Antibody data
- Antibody Data
- Antigen structure
- References [2]
- Comments [0]
- Validations
- Chromatin Immunoprecipitation [1]
Submit
Validation data
Reference
Comment
Report error
- Product number
- HPA031455 - Provider product page

- Provider
- Atlas Antibodies
- Proper citation
- Atlas Antibodies Cat#HPA031455, RRID:AB_10670702
- Product name
- Anti-HSF2
- Antibody type
- Polyclonal
- Description
- Polyclonal Antibody against Human HSF2, Gene description: heat shock transcription factor 2, Validated applications: WB, IHC, ChIP, Uniprot ID: Q03933, Storage: Store at +4°C for short term storage. Long time storage is recommended at -20°C.
- Reactivity
- Human
- Host
- Rabbit
- Conjugate
- Unconjugated
- Isotype
- IgG
- Vial size
- 100 µl
- Concentration
- 0.1 mg/ml
- Storage
- Store at +4°C for short term storage. Long time storage is recommended at -20°C.
- Handling
- The antibody solution should be gently mixed before use.
Submitted references Comprehensive analysis of human tissues reveals unique expression and localization patterns of HSF1 and HSF2
Gambogic acid and gambogenic acid induce a thiol-dependent heat shock response and disrupt the interaction between HSP90 and HSF1 or HSF2
Joutsen J, Pessa J, Jokelainen O, Sironen R, Hartikainen J, Sistonen L
Cell Stress and Chaperones 2024;29(2):235-271
Cell Stress and Chaperones 2024;29(2):235-271
Gambogic acid and gambogenic acid induce a thiol-dependent heat shock response and disrupt the interaction between HSP90 and HSF1 or HSF2
Pesonen L, Svartsjö S, Bäck V, de Thonel A, Mezger V, Sabéran-Djoneidi D, Roos-Mattjus P
Cell Stress and Chaperones 2021;26(5):819-833
Cell Stress and Chaperones 2021;26(5):819-833
No comments: Submit comment
Supportive validation
- Submitted by
- Atlas Antibodies (provider)
- Main image
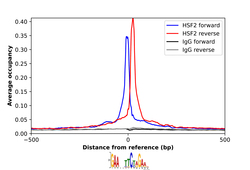
- Experimental details
- ChIP-Exo-Seq composite graph for Anti-HSF2 (HPA031455, Lot B116429) tested in K562 cells. Strand-specific reads (blue: forward, red: reverse) and IgG controls (black: forward, grey: reverse) are plotted against the distance from a composite set of reference binding sites. The antibody exhibits robust target enrichment compared to a non-specific IgG control and precisely reveals its structural organization around the binding site. Data generated by Prof. B. F. Pugh´s Lab at Cornell University.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Chromatin Immunoprecipitation
Chromatin Immunoprecipitation