Antibody data
- Antibody Data
- Antigen structure
- References [18]
- Comments [0]
- Validations
- Immunohistochemistry [1]
- Flow cytometry [2]
- Other assay [15]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-0479-82 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD47 Monoclonal Antibody (B6H12), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The monoclonal antibody B6H12 reacts to CD47 also known as integrin-associated protein (IAP), and neurophilin. CD47 is a glycosylated five transmembrane protein with a small alternatively spliced cytoplasmic domain. CD47 is involved in adhesion through interactions with SIRP (signal regulator protein) and is non-covalently associated with beta3 integrins CD51/CD61 and CD41/CD61. Furthermore this interaction can mediate bi-directional signaling to modify neural synaptic activity and regulate the phagocytic activities of macrophages. CD47 is the receptor for thrombospondin. T cell expression of CD47 can mediate activation or apoptosis (in the presence of high levels of thrombospondin). Recently stimulation of CD47 by monoclonal antibody has been shown to induce CD4+CD25- suppressive activity also increasing expression of Foxp3. Expression is found in the majority of hematopoietic cells including T and B cells, monocytes, platelets and erythrocytes (as part of the Rh complex). Expression is also found in non-hematopoietic cells. This antibody has been reported to have neutralizing activity. Applications Reported: This B6H12 antibody has been reported for use in flow cytometric analysis, immunoprecipitation, western blotting, and immunohistochemical staining of formalin-fixed paraffin embedded tissue sections. Applications Tested: This B6H12 antibody has been tested by flow cytometric analysis of normal human peripheral blood cells. This can be used at less than or equal to 1 µg per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. The B6H12 antibody has been tested by immunohistochemistry of formalin-fixed paraffin embedded human tissue using low pH antigen retreival and can be used at less than or equal to 40 µg/mL. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- B6H12
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- 4°C
Submitted references CD47 Blockade Accelerates Blood Clearance and Alleviates Early Brain Injury After Experimental Subarachnoid Hemorrhage.
Schweinfurthin induces ICD without ER stress and caspase activation.
Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic Kras(G12D) in pancreatic cancer.
Anti-CD47 antibody synergizes with cisplatin against laryngeal cancer by enhancing phagocytic ability of macrophages.
LSD1 silencing contributes to enhanced efficacy of anti-CD47/PD-L1 immunotherapy in cervical cancer.
Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity.
CD47 prevents the elimination of diseased fibroblasts in scleroderma.
Regulation of senescence escape by TSP1 and CD47 following chemotherapy treatment.
Generation and testing of clinical-grade exosomes for pancreatic cancer.
Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer.
CD47 promotes ovarian cancer progression by inhibiting macrophage phagocytosis.
SIRPα-antibody fusion proteins stimulate phagocytosis and promote elimination of acute myeloid leukemia cells.
CD47 is an adverse prognostic factor and a therapeutic target in gastric cancer.
Nanoparticle biointerfacing by platelet membrane cloaking.
Exploiting light chains for the scalable generation and platform purification of native human bispecific IgG.
Cells expressing the C/EBPbeta isoform, LIP, engulf their neighbors.
CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation.
Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25- T cells in response to inflammation.
Xu CR, Li JR, Jiang SW, Wan L, Zhang X, Xia L, Hua XM, Li ST, Chen HJ, Fu XJ, Jing CH
Frontiers in immunology 2022;13:823999
Frontiers in immunology 2022;13:823999
Schweinfurthin induces ICD without ER stress and caspase activation.
Zhang R, Neighbors JD, Schell TD, Hohl RJ
Oncoimmunology 2022;11(1):2104551
Oncoimmunology 2022;11(1):2104551
Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic Kras(G12D) in pancreatic cancer.
McAndrews KM, Xiao F, Chronopoulos A, LeBleu VS, Kugeratski FG, Kalluri R
Life science alliance 2021 Sep;4(9)
Life science alliance 2021 Sep;4(9)
Anti-CD47 antibody synergizes with cisplatin against laryngeal cancer by enhancing phagocytic ability of macrophages.
Wang J, Zhang H, Yin X, Bian Y
Clinical and experimental immunology 2021 Sep;205(3):333-342
Clinical and experimental immunology 2021 Sep;205(3):333-342
LSD1 silencing contributes to enhanced efficacy of anti-CD47/PD-L1 immunotherapy in cervical cancer.
Xu S, Wang X, Yang Y, Li Y, Wu S
Cell death & disease 2021 Mar 17;12(4):282
Cell death & disease 2021 Mar 17;12(4):282
Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity.
von Roemeling CA, Wang Y, Qie Y, Yuan H, Zhao H, Liu X, Yang Z, Yang M, Deng W, Bruno KA, Chan CK, Lee AS, Rosenfeld SS, Yun K, Johnson AJ, Mitchell DA, Jiang W, Kim BYS
Nature communications 2020 Mar 20;11(1):1508
Nature communications 2020 Mar 20;11(1):1508
CD47 prevents the elimination of diseased fibroblasts in scleroderma.
Lerbs T, Cui L, King ME, Chai T, Muscat C, Chung L, Brown R, Rieger K, Shibata T, Wernig G
JCI insight 2020 Aug 20;5(16)
JCI insight 2020 Aug 20;5(16)
Regulation of senescence escape by TSP1 and CD47 following chemotherapy treatment.
Guillon J, Petit C, Moreau M, Toutain B, Henry C, Roché H, Bonichon-Lamichhane N, Salmon JP, Lemonnier J, Campone M, Verrièle V, Lelièvre E, Guette C, Coqueret O
Cell death & disease 2019 Feb 27;10(3):199
Cell death & disease 2019 Feb 27;10(3):199
Generation and testing of clinical-grade exosomes for pancreatic cancer.
Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, Marszalek JR, Maitra A, Yee C, Rezvani K, Shpall E, LeBleu VS, Kalluri R
JCI insight 2018 Apr 19;3(8)
JCI insight 2018 Apr 19;3(8)
Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer.
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R
Nature 2017 Jun 22;546(7659):498-503
Nature 2017 Jun 22;546(7659):498-503
CD47 promotes ovarian cancer progression by inhibiting macrophage phagocytosis.
Liu R, Wei H, Gao P, Yu H, Wang K, Fu Z, Ju B, Zhao M, Dong S, Li Z, He Y, Huang Y, Yao Z
Oncotarget 2017 Jun 13;8(24):39021-39032
Oncotarget 2017 Jun 13;8(24):39021-39032
SIRPα-antibody fusion proteins stimulate phagocytosis and promote elimination of acute myeloid leukemia cells.
Ponce LP, Fenn NC, Moritz N, Krupka C, Kozik JH, Lauber K, Subklewe M, Hopfner KP
Oncotarget 2017 Feb 14;8(7):11284-11301
Oncotarget 2017 Feb 14;8(7):11284-11301
CD47 is an adverse prognostic factor and a therapeutic target in gastric cancer.
Yoshida K, Tsujimoto H, Matsumura K, Kinoshita M, Takahata R, Matsumoto Y, Hiraki S, Ono S, Seki S, Yamamoto J, Hase K
Cancer medicine 2015 Sep;4(9):1322-33
Cancer medicine 2015 Sep;4(9):1322-33
Nanoparticle biointerfacing by platelet membrane cloaking.
Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L
Nature 2015 Oct 1;526(7571):118-21
Nature 2015 Oct 1;526(7571):118-21
Exploiting light chains for the scalable generation and platform purification of native human bispecific IgG.
Fischer N, Elson G, Magistrelli G, Dheilly E, Fouque N, Laurendon A, Gueneau F, Ravn U, Depoisier JF, Moine V, Raimondi S, Malinge P, Di Grazia L, Rousseau F, Poitevin Y, Calloud S, Cayatte PA, Alcoz M, Pontini G, Fagète S, Broyer L, Corbier M, Schrag D, Didelot G, Bosson N, Costes N, Cons L, Buatois V, Johnson Z, Ferlin W, Masternak K, Kosco-Vilbois M
Nature communications 2015 Feb 12;6:6113
Nature communications 2015 Feb 12;6:6113
Cells expressing the C/EBPbeta isoform, LIP, engulf their neighbors.
Abreu M, Sealy L
PloS one 2012;7(7):e41807
PloS one 2012;7(7):e41807
CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation.
Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE
The Journal of neuroscience : the official journal of the Society for Neuroscience 2009 Sep 23;29(38):11982-92
The Journal of neuroscience : the official journal of the Society for Neuroscience 2009 Sep 23;29(38):11982-92
Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25- T cells in response to inflammation.
Grimbert P, Bouguermouh S, Baba N, Nakajima T, Allakhverdi Z, Braun D, Saito H, Rubio M, Delespesse G, Sarfati M
Journal of immunology (Baltimore, Md. : 1950) 2006 Sep 15;177(6):3534-41
Journal of immunology (Baltimore, Md. : 1950) 2006 Sep 15;177(6):3534-41
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
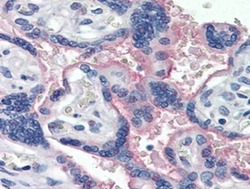
- Experimental details
- Immunohistochemistry of formalin-fixed paraffin embedded human placenta stained with 40 µg/mL Anti-Human CD47 Purified followed by Anti-Mouse IgG Biotin, Streptavidin Alkaline Phosphatase, and Fast Red. Nuclei are counterstained with hematoxylin.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
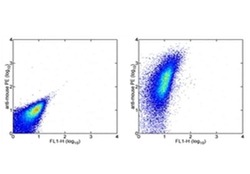
- Experimental details
- Staining of normal human peripheral blood cells with 0.5 µg of Mouse IgG1 kappa Isotype Control Purified (Product # 14-4714-82) (left) or 0.5 µg of Anti-Human CD47 Purified (center) followed by F (ab')2 Anti-Mouse IgG PE (Product # 12-4012). Lymphocytes were used for analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
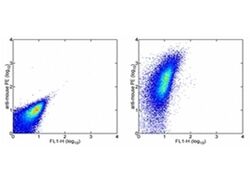
- Experimental details
- Staining of normal human peripheral blood cells with 0.5 µg of Mouse IgG1 kappa Isotype Control Purified (Product # 14-4714-82) (left) or 0.5 µg of Anti-Human CD47 Purified (center) followed by F (ab')2 Anti-Mouse IgG PE (Product # 12-4012). Lymphocytes were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
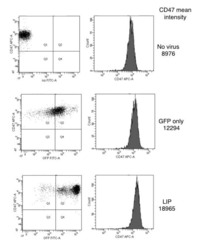
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
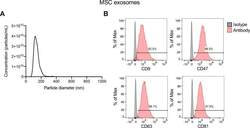
- Figure S5. Characterization of mesenchymal stem cell (MSC) exosomes. (A) Representative nanoparticle tracking analysis plot of MSC exosomes. (B) Representative histogram of flow cytometry analyses of exosomal surface markers (CD9, CD47, CD63, and CD81, indicated in red; isotype control indicated in gray) on MSC exosomes bound to beads. Numbers represent the percentage of positive beads. Source data are available for this figure. Source Data for Figure S5
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
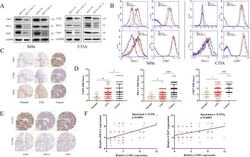
- Fig. 1 LSD1 is positively correlated with CD47/PD-L1 in cervical cancer cells and tissues. The positive correlation between LSD1 and CD47/PD-L1 expression was demonstrated using western blotting ( A ) and flow cytometry ( B ). Both IHC staining intensity ( C ) and IHC score ( D ) for LSD1, as for CD47/PD-L1, showed a steady increase during the progression of cervical cancer. IHC staining intensity ( E ) and Spearman correlation analysis ( F ) also confirmed the significant correlation between LSD1 and CD47/PD-L1 expressionfrom a clinical perspective. The western blotting and flow cytometric processes were repeated three times. * p < 0.05; ** p < 0.01; **** p < 0.0001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
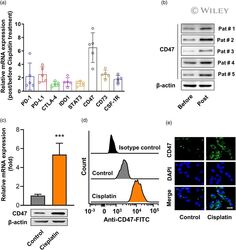
- Experimental details
- 1 FIGURE Cisplatin treatment increased CD47 expression in cells from laryngeal cancer tissue from patients and the human laryngeal cancer cell line. (a) Gene expression in patient-derived laryngeal cancer cells post-cisplatin treatment. Five laryngeal cancer samples obtained from five patients underwent an enzymatic digestion and digested single cells were cultured in complete Dulbecco's modified Eagle's medium (DMEM) medium containing 7.5 muM cisplatin; 24 h later, cells were harvested for the examination of gene expression. The mRNA levels of gene associated with immunosuppression [including programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), cytotoxic T lymphocyte antigen (CTLA-4), indoleamine 2,3-dioxygenase 1 (IDO1), signal transducer and activator of transcription (STAT)-3, CD47, CD73 and colony-stimulating factor 1 receptor (CSF-1R)] were examined by real-time polymerase chain reaction (PCR). GAPDH was detected as control. n = 5. (b) The protein levels of CD47 in patient-derived laryngeal cancer cells post-cisplatin treatment were examined by Western blot. beta-actin was used as loading control. Human laryngeal cancer cell line human epithelial type 2 (Hep-2) cells were treated with 7.5 muM cisplatin for 24 h, the expression of CD47 in mRNA level (up) and protein level (low) were examined by real-time PCR and Western blot (c); n = 3. Data represent means +- standard deviation (SD). *** p < 0.001 ( versus cisplatin group). The CD47 expressions were a
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
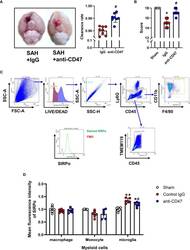
- Experimental details
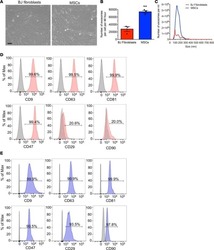
- Effects of CD47 blocking antibody on blood clearance and neurological function. (A) Representative brain images and quantification analysis of residual hemorrhage in brain samples. n = 8 per group. Mean +- SD. # P < 0.05 vs. SAH + IgG group. (B) Summary of modified Garcia scores of Sham, SAH + IgG, and SAH + CD47. n = 8 per group. Medians +- IQR. * P < 0.05 vs. Sham group, # P < 0.05 vs. SAH + IgG group. (C) Gating strategy to distinguish monocytes (CD45+, CD11b+, F4/80-), macrophages (Ly6G-, CD45+, CD11b+, F4/80+), and microglia (CD45-, Ly6G-, Tmem119+). SIRPalpha was then assessed based on FMO control. (D) Mean fluorescence index (MFi) of SIRPalpha in various groups. n = 6 per group. Mean +- SD. *P < 0.05 vs. Sham group; **P < 0.01 vs. Sham group; # P < 0.05 vs. SAH+IgG group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
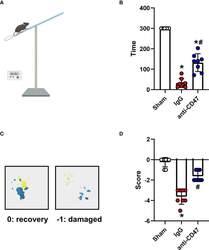
- Experimental details
- Effects of CD47 blocking antibody on motor deficiencies day 7 after SAH. (A) Schematic diagram of the holding time test. (B) The holding capacity of holding time test in various groups. n = 8 per group. Data are expressed as mean+- SD. *P < 0.05 vs. Sham group; # P < 0.05 vs. SAH+IgG group. (C) Yellow paint on the two forepaws and blue paint on the two hind paws. (D) Scores of footprint test in various groups. n = 8 per group. Data are expressed as mean+- SD. *P < 0.05 vs. Sham group; # P < 0.05 vs. SAH+IgG group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
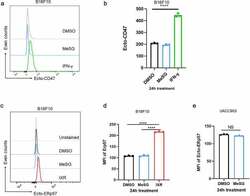
- Experimental details
- Figure 4. MeSG treatment doesn't induce exposure of CD47 and ERp57 on surface of B16F10 and UACC903 cells. (a,b) B16F10 cells were treated with MeSG at 100 nM, or with vehicle control DMSO for 24 hours. Cell surface CD47 was determined by flow cytometry among viable cells (7AAD negative) and presented in histograms. MFI of surface CD47 were shown as Mean +- SD (n = 3) in bar graph. Significance was determined by One-way ANOVA with Tukey correction. (c,d) B16F10 cells were treated with MeSG at 100 nM, or with DMSO, for 24 hours, or 10,000 rads gamma irradiation. Cell surface ERp57 was determined by flow cytometry among viable cells (7AAD negative) and presented in histograms. MFI of surface ERp57 are shown as Mean +- SD (n = 3) in bar graph. Significance was determined by One-way ANOVA with Tukey correction. (e) UACC903 cells were treated with MeSG at 100 nM, or with DMSO, for 24 hours. Cell surface ERp57 levels were measured by flow cytometry. The quantification of ERp57 MFI are reported as Mean +- SD (n = 3) in bar graph. Significance was determined by t-test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
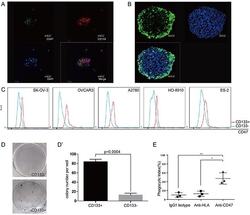
- Figure 4 CD47 expression is elevated in the ovarian cancer stem cell-like population (A) Tumor cells obtained from patient ascites samples were used for sphere forming assays. CD47 and CD133 expression on the spheres was analyzed by immunofluorescence staining (scale bar = 20 mum). (B) Ovarian cancer sphere cells showed aldehyde dehydrogenase (scale bar = 20 mum). (C) The CD47 expression levels in CD133 + and CD133 - sub-populations was measured by mean fluorescence intensity in 5 epithelial ovarian cancer cell lines. (D) Representative colony formation assay for HO-8910 CD133 + and CD133 - sub-populations. (D') The number of colonies was quantified and plotted (Unpaired Student's-t-test, two-tailed, **p < 0.005). (E) The anti-CD47 mAb promoted phagocytosis of the CD133 + sub-population of cells isolated from the HO-8910 cell line by macrophages.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
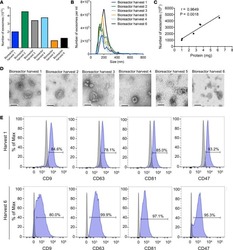
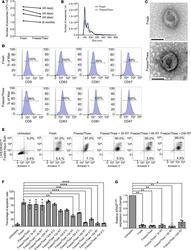
- Figure 8 GMP-grade iExosomes stability. ( A ) Comparison of the number of MSC exosomes, quantified by NanoSight, prior to freezing and after thawing of frozen exosomes ( n = 4 distinct exosomes aliquots). The times listed refer to the times that exosomes were stored for at -80degC. ( B ) Particle size distribution analysis of fresh and frozen (45 days) and then thawed MSC exosomes by NanoSight. ( C ) TEM of MSC exosomes, prior to freezing (fresh) and after freezing (45 days) and thawing. Scale bar: 100 nm. ( D ) Representative histogram of flow cytometry analyses of exosomal markers (CD9, CD63, CD81, CD47) on fresh versus freeze (45 days) and thaw MSC exosomes. Numbers represent the percentage of positive beads (gray, isotype control). ( E and F ) Representative dot plot of flow cytometry analyses ( E ) and quantification ( F ) of apoptosis in Panc-1 cells induced by MSCs siKras G12D iExo (48 hours following iExo treatment), comparing the efficacy of freeze (3 months) and thaw iExosomes that were allowed to incubate for 3, 6, and 24 hours and 2, 3, 4, and 5 days at room temperature (RT) or 4degC ( n = 2-3 independent experiments). Numbers represent the percentage of positive cells. One-way ANOVA compared with fresh exosomes. ( G ) KRAS G12D transcript levels in Panc-1 cells treated with MSCs siKras G12D iExo after 3 hours, comparing the efficacy of freeze (3 months) and thaw of iExosomes, under the listed conditions ( n = 3 independent experiments, 1
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
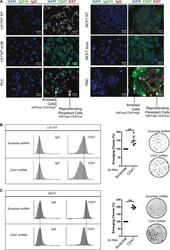
- Fig. 5 CD47 expression is downregulated in emergent clones. a Analysis of CD47 and Ki67 expressions by immunofluorescence in parental LS174T and MCF7 cells or emergent populations (PLC/PMC). Ki67 staining identifies the clones that have restarted proliferation in the middle of senescent cells ( n = 3). b CD47 expression was analyzed by flow cytometry (one experiment representative of three, left part) in LS174T cells that express an shRNA directed against CD47 or a non-targeting control. In parallel, cells have been treated with sn38 to induce senescence and 10% FBS was added after 4 days to allow emergence ( n = 3, Kolmogorov-Smirnov test, ** = p < 0.01). c CD47 expression was analyzed by flow cytometry in MCF7 clones expressing an shRNA directed against CD47 or a non-targeting control shRNA. Emergence was induced as described above ( n = 3, Kolmogorov-Smirnov test, ** = p < 0.01)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
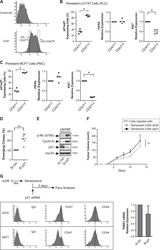
- Fig. 6 p21waf1 inactivation increases emergence and generates CD47 low cells. a Flow cytometry analysis of CD47 expression. The image describes the gating position of the 10% cells corresponding to CD47 low or CD47 high cells. b , c Following senescence induction, emergence was induced by serum addition. The dividing and senescent clones were then recovered and analyzed by intracellular flow cytometry. p21waf1, KI67, and g-H2AX expressions were quantified by flow cytometry in CD47 low cells or CD47 high cells and normalized to control IgG staining ( n = 4, LS174T ( b ) and MCF7 ( c ), Kolmogorov-Smirnov test, * = p < 0.05). d Senescence was induced as decribed above in LS174T cells and after 4 days, cells were transfected with control siRNA or siRNA directed against p21waf1. Ten-percent FBS was added to allow cell emergence ( n = 6, Kolmogorov-Smirnov test, ** = p < 0.01). e LS174T cells were treated as above and cell extracts were recovered 2 days after p21waf1 inactivation by siRNA. The expression of the indicated proteins was analyzed by western blot ( n = 4). f CIS was induced in 4T1 cells with doxorubicine and after 4 days, cells were transfected with a control siRNA or siRNA directed against p21waf1. After 24 h, cells were mixed with untreated 4T1 cells and injected in Balb/c mice ( n = 7, two-way Anova with a Bonferroni's multiple comparison test: ** = p < 0.01, p = 0.0075). Tumor growth was monitored in the mammary fat pad. g LS174T cells were treated as above to indu
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
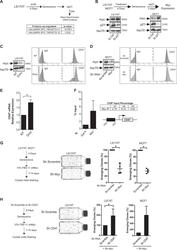
- Fig. 7 p21waf1 prevents the generation of CD47 low cells by downregulating Myc expression. a Following senescence induction, emergence was induced by serum addition, in the presence or absence of a control siRNA or a siRNA directed against p21 ( n = 3). Cell extracts were analyzed by SWATH quantitative proteomics and GSEA analysis. b Validation of Myc induction by western blot analysis following p21 downregulation and emergence ( n = 3). c , d Myc expression was downregulated by an inducible shRNA in LS174T cells or by a transient infection of a different shRNA in MCF7 cells. CD47 expression was then analyzed by flow cytometry. One experiment representative of 4 is presented. e Myc was downregulated as described above in LS174T cells and the expression of the CD47 mRNA was evaluated by RT-QPCR ( n = 4 +/- sd, Kolmogorov-Smirnov test, * = p < 0.05). f ChIP assays were performed following Myc or Gal4 immunoprecipitation using the indicated primers and analyzed by quantitative PCR ( n = 3). Each amplification is also shown compared with the Gal4 signal. g Senescence was induced as decribed above and after 4 days, 10% FBS was added to allow LS174T or MCF7 cell emergence, in the presence of control shRNAs or shRNAs directed against Myc ( n = 4, Kolmogorov-Smirnov test, * = p < 0.05). h LS174T or MCF7 cells were infected with a lentivirus expressing an shRNA targeting CD47 or a control sequence. Senescence was then induced as described and after 4 days, 10% FBS was added to allow L
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
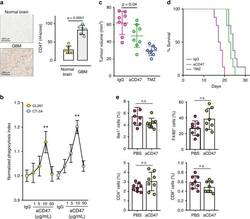
- Fig. 1 Blockade of CD47, an anti-phagocytosis molecule over-expressed in glioblastoma (GBM), does not produce significant antitumor effect. a Immunohistochemistry analyses showing that CD47 expression is significantly elevated in GBM as compared to normal brain tissues from patient samples. Scale bar = 200 mum. n = 6/group. Unpaired student's t test. Error bar = mean +- standard deviation. b CD47 blockade resulted in a modest increase in murine GBM cell phagocytosis by BM phagocytes. n = 5/group. ** p < 0.01 compared to IgG (unpaired Student's t test). c The tumor growth inhibitory effect of CD47 blockade was less than temozolomide. n = 8/group. d CD47 blockade did not result in improved animal survival over temozolomide treatment. n = 8/group. p = 0.1 (log-rank test). e No significant changes were noted in the intratumoral total (F4/80) or activated BM phagocytes (Iba1), CD4 + or CD8 + T cells after anti-CD47 antibody treatment. n = 8/group. Error bar = mean +- standard deviation. n.s. not significant.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunohistochemistry
Immunohistochemistry