHC expression only, 41 genes repressed cTnT expression only, and 33 genes repressed both alphaMHC-GFP and cTnT expression. ( D ) Representative immunocytochemistry images of TTFs from adult alphaMHC-GFP transgenic mice treated with 5F and either empty virus or viruses encoding activators (ZNF281 or PHF7) and inhibitors (FOXA3 or SOX9). Cells were fixed and stained for alphaMHC-GFP (green), cTnT (red), and Hoechst (blue) 9 d after infection. Bars, 2 mm. ( E ) Pathways enriched in activators ( n = 49) and inhibitors ( n = 129), respectively, by DAVID pathway analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
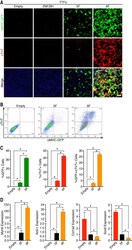
- Experimental details
- Figure 2. ZNF281 enhances cardiac reprogramming of adult fibroblasts. ( A ) Immunocytochemistry images of adult alphaMHC-GFP transgenic TTFs 7 d after infection with empty, ZNF281, 5F, or 6F retroviruses show that ZNF281 enhances expression of cardiac markers with 5F. (Green) alphaMHC-GFP; (red) cTnT; (blue) Hoechst. Bars, 500 um. ( B , C ) Representative flow cytometry plot ( B ) and analyses ( C ) of alphaMHC-GFP + and cTnT + TTFs 7 d after infection with empty, 5F, or 6F. ( D ) TTFs were infected with empty, 5F plus empty, and ZNF281 for 7 d. Transcript levels of cardiac marker genes ( Myh6 and Actc1 ) and fibroblast marker genes ( Col1a2 and Sox9 ) were determined by qPCR. (*) P < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
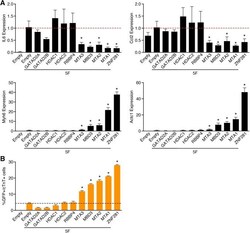
- Experimental details
- Figure 6. ZNF281 represses the inflammatory response through the NuRD complex. ( A ) TTFs were infected with empty, 5F plus empty, ZNF281, or each individual NuRD complex subunit retrovirus for 7 d. Transcript levels of inflammatory ( IL6 and Ccl2 ) and cardiac ( Myh6 and Actc1 ) marker genes were determined by qPCR. ( B ) Representative flow cytometry plot of alphaMHC-GFP + and cTnT + TTFs 7 d after infection with empty, 5F plus empty, ZNF281, or each individual NuRD complex subunit retroviruses. The dashed line indicates control. (*) P < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
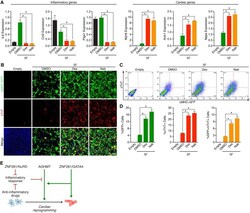
- Experimental details
- Figure 7. Anti-inflammatory drugs promote cardiac reprogramming. ( A ) 5F reprogrammed TTFs treated with either DMSO or the indicated anti-inflammatory drugs for 7 d after infection. Expression of inflammatory genes ( IL6 , Ccl2 , and Ptgs1 ), and cardiac genes ( Myh6 , Actc1 , and Nppa ) was determined by qPCR. (Dex) 10 uM Dex; (Nab) 10 uM Nab. ( B ) Immunocytochemistry images of 5F reprogrammed adult alphaMHC-GFP transgenic TTFs treated with DMSO or the indicated anti-inflammatory drugs for 7 d. (Green) alphaMHC-GFP; (red) cTnT; (blue) Hoechst. Bars, 500 um. ( C , D ) Representative flow cytometry plot ( C ) and analyses ( D ) of alphaMHC-GFP + and cTnT + cells in 5F reprogrammed adult alphaMHC-GFP transgenic TTFs treated with DMSO or the indicated anti-inflammatory drugs for 7 d. (*) P < 0.05. ( E ) Model showing the mechanism of action of ZNF281 on 5F-mediated direct cardiac reprogramming. ZNF281 is a cardiac transcription coactivator recruited by GATA4 to cardiac enhancers to activate cardiac gene expression. ZNF281 also represses the inflammatory response, which acts as a barrier pathway to cardiac reprogramming.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 1 ( A ) Schematic representation of direct cardiac reprogramming protocol. MEFs or CFs were plated on Matrigel pre-coated plates in fibroblast growth medium containing 1 muM PTC-209 (PTC) or DMSO (NT) one day before the addition of Cardiac Reprogramming Medium (CRM) containing the small molecule cocktails. At day 16 for MEFs or 20 for CFs, the medium was changed into Cardiomyocyte Maintaining Medium (CMM) for at list 10 days more. Samples were analysed at final stage of reprogramming (day 24 or 28, for MEFs and CFs respectively). ( B,C ) Representative 2D scatter plots showing end-stage reprogrammed cell population upon 24 h PTC-209 pre-treatment (PTC) or DMSO (NT). Green dots represent alpha-MHC + cells. Typical results obtained starting from MEFs are in ( B ); those starting from CFs are in ( C ). ( D,E ) Representative plots showing fluorescent cells vs total cell counts, with the percentage of alpha-MHC + cells highlighted in green, at final stage of reprogramming. The increasing in alpha-MHC + cells upon PTC-209 pre-treatment is shown in ( D ) for MEFs and in ( E ) for CFs. ( F,G ) Box plots showing the percentage of alpha-MHC + cells, normalized to cell count, at final stage of reprogramming from MEFs ( F ) or CFs ( G ). For each data set: n = 4; Delta = mean of % of alpha-MHC + cell increase upon 24 h PTC-209 pre-treatment. *p < 0.05, **p < 0.01. ( H ) Representative immunostaining of cardiac marker alpha-MHC (green). ( I ) Representative immunostaining of cardi
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 7. Heterogeneity and Distinct Injury Response of Immune Cells (A) UMAP representation of different myeloid cell populations analyzed. (B) Heatmap showing expression of top enriched genes for each myeloid cell sub-population. (C-G) UMAP plots showing expression of Cd86 , an M1-macrophage marker gene (C); Arg1 , an M2-macrophage marker gene (D); Plac8 , a monocyte-enriched gene (E); Ly6c2 , an M1 monocyte-enriched gene (F); and Naaa , a DC-like cell enriched gene (G). (H) Percentage of each immune cell population over total nonmyocytes within each sample. (I) UMAP plot showing expression of Clcf1 among different cell types present in the P1+1 dpi and dps scRNA-seq data. (J) Representative images showing EdU incorporation (magenta) and cardiac troponin T (cTnT) immunofluorescent staining (green) of NRVM cells treated with 200 ng/mL BSA (negative control), 20 ng/mL recombinant insulin-like growth factor 2 (IGF2) (positive control), or different concentrations of recombinant CLCF1 (5, 10, 25, and 50 ng/mL). Scale bar, 100 mum. (K) Quantification showing the proportion of EdU-positive cells among cTnT-positive cells (CM) after treatment of BSA, CLCF1 recombinant protein, or recombinant IGF2 (n = 4 per each group; **p < 0.01, ****p < 0.0001). See also Figure S7 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- FIGURE 3 Cardiomyocyte-like cells induced by GMTMS from MICFs express multiple structural proteins. (A) Immunostaining for cTnT, cTnI, and alpha-Actinin at day 21 after GMTMS transduction in MICFs derived from Myh6 -mCherry mice. mCherry + cells expressed cardiac proteins with well-organized sarcomeric structures. (B) Representative images of GMTMS-induced iCMs co-expressing cTnT and cTnI. (C) Immunofluorescence for cTnT (red) and cTnI (green) was performed 3 weeks after transduction of MICFs (from C57 mice, 8 weeks old) with GMT, GMTM, GMTS, GMTMS, and GMTMM. (D) Quantification of cTnT and cTnI double positive cells ( n = 3). All data are presented as mean +- SD. ** p < 0.01 vs. relevant control. Scale bars: 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- FIGURE 6 GMTMS significantly increase the iCMs reprogramming efficiency from myofibroblasts traced by Postn . (A,B) Immunofluorescence for cTnI and cTnT was performed 3 weeks after transduction of indicated cocktails with MICFs (from Postn -MCM +/- ; Rosa26 -lsl-tdTomato +/- mice, 8 weeks old). (C) Quantification of cTnI- or cTnT- positive iCMs induced from Postn -tdTomato + myofibroblast by GMTMS and GMT ( n = 5). (D) Quantification of cTnI- or cTnT- positive iCMs induced by GMTMS from Postn -tdTomato + myofibroblast or Postn -tdTomato - MICFs ( n = 5). All data are presented as mean +- SD. ** p < 0.01 vs. relevant control and ns means no significant difference. Scale bars: 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
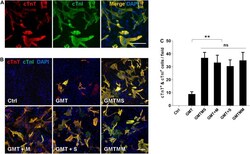
- Experimental details
- FIGURE 7 Myocd or Sall4 significantly enhance iCMs reprogramming efficiency from adult cardiac fibroblasts. (A) Representative images of GMTMS-induced iCMs co-expressing cTnT and cTnI. (B) Immunofluorescence for cTnT (red) and cTnI (green) was performed 3 weeks after transduction of ACFs (from C57 mice without injury, 8 weeks old) with GMT, GMTM, GMTS, GMTMS, and GMTMM. (C) Quantification of cTnT and cTnI double positive cells ( n = 3). Note that four factors (GMTM or GMTS) are enough to reprogram abundant iCMs co-expressing cTnT and cTnI. ** p < 0.01 vs. relevant control and ns means no significant difference. Scale bars: 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
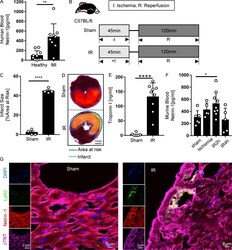
- Experimental details
- Figure 1. Blood netrin-1 increases in MI patients and mice with myocardial IR surgery. (A) Blood netrin-1 levels of healthy donors and MI patients ( n = 10 for healthy control, n = 9 for MI; two-tailed Welch's t test). (B) Experimental strategy for the murine myocardial IR model. (C) Infarct sizes of IR group compared with the sham group ( n = 4 per group; two-tailed unpaired t test). (D) Representative infarct staining results for the IR model. The infarct area is outlined by a green line; the blue line marks the AAR (scale bar = 1 mm). (E) cTnI levels after surgery ( n = 6 for sham group, n = 9 for IR group; two-tailed Welch's t test). (F) Murine blood netrin-1 levels were measured in sham, ischemia (45 min), IR2h (45 min of ischemia + 2 h of reperfusion), and IR4h (45 min of ischemia + 4 h of reperfusion) groups ( n = 6 for sham group, n = 5 for ischemia group, n = 9 for IR2h group, and n = 5 for IR4h group; one-way ANOVA with Bonferroni post hoc tests). (G) Immunofluorescence costaining of netrin-1 with cTnT and Ly6G in WT mice after sham or IR surgery shows netrin-1 in cardiomyocytes and neutrophils. (For single-channel pictures, scale bar = 2 um; for merged channel pictures, scale bar = 8 um.) *, P < 0.05; **, P < 0.01; ****, P < 0.0001. Data are presented as mean +- SD.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
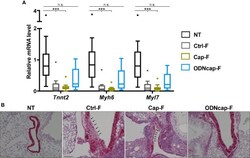
- Experimental details
- Figure 6 ODNcap-F protects pulmonary vein cardiomyocytes from allergic injury. Lung total RNA and sections were prepared on Day 70. (A) Expression levels of cardiac muscle-specific genes in the lung were measured by real-time quantitative PCR ( n = 12). Data are shown as Tukey's box plots. *** P < 0.001; n.s., not significant by Kruskal-Wallis test with Dunn's multiple comparisons. (B) Lung sections prepared on Day 70 were stained with anti-cardiac muscle troponin T (cTnT) antibody ( n = 6). Representative images are shown. Positive reaction of the antibody (red) was observed around the large pulmonary veins. Injured areas (arrows) of the cTnT-positive cells were observed in Ctrl-F and Cap-F, but not in NT or ODNcap-F. Scale bars, 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
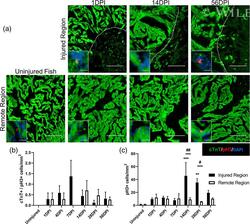
- Experimental details
- 5 FIGURE Local proliferation of noncardiomyocytes contributes to fibrosis. (a) Co-staining of cTnT+ cardiomyocytes and pH 3+ cells reveals an increase in nonmyocyte proliferation at 14 DPI specifically in the injured zone (note: DAPI omitted in larger panels; insets show higher magnification views within the same region of the heart, but are not enlargements of the larger panels). Quantification of proliferating cell types reveals that (b) cardiomyocytes do not proliferate to repopulate the injured region at any point after injury (proliferative cardiomyocytes quantified as those positive for both pH 3 and cTnT; Injured Region: n = 4 for 1, 4, 7, and 14 DPI; n = 5 for 28 and 56 DPI; n = 6 for uninjured; Remote Region: n = 4 for 1, 4, and 7 DPI; n = 5 for 14, 28, and 56 DPI; n = 6 for uninjured), but (c) a proliferating nonmyocyte population within the injured zone is present at 14 and 28 DPI (Injured Region: n = 3 for 1 and 4 DPI; n = 4 for 7 and 14 DPI; n = 5 for 28 and 56 DPI; n = 6 for uninjured; Remote Region: n = 4 for 1, 4, and 7 DPI; n = 5 for 14, 28, and 56 DPI; n = 6 for uninjured; large scale bar = 100 mum, small scale bar = 5 mum; ** p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Morphological analysis of EHTs in four different media at day 21 of culture. ( A ) Brightfield images of full EHTs in BPEL+HS, DMEM+HS, MM+HS, or MM(SF). Scale bar = 0.5 mm. ( B ) Confocal images of whole-mount tissue immunostaining for cardiac troponin T (cTnT, red) counterstained with DAPI (nuclei, blue). Scale bars, 25 um. HS, horse serum and SF, serum-free.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
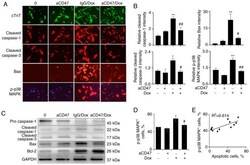
- Experimental details
- Figure 5 aCD47 reduces the expression of Bax and p-p38 MAPK in Dox-treated cardiomyocytes. (A) Immunostaining for the expression of cTnT, cleaved caspase-1/3, Bax and p-p38 MAPK in the treated cardiomyocytes. Cardiomyocytes were identified as cTnT-positive cells (green). Red, cells positively stained for cleaved caspase-1/3, Bax and p-p38 MAPK. Representative images with x200 magnification. (B) Semi-quantitative analysis of positively stained cells by ImageJ software. Data are presented as the relative intensity of positively stained cells over untreated controls. (C) Western blot analysis for the expression of pro-caspase-1, cleaved caspase-1, cleaved caspase-3, Bax and Bcl-2 in the treated cardiomyocytes. GAPDH was internal loading control. One representative blot. (D) p-p38 MAPK + cells after Dox and aCD47 treatment were analyzed by flow cytometry. Data are presented as the percentage of p-p38 MAPK + cells. (E) Association between the percentage of p-p38 MAPK + cells and apoptotic cardiomyocytes after treatment. Bar plot data in all panels are represented as mean +- standard error. n=3. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
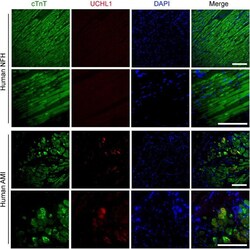
- Experimental details
- FIGURE 3 Post-MI myocardial upregulation of UCHL1 occurred primarily in the cardiomyocytes of the peri-infarct zone in humans. Paraffin-embedded and formalin-fixed ventricular myocardial sections (4-mum thick) from human non-failing donor hearts (NFH) or from the tissue cylinder core obtained from acute myocardial infarction (AMI) patients during the installation of a LV assisting device were subjected to deparaffinization, rehydration, antigen recovery, and indirect immunofluorescence staining for cardiac troponin T (cTnT) to identify cardiomyocytes (green) and for UCHL1 (red) as described in the Methods section. Nuclei are counter-stained with DAPI (blue). Shown are the representative confocal images. Scale bar, 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
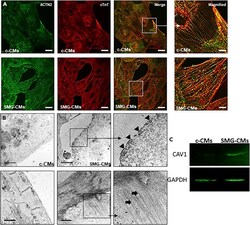
- Experimental details
- Exposure to SMG does not cause cytoskeletal deteriorations in CMs (A) A representative immunofluorescence staining of sarcomeric alpha-actinin (ACTN2) and cardiac troponin T (cTnT) in c-CMs and SMG-CMs (scale bar: 10 mum). (B) TEM images of SMG-CMs and c-CMs. Black arrow heads indicate caveolae structures (black arrows show stress fibers (scale bar: 500 nm). (C) Western blot analysis of Caveolin (CAV1) in control and SMG-exposed CMs (molecular weight of 22 kDa). GAPDH (36 kDa) was used as an internal protein loading control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
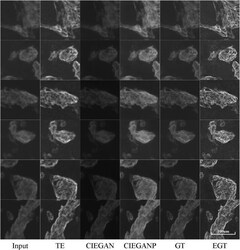
- Experimental details
- FIGURE 7 Results of cTnT fluorescence microscopy images of the iPSC-CM differentiation experiment. TE stands for the traditional enhancement method, CIEGAN, CIEGAN plus (CIEGANP) is our method, GT is the ground truth, and EGT is the enhanced ground truth.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- FIGURE 3. TRPV1 activation protected against PE-induced cardiomyocyte hypertrophy in vitro. A, Representative images and analysis of the cell surface area of neonatal rat cardiomyocytes stained with cTnT (green) and DAPI (blue) (n = 5, scale bar = 10 mum). B, The ANP, BNP, beta-MHC protein expression in primary cardiomyocytes treated with capsaicin and PE (n = 5). C, Quantitative polymerase chain reaction analyses of the mRNA levels of ANP, BNP, beta-MHC in the indicated groups. n = 5 per group. * P < 0.05 compared with control, # P < 0.05 compared with PE group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- FIGURE 6. Disruption of MAM by siMFN2 abolished capsaicin-mediated mitochondria protection. A, MMP was assessed using a JC-1 kit. The JC-1 aggregate/JC-1 monomer ratio was measured for MMP (n = 6, scale bar = 10 mum). B, Effects of capsaicin on ROS production in PE-treated cardiomyocytes using MitoSOX red staining (n = 5, scale bar = 10 mum). C, ATP levels in cultured cardiomyocytes (n = 6). D, Representative images and analysis of the cell surface area of neonatal rat cardiomyocytes stained with cTnT (green) and DAPI (blue) (n = 5, scale bar = 10 mum). * P < 0.05 compared with control, # P < 0.05 compared with PE group, $ P < 0.05 compared with PE + CAP group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. 8 TBX5-Lineage tracing confirms devCellPy prediction of a predominantly left ventricular cardiomyocytes differentiation of hiPSC-CMs in vitro. A human iPSC line was genome-edited to introduce a TBX5 - Cre /LoxP lineage tracing system. Genome-edited hiPSCs were differentiated and collected for scRNA-seq using the ICELL8 Smart-seq2 system. devCellPy models used for murine ventricular cardiomyocyte subtype predictions were applied for the prediction of the hiPSC-CMs. a Schematic of dual TBX5-Cre / MYL2-TdTomato lineage tracing system. b workflow for collection of day 15 ICELL8 data from genome-edited cell line. c Feature plots showing the expression of TurboGFP , LV marker HAND1 , ventricular markers (MYL3, MYL2, MYH7, MPPED2), and atrial markers ( NR2F1, KCNA5, VSNL1, STARD10) . d Left, Representative flow cytometry plot of cardiac troponin T (TNNT2) and TurboGFP expression at day 35 of cardiomyocytes containing TBX5 -lineage tracing system. Right, Quantification of GFP-positive percentage among TNNT2 + cardiomyocytes among 17 independent biological replicate differentiations. Error bars represent standard error around the mean. e Left, Representative flow cytometry plot of MYL2-TdTomato and TurboGFP expression at day 35 of cardiomyocytes. Right, Quantification of GFP-positive percentage among TdTomato+ cardiomyocytes among 17 independent biological replicate differentiations. Error bars represent standard error around the mean. f Predictions of cardiomyocyte subtypes for
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
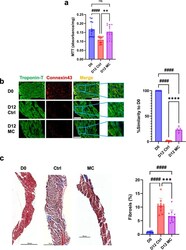
- Experimental details
- Mechanical stimulation partially improves tissue slice viability and structural integrity after 12 days in culture. a Bar graph shows quantification of the MTT viability of fresh heart slices (D0) or heart slices culture for 12 days either in static culture (D12 Ctrl) or in CTCM (D12 MC) ( n = 18 (D0), 15 (D12 Ctrl), 12 (D12 MC) slices/group from different pigs, one way ANOVA test is performed; #### p < 0.0001 compared to D0 and ** p < 0.01 compared to D12 Ctrl). b Representative immunofluorescence images for troponin-T (green), connexin 43 (red), and DAPI (blue) for freshly isolated heart slices (D0) or heart slices cultured for 12 days under static conditions (Ctrl) or CTCM conditions (MC) (Scale bare = 100 um). Artificial intelligence quantification of the heart tissue structural integrity ( n = 7 (D0), 7 (D12 Ctrl), 5 (D12 MC) slices/group each from different pig, one-way ANOVA test is performed; #### p < 0.0001 compared to D0 and **** p < 0.0001 compared to D12 Ctrl). c Representative images (left) and quantification (right) for heart slices stained with Masson's trichrome stain (Scale bare = 500 um) ( n = 10 slices/group each from different pig, one-way ANOVA test is performed; #### p < 0.0001 compared to D0 and *** p < 0.001 compared to D12 Ctrl). Error bars are representative of the Mean +- SD.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
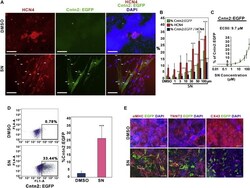
- Experimental details
- Figure 2 Sodium Nitroprusside Enhances Generation of Cardiac Conduction System Cells (A) Expression of cardiac conduction system markers, HCN4 and Cntn2:EGFP, was examined by immunofluorescence staining using antibodies against either HCN4 or GFP. Scale bar, 200 mum. DMSO was used as a control. Arrows indicate cells that are double positive deriving from the SN-treated cultures. (B) Quantification of immunofluorescence staining of HCN4 + , Cntn2:EGFP + , and HCN4 + /Cntn2:EGFP + double-positive cells by MetaExpress image analysis. Results represent the combined data from three independent differentiation experiments. Error bars show SD. (C) Efficacy curve of SN in the Cntn2:egfp cell line. Results represent the combined data from three independent differentiation experiments. Error bars show SD. (D) Flow Cytometry analysis of Cntn2:EGFP expression. Following 5 days treatment of either SN or DMSO, cells were harvested at day 25 of differentiation. Quantification of Cntn2:EGFP expression is shown in the right panel. Results are from five independent experiments. Error bars show SD. (E) SN- or DMSO-treated cells co-express cardiomyocyte markers alphaMHC or TNNT2 with Cntn2:EGFP. Cntn2:EGFP + cells express relatively weak levels of CX43 (right panel). Scale bar, 200 mum. Asterisks indicate significant differences compared with DMSO. Statistical significance is indicated: * p < 0.05, ** p < 0.01, *** p < 0.001. See also Figure S2 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
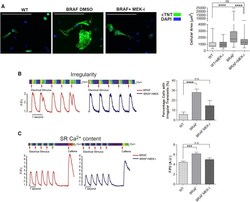
- Experimental details
- Figure 7 Inhibition of MEK Reverses the Hypertrophic Phenotype in BRAF-Mutant CMs (A) Dissociated EBs were treated with the MEK inhibitor U0126 and cellular area of cTNT + cells quantified. Exposure to U0126 (n = 130) significantly decreased the cellular area of BRAF-mutant CMs (n = 142) by 36% ( **** p < 0.0001), but had no significant effect (ns) on the cellular area of WT CMs (n = 57). Box-and-whisker plots show the median to the first and third quartiles and the minimum and maximum values. Scale bars, 200 mum. (B and C) Purified BRAF-mutant CMs exposed to U0126 displayed (B) decreased prevalence of irregular transients (n = 34) ( **** p < 0.0001) and (C) decreased SR Ca 2+ content (n = 12) ( *** p < 0.001). Data are presented as means +- SEM. n.s., not significant. Data represent two (WT2, 3) and three (BRAF1, 2, 3) biological and four technical replicates. See also Figure S7 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
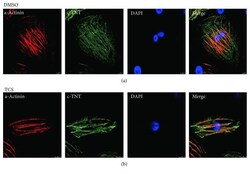
- Experimental details
- Figure 1 Structural characterization of H9-derived CMs. CMs were generated from H9 hESCs using the monolayer-based directed differentiation protocol. At day 20, CMs were immunostained for alpha -actinin (red) and cTnT (green). The cell nuclei were stained with DAPI (blue). Scale bar = 50 mu m.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
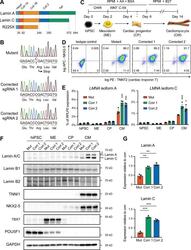
- Experimental details
- Figure 1. Generation of lamin A/C haploinsufficient hiPSC-CMs. (A) Predicted effect of the LMNA R225X mutation on the two splicing products lamin A and C. (B) Sanger sequencing of LMNA exon 4 in hiPSCs with heterozygous R225X mutation (top), or in hiPSCs obtained after CRISPR/Cas9-based scarless correction of the mutation (bottom). (C) Schematic of the protocol for step-wise directed differentiation of hiPSC-CMs. CHIR, CHIR-99021; AA, ascorbic acid. (D) Quantification of cardiac differentiation efficiency by flow cytometry for TNNT2 and NKX2-5 on hiPSC-CMs at day 14 of differentiation. (E) RT-qPCR analyses at the indicated stages of hiPSC-CM differentiation (see panel C). Differences versus mutant were calculated by two-way ANOVA with post hoc Holm-Sidak binary comparisons (*, P < 0.05; ***, P < 0.001; n = 3 differentiations; average +- SEM). (F) Representative Western blot for A- and B-type lamins and differentiation markers during iPSC-CM differentiation. (G) Quantification of lamin A/C expression in hiPSC-CMs from Western blot densitometries. Differences versus mutant were calculated by one-way ANOVA with post hoc Holm-Sidak binary comparisons (**, P < 0.01; ***, P < 0.001; n = 3 differentiations; average +- SEM). Throughout the figure (and in all other figures), Mut or Mutant indicates LMNA R225X hiPSCs, and Corr.1/2 or Corrected 1/2 indicates the two isogenic corrected control LMNA R225R hiPSC lines.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
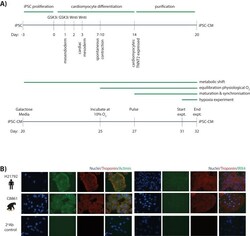
- Experimental details
- Figure 1--figure supplement 1. Cardiomyocytes can be differentiated from iPSCs from both humans and chimpanzees. ( A ) Schematic representation of the protocol used to differentiate cardiomyocytes from human and chimpanzee iPSC lines (see Materials and methods for a detailed description). ( B ) Immunostaining of cardiomyocyte-specific markers in iPSC-CMs from a representative human and chimpanzee individual. The cytoplasmic markers TNNT2 (green) and ACTN1 (red) are shown in the left panel. The nuclear marker IRX4 (green), together with TNNT2 is shown in the right panel. Nuclei are stained with Hoechst. Staining of iPSC-CMs incubated with a fluorescently-tagged secondary antibody, without a prior incubation with primary antibody, is included as a negative control in the bottom panel.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
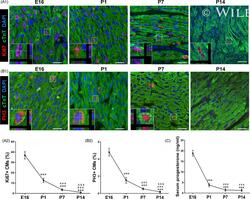
- Experimental details
- 1 Figure Serum progesterone concentration declines after birth and is associated with decrease in CM proliferation. A,B, Evaluation of CM proliferation by Ki67 and PH3 immunostaining at E16, P1, P7 and P14 hearts. Representative images with z-stacking are shown in A1 and B1, and quantification of percentages of Ki67 + and PH3 + CMs is shown in A2 and B2, respectively (n = 5). Scale bars are 20 um. cTnT indicates cardiac troponin T. C, Serum concentrations of progesterone in mice at E16, P1, P7 and P14 detected using ELISA kit (n = 5-8). *** P < .001 vs E16; ### P < .001 vs P1; &&& P < .001 vs P7. CM, cardiomyocyte
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
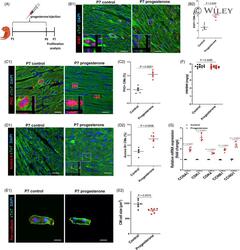
- Experimental details
- 2 Figure Progesterone supplementation promotes postnatal CM proliferation in vivo. A, Overview of the experimental set-up of the daily intraperitoneal injection of progesterone (8 mg/kg) or control vehicle (corn oil) from P1 to P6. Hearts were harvested at P7. B-D, CM proliferation was evaluated by Ki67, PH3 and Aurora B immunostaining. Representative images with z-stacking are shown in B1, C1 and D1, and quantification of percentages of Ki67 + , PH3 + or Aurora B + CMs is shown in B2, C2 and D2, respectively (n = 6). Scale bars are 20 um. E, Representative images (E1) and quantification (E2) of the cell size of CMs (labelled by cTnT and pan-cadherin to show intact cardiomyocytes) isolated from P7 heart. Eighty to one hundred cells were randomly selected per heart (n = 6). Scale bars are 20 um. F, Heart weight and body weight (HW/BW) ratio (n = 10-11). G, The mRNA expression of cell cycle activators in mouse hearts was analysed by real-time PCR (n = 5). CM, cardiomyocyte
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
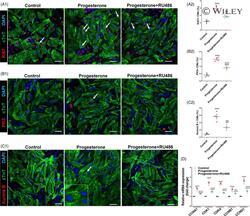
- Experimental details
- 3 Figure Progesterone promotes CM proliferation in vitro in a progesterone receptor-dependent manner. Cultured P7 CMs were treated with control vehicle (DMSO) or progesterone (10 -7 M), alone or in combination with the progesterone receptor inhibitor RU486 (10 -6 M). CM proliferation was analysed 24 h after progesterone stimulation. A-C, CM proliferation was evaluated by Ki67, PH3 and Aurora B immunostaining. Representative images are shown in A1, B1 and C1, and quantification of percentages of Ki67 + , PH3 + or Aurora B + CMs is shown in A2, B2 and C2, respectively. (3000-5000 cells were randomly selected and analysed for each group, and four independent experiments were conducted). Scale bars are 40 um. D, The mRNA expression of cell cycle activators in P7 CMs was analysed by quantitative PCR (n = 5). ( ** P < .01, *** P < .001) vs Control; ( ## P < .01, ### P < .001) vs Progesterone. CM, cardiomyocyte
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
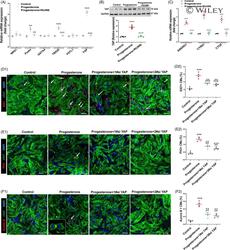
- Experimental details
- 5 Figure Progesterone promotes CM proliferation through up-regulation of YAP expression. Cultured P7 CMs were treated with control (vehicle, DMSO) or progesterone (10 -7 M), alone or in combination with RU486 (A-C) or YAP siRNAs (D-F). A, Proliferation regulator mRNA expressions of CM were analysed by quantitative PCR (n = 4). B, Representative immunoblots (B1) and quantification (B2) of YAP protein expression were analysed by Western blotting (GAPDH was used as internal control) (n = 4). C, The mRNA expression of YAP target genes ANKRD1, CYR61 and CTGF was analysed by quantitative real-time PCR (n = 4). D-F, CM proliferation was evaluated by Ki67, PH3 and Aurora B immunostaining. Representative images are shown in D1, E1 and F1, and quantification of percentages of Ki67 + , PH3 + or Aurora B + CMs is shown in D2, E2 and F2, respectively (3000-5000 cells were randomly selected and analysed for each group) (n = 4). Scale bars are 40 um ( ** P < .01, *** P < .001) vs Control; ( ## P < .01, ### P < .001) vs Progesterone. CM, cardiomyocyte; YAP, yes-associated protein
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
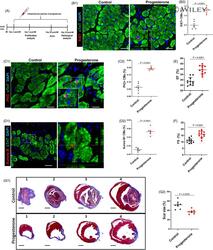
- Experimental details
- 7 Figure Progesterone promotes adult CM proliferation and improves cardiac function after MI. A, Adult mice were subjected to MI by ligation of the left anterior descending coronary artery and intraperitoneally injected daily with progesterone (8 mg/kg) or control vehicle (corn oil) from day 2 to day 35 post-MI. B-D, Hearts were harvested at day 7 post-MI; CM proliferation in the peri-infarcted area was evaluated by Ki67, PH3 and Aurora B staining in CMs. Representative images with z-stacking and quantification of percentages of those markers are shown (more than 5000 cells were randomly selected in each heart, and 6 mice were analysed in each group). Scale bars are 20 um. E,F, Echocardiographic (Echo) analyses of the effect of progesterone on cardiac function were performed 28 days after MI. Quantitative analysis of left ventricular ejection fraction (EF) and left ventricular fraction shortening (FS) is shown (n = 11-12). G, Hearts were harvested at day 35 post-MI. Representative images (G1) and quantification (G2) of fibrotic scar of heart sections were analysed using Masson's trichrome staining (n = 8). Scale bars are 1 mm. CM, cardiomyocyte; MI, myocardial infarction
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
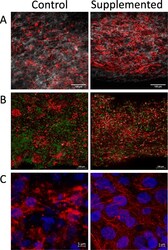
- Experimental details
- Fig. 2 Organization and microstructure of hECTs. Representative microscopy images of hECTs obtained along the horizontal axis of the hECT. a Single slice image obtained on a two-photon microscope, showing alpha-actinin (red) and collagen fibers (white), scale bar = 100 mum. b Confocal max projection showing Cardiac Troponin T (red), and nuclei stained with anti-histone H2B (green), scale bar = 100 mum. c Confocal max projection showing myofibril organization, with cardiac Troponin T (red), and nuclei stained with DAPI (blue,) scale bar = 5 mum
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. 2 In vitro 2D and 3D cardiomyocyte cultures. (A) Timeline of the presented liquid marble procedure from start (0h) making the marble, until 96h after attachment of cardiosphere used for immunofluorescent staining. (B) 20-day-old cardiomyocytes grown in 2D were passaged to a glass coverslip and immunofluorescent staining after 96h of attachment shows expression of cardiac markers cTnT (green), NKX2.5 (red), a counterstain with Hoechst (blue) was performed. (C) Cardiospheres (indicated by the black arrow) were produced after 24h in a 50 µL Liquid Marble (LM) of different cardiac culture concentration (500, 1000 and 2000 cells/ ul). Phase-contrast pictures show attachment after cardiosphere harvesting after 24h and 96h. Maximal intensity projection images show cardiac marker expression for cTnT (green) and NKX2.5 (red) and nuclei (blue). Bar in phase-contrast images indicates 500 um, and 100 um in confocal images. (For interpretation of the references to color in the text, the reader is referred to the web version of this article.) Fig 2
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. 3 Purification of cardiac cultures using MACS. Phase contrast images show 2D cardiac cultures 48 h after passaging without purification and with MACS purification. Cardiac markers cTnT (green) and NKX2.5 (red) are showed in the immunofluorescent images, a counter stain for nuclei with Hoechst (blue) was performed. Bar indicates 500 um in phase-contrast images and 100 um in confocal images. (For interpretation of the references to color in the text, the reader is referred to the web version of this article.) Fig 3
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry