Antibody data
- Antibody Data
- Antigen structure
- References [35]
- Comments [0]
- Validations
- Immunocytochemistry [6]
- Other assay [39]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-757 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- BACE1 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA1-757 detects transfected beta-secretase 1. PA1-757 has been successfully used in ELISA, ICC/IF, Western blot and immunoprecipitation applications. The molecular weight of BACE1 detected may vary depending on the lysate tested. This may be due to glycosylation differences in various cell lines. The PA1-757 immunizing peptide (Cat. # PEP-093) is available for use in neutralization and control experiments.
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Alzheimer's disease protease-containing plasma extracellular vesicles transfer to the hippocampus via the choroid plexus.
Activation of the hypoxia response protects mice from amyloid-β accumulation.
Upregulation of APP endocytosis by neuronal aging drives amyloid-dependent synapse loss.
Protein farnesylation is upregulated in Alzheimer's human brains and neuron-specific suppression of farnesyltransferase mitigates pathogenic processes in Alzheimer's model mice.
Amyloidogenic Processing of Amyloid Precursor Protein Drives Stretch-Induced Disruption of Axonal Transport in hiPSC-Derived Neurons.
A cellular complex of BACE1 and γ-secretase sequentially generates Aβ from its full-length precursor.
Bin1 and CD2AP polarise the endocytic generation of beta-amyloid.
Relationship between ubiquilin-1 and BACE1 in human Alzheimer's disease and APdE9 transgenic mouse brain and cell-based models.
Cathepsin L Mediates the Degradation of Novel APP C-Terminal Fragments.
Elucidation of the BACE1 regulating factor GGA3 in Alzheimer's disease.
Involvement of receptor tyrosine kinase Tyro3 in amyloidogenic APP processing and β-amyloid deposition in Alzheimer's disease models.
Depletion of GGA1 and GGA3 mediates postinjury elevation of BACE1.
BACE1 protein endocytosis and trafficking are differentially regulated by ubiquitination at lysine 501 and the Di-leucine motif in the carboxyl terminus.
Calsyntenin-1 shelters APP from proteolytic processing during anterograde axonal transport.
Minocycline corrects early, pre-plaque neuroinflammation and inhibits BACE-1 in a transgenic model of Alzheimer's disease-like amyloid pathology.
Development of a specific ELISA to measure BACE1 levels in human tissues.
The contribution of activated astrocytes to Aβ production: implications for Alzheimer's disease pathogenesis.
Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer's disease models.
Ubiquitin regulates GGA3-mediated degradation of BACE1.
Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in Alzheimer's mouse model.
Focal cerebral ischemia in rats alters APP processing and expression of Abeta peptide degrading enzymes in the thalamus.
Down-regulation of seladin-1 increases BACE1 levels and activity through enhanced GGA3 depletion during apoptosis.
Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis.
Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer's disease pathogenesis.
Ubiquilin 1 modulates amyloid precursor protein trafficking and Abeta secretion.
Temporal memory deficits in Alzheimer's mouse models: rescue by genetic deletion of BACE1.
BACE is degraded via the lysosomal pathway.
Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer's disease pathogenesis.
Statins cause intracellular accumulation of amyloid precursor protein, beta-secretase-cleaved fragments, and amyloid beta-peptide via an isoprenoid-dependent mechanism.
Presenilin 1 stabilizes the C-terminal fragment of the amyloid precursor protein independently of gamma-secretase activity.
The metabolism of beta-amyloid converting enzyme and beta-amyloid precursor protein processing.
Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production.
Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE.
Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE.
Identification of a novel aspartic protease (Asp 2) as beta-secretase.
Lee JH, Ostalecki C, Oberstein T, Schierer S, Zinser E, Eberhardt M, Blume K, Plosnita B, Stich L, Bruns H, Coras R, Vera-Gonzales J, Maler M, Baur AS
EBioMedicine 2022 Mar;77:103903
EBioMedicine 2022 Mar;77:103903
Activation of the hypoxia response protects mice from amyloid-β accumulation.
Ollonen T, Kurkela M, Laitakari A, Sakko S, Koivisto H, Myllyharju J, Tanila H, Serpi R, Koivunen P
Cellular and molecular life sciences : CMLS 2022 Jul 19;79(8):432
Cellular and molecular life sciences : CMLS 2022 Jul 19;79(8):432
Upregulation of APP endocytosis by neuronal aging drives amyloid-dependent synapse loss.
Burrinha T, Martinsson I, Gomes R, Terrasso AP, Gouras GK, Almeida CG
Journal of cell science 2021 May 1;134(9)
Journal of cell science 2021 May 1;134(9)
Protein farnesylation is upregulated in Alzheimer's human brains and neuron-specific suppression of farnesyltransferase mitigates pathogenic processes in Alzheimer's model mice.
Jeong A, Cheng S, Zhong R, Bennett DA, Bergö MO, Li L
Acta neuropathologica communications 2021 Jul 27;9(1):129
Acta neuropathologica communications 2021 Jul 27;9(1):129
Amyloidogenic Processing of Amyloid Precursor Protein Drives Stretch-Induced Disruption of Axonal Transport in hiPSC-Derived Neurons.
Chaves RS, Tran M, Holder AR, Balcer AM, Dickey AM, Roberts EA, Bober BG, Gutierrez E, Head BP, Groisman A, Goldstein LSB, Almenar-Queralt A, Shah SB
The Journal of neuroscience : the official journal of the Society for Neuroscience 2021 Dec 8;41(49):10034-10053
The Journal of neuroscience : the official journal of the Society for Neuroscience 2021 Dec 8;41(49):10034-10053
A cellular complex of BACE1 and γ-secretase sequentially generates Aβ from its full-length precursor.
Liu L, Ding L, Rovere M, Wolfe MS, Selkoe DJ
The Journal of cell biology 2019 Feb 4;218(2):644-663
The Journal of cell biology 2019 Feb 4;218(2):644-663
Bin1 and CD2AP polarise the endocytic generation of beta-amyloid.
Ubelmann F, Burrinha T, Salavessa L, Gomes R, Ferreira C, Moreno N, Guimas Almeida C
EMBO reports 2017 Jan;18(1):102-122
EMBO reports 2017 Jan;18(1):102-122
Relationship between ubiquilin-1 and BACE1 in human Alzheimer's disease and APdE9 transgenic mouse brain and cell-based models.
Natunen T, Takalo M, Kemppainen S, Leskelä S, Marttinen M, Kurkinen KMA, Pursiheimo JP, Sarajärvi T, Viswanathan J, Gabbouj S, Solje E, Tahvanainen E, Pirttimäki T, Kurki M, Paananen J, Rauramaa T, Miettinen P, Mäkinen P, Leinonen V, Soininen H, Airenne K, Tanzi RE, Tanila H, Haapasalo A, Hiltunen M
Neurobiology of disease 2016 Jan;85:187-205
Neurobiology of disease 2016 Jan;85:187-205
Cathepsin L Mediates the Degradation of Novel APP C-Terminal Fragments.
Wang H, Sang N, Zhang C, Raghupathi R, Tanzi RE, Saunders A
Biochemistry 2015 May 12;54(18):2806-16
Biochemistry 2015 May 12;54(18):2806-16
Elucidation of the BACE1 regulating factor GGA3 in Alzheimer's disease.
Natunen T, Parrado AR, Helisalmi S, Pursiheimo JP, Sarajärvi T, Mäkinen P, Kurkinen KM, Mullin K, Alafuzoff I, Haapasalo A, Bertram L, Soininen H, Tanzi RE, Hiltunen M
Journal of Alzheimer's disease : JAD 2013;37(1):217-32
Journal of Alzheimer's disease : JAD 2013;37(1):217-32
Involvement of receptor tyrosine kinase Tyro3 in amyloidogenic APP processing and β-amyloid deposition in Alzheimer's disease models.
Zheng Y, Wang Q, Xiao B, Lu Q, Wang Y, Wang X
PloS one 2012;7(6):e39035
PloS one 2012;7(6):e39035
Depletion of GGA1 and GGA3 mediates postinjury elevation of BACE1.
Walker KR, Kang EL, Whalen MJ, Shen Y, Tesco G
The Journal of neuroscience : the official journal of the Society for Neuroscience 2012 Jul 25;32(30):10423-37
The Journal of neuroscience : the official journal of the Society for Neuroscience 2012 Jul 25;32(30):10423-37
BACE1 protein endocytosis and trafficking are differentially regulated by ubiquitination at lysine 501 and the Di-leucine motif in the carboxyl terminus.
Kang EL, Biscaro B, Piazza F, Tesco G
The Journal of biological chemistry 2012 Dec 14;287(51):42867-80
The Journal of biological chemistry 2012 Dec 14;287(51):42867-80
Calsyntenin-1 shelters APP from proteolytic processing during anterograde axonal transport.
Steuble M, Diep TM, Schätzle P, Ludwig A, Tagaya M, Kunz B, Sonderegger P
Biology open 2012 Aug 15;1(8):761-74
Biology open 2012 Aug 15;1(8):761-74
Minocycline corrects early, pre-plaque neuroinflammation and inhibits BACE-1 in a transgenic model of Alzheimer's disease-like amyloid pathology.
Ferretti MT, Allard S, Partridge V, Ducatenzeiler A, Cuello AC
Journal of neuroinflammation 2012 Apr 2;9:62
Journal of neuroinflammation 2012 Apr 2;9:62
Development of a specific ELISA to measure BACE1 levels in human tissues.
Gonzales A, Decourt B, Walker A, Condjella R, Nural H, Sabbagh MN
Journal of neuroscience methods 2011 Oct 30;202(1):70-6
Journal of neuroscience methods 2011 Oct 30;202(1):70-6
The contribution of activated astrocytes to Aβ production: implications for Alzheimer's disease pathogenesis.
Zhao J, O'Connor T, Vassar R
Journal of neuroinflammation 2011 Nov 2;8:150
Journal of neuroinflammation 2011 Nov 2;8:150
Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer's disease models.
Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG
PloS one 2011 Jan 5;6(1):e15816
PloS one 2011 Jan 5;6(1):e15816
Ubiquitin regulates GGA3-mediated degradation of BACE1.
Kang EL, Cameron AN, Piazza F, Walker KR, Tesco G
The Journal of biological chemistry 2010 Jul 30;285(31):24108-19
The Journal of biological chemistry 2010 Jul 30;285(31):24108-19
Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in Alzheimer's mouse model.
Karuppagounder SS, Xu H, Shi Q, Chen LH, Pedrini S, Pechman D, Baker H, Beal MF, Gandy SE, Gibson GE
Neurobiology of aging 2009 Oct;30(10):1587-600
Neurobiology of aging 2009 Oct;30(10):1587-600
Focal cerebral ischemia in rats alters APP processing and expression of Abeta peptide degrading enzymes in the thalamus.
Hiltunen M, Mäkinen P, Peräniemi S, Sivenius J, van Groen T, Soininen H, Jolkkonen J
Neurobiology of disease 2009 Jul;35(1):103-13
Neurobiology of disease 2009 Jul;35(1):103-13
Down-regulation of seladin-1 increases BACE1 levels and activity through enhanced GGA3 depletion during apoptosis.
Sarajärvi T, Haapasalo A, Viswanathan J, Mäkinen P, Laitinen M, Soininen H, Hiltunen M
The Journal of biological chemistry 2009 Dec 4;284(49):34433-43
The Journal of biological chemistry 2009 Dec 4;284(49):34433-43
Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis.
O'Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, Eimer WA, Hitt B, Bembinster LA, Lammich S, Lichtenthaler SF, Hébert SS, De Strooper B, Haass C, Bennett DA, Vassar R
Neuron 2008 Dec 26;60(6):988-1009
Neuron 2008 Dec 26;60(6):988-1009
Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer's disease pathogenesis.
Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O'Connor T, Logan S, Maus E, Citron M, Berry R, Binder L, Vassar R
The Journal of neuroscience : the official journal of the Society for Neuroscience 2007 Apr 4;27(14):3639-49
The Journal of neuroscience : the official journal of the Society for Neuroscience 2007 Apr 4;27(14):3639-49
Ubiquilin 1 modulates amyloid precursor protein trafficking and Abeta secretion.
Hiltunen M, Lu A, Thomas AV, Romano DM, Kim M, Jones PB, Xie Z, Kounnas MZ, Wagner SL, Berezovska O, Hyman BT, Tesco G, Bertram L, Tanzi RE
The Journal of biological chemistry 2006 Oct 27;281(43):32240-53
The Journal of biological chemistry 2006 Oct 27;281(43):32240-53
Temporal memory deficits in Alzheimer's mouse models: rescue by genetic deletion of BACE1.
Ohno M, Chang L, Tseng W, Oakley H, Citron M, Klein WL, Vassar R, Disterhoft JF
The European journal of neuroscience 2006 Jan;23(1):251-60
The European journal of neuroscience 2006 Jan;23(1):251-60
BACE is degraded via the lysosomal pathway.
Koh YH, von Arnim CA, Hyman BT, Tanzi RE, Tesco G
The Journal of biological chemistry 2005 Sep 16;280(37):32499-504
The Journal of biological chemistry 2005 Sep 16;280(37):32499-504
Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer's disease pathogenesis.
Velliquette RA, O'Connor T, Vassar R
The Journal of neuroscience : the official journal of the Society for Neuroscience 2005 Nov 23;25(47):10874-83
The Journal of neuroscience : the official journal of the Society for Neuroscience 2005 Nov 23;25(47):10874-83
Statins cause intracellular accumulation of amyloid precursor protein, beta-secretase-cleaved fragments, and amyloid beta-peptide via an isoprenoid-dependent mechanism.
Cole SL, Grudzien A, Manhart IO, Kelly BL, Oakley H, Vassar R
The Journal of biological chemistry 2005 May 13;280(19):18755-70
The Journal of biological chemistry 2005 May 13;280(19):18755-70
Presenilin 1 stabilizes the C-terminal fragment of the amyloid precursor protein independently of gamma-secretase activity.
Pitsi D, Octave JN
The Journal of biological chemistry 2004 Jun 11;279(24):25333-8
The Journal of biological chemistry 2004 Jun 11;279(24):25333-8
The metabolism of beta-amyloid converting enzyme and beta-amyloid precursor protein processing.
Benjannet S, Cromlish JA, Diallo K, Chrétien M, Seidah NG
Biochemical and biophysical research communications 2004 Dec 3;325(1):235-42
Biochemical and biophysical research communications 2004 Dec 3;325(1):235-42
Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production.
Benjannet S, Elagoz A, Wickham L, Mamarbachi M, Munzer JS, Basak A, Lazure C, Cromlish JA, Sisodia S, Checler F, Chrétien M, Seidah NG
The Journal of biological chemistry 2001 Apr 6;276(14):10879-87
The Journal of biological chemistry 2001 Apr 6;276(14):10879-87
Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE.
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M
Science (New York, N.Y.) 1999 Oct 22;286(5440):735-41
Science (New York, N.Y.) 1999 Oct 22;286(5440):735-41
Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE.
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M
Science (New York, N.Y.) 1999 Oct 22;286(5440):735-41
Science (New York, N.Y.) 1999 Oct 22;286(5440):735-41
Identification of a novel aspartic protease (Asp 2) as beta-secretase.
Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, Smith TS, Simmons DL, Walsh FS, Dingwall C, Christie G
Molecular and cellular neurosciences 1999 Dec;14(6):419-27
Molecular and cellular neurosciences 1999 Dec;14(6):419-27
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
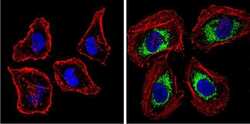
- Experimental details
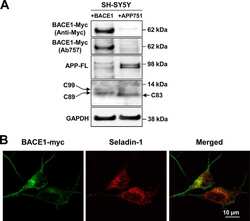
- Immunofluorescent analysis of BACE1 (green) showing staining in the Golgi apparatus of HeLa cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a BACE1 polyclonal antibody (Product # PA1-757) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
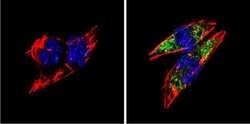
- Experimental details
- Immunofluorescent analysis of BACE1 (green) showing staining in the Golgi apparatus of Neuro-2a cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a BACE1 polyclonal antibody (Product # PA1-757) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
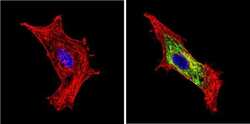
- Experimental details
- Immunofluorescent analysis of BACE1 (green) showing staining in the Golgi apparatus of SH-SY5Y cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a BACE1 polyclonal antibody (Product # PA1-757) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
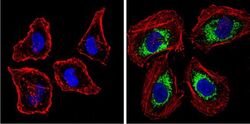
- Experimental details
- Immunofluorescent analysis of BACE1 (green) showing staining in the Golgi apparatus of HeLa cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a BACE1 polyclonal antibody (Product # PA1-757) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
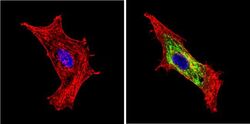
- Experimental details
- Immunofluorescent analysis of BACE1 (green) showing staining in the Golgi apparatus of Neuro-2a cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a BACE1 polyclonal antibody (Product # PA1-757) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of BACE1 (green) showing staining in the Golgi apparatus of SH-SY5Y cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a BACE1 polyclonal antibody (Product # PA1-757) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
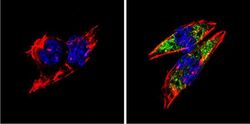
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
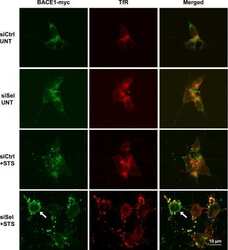
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
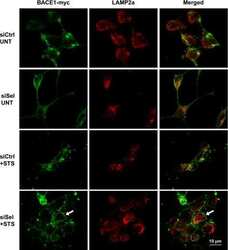
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
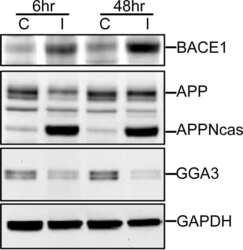
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
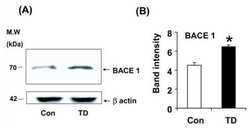
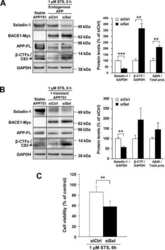
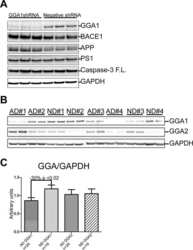
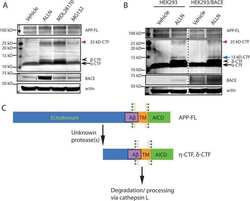
- Fig. 6. Downregulation of calsyntenin-1 by RNAi results in enhanced APP processing and increased secretion of Abeta. Cortical neurons were infected with rAAVs expressing a hairpin targeting calsyntenin-1 (shCst1) or a nonsense hairpin (shNS), together with eGFP. ( A ) Western blot of cell lysates. The signal indicated equal expression of the recombinant proteins. ( B ) Quantification of Western blots. Values indicate mean+-s.e.m. (*, P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
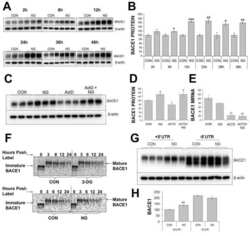
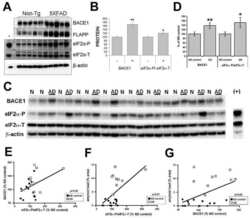
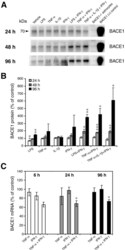
- Figure 2 Combinations of pro-inflammatory cytokines elevate endogenous BACE1 levels in mouse primary astrocyte cultures . (A, B) Cell lysates of cytokine-stimulated mouse primary astrocytes analyzed in Fig. 1A-C were analyzed for BACE1 by immunoblot. (A) BACE1 immunoblot images. The mature BACE1 band at 70 kDa is indicated by the arrowhead. Pro-inflammatory agents (alone and combinations) and stimulation times are indicated. Cell lysate from un-stimulated BACE1 -/- primary astrocytes was used as a negative control, while cell lysate from a stable BACE1-overexpressing HEK-293 cell line was used as a positive control. GFAP and beta-actin immunosignals were loading controls as in Fig 1A-C. (B) Histograms represent quantifications of BACE1 immunoblot signals in (A) expressed as percent of vehicle control. Note that cytokine combinations including TNF-alpha and IFN-gamma were generally more potent at increasing endogenous BACE1 levels in astrocytes over time in culture, raising BACE1 levels to ~600% of control. (C) mRNAs prepared from cytokine-stimulated primary astrocytes in Fig 1D were analyzed for endogenous BACE1 mRNA levels by TaqMan quantitative RT-PCR. Histograms represent quantifications of BACE1 mRNA levels expressed as percent of vehicle control. Note that astrocytic BACE1 mRNA levels were significantly reduced by TNF-alpha+IFN-gamma stimulation (C), even though BACE1 protein levels were increased several fold in similarly treated astrocytes (B). Statistical analysis was
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
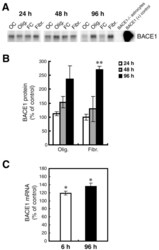
- Figure 7 Oligomeric and fibrillar Abeta42 increase levels of endogenous BACE1 in mouse primary astrocyte cultures . Lysates of C57BL/6J mouse primary astrocyte cultures treated with 10 muM oligomeric or fibrillar Abeta42 from Fig. 6 were prepared for BACE1 immunoblot (A, B) or mRNA quantification by TaqMan RT-PCR (C). Treatment times are indicated. Olig., Abeta42 oligomer treatment; Fibr., Abeta42 fibril treatment; OC, oligomer vehicle control; FC, fibril vehicle control. Cell lysate from un-treated BACE1 -/- primary astrocytes was used as a negative control, while cell lysate from a stable BACE1-overexpressing HEK-293 cell line was used as a positive control. GFAP immunosignals were loading controls as in Fig. 6A. Abeta42 oligomers and fibrils elevated BACE1 protein levels at 48 h and 96 h of treatment (A, B). BACE1 levels peaked at nearly 300% of control at 96 h that correlated with a rise in BACE1 mRNA level (C). Unlike APP, levels of both BACE1 protein and mRNA did not return to normal but remained elevated at 96 h. Asterisks indicate significant differences as compared to vehicle controls (*: p < 0.05; **: p < 0.01; n = 3). Error bars, SEM.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
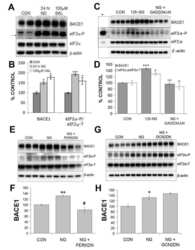
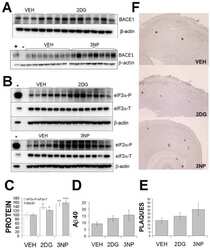
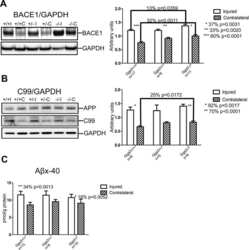
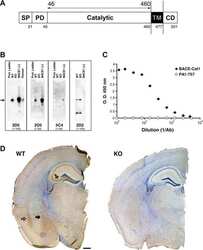
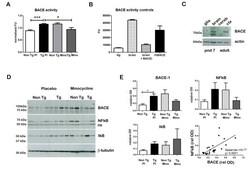
- Figure 3 Minocycline inhibits beta-site APP cleaving enzyme 1 in young, pre-plaque Tg mice . (A) BACE-1 activity was quantified from hippocampal homogenates using a well-characterized fluorometric assay. Fluorescent units (FU) data were normalized on Non Tg Placebo values. Note the significant up-regulation of BACE-1 activity in Tg Placebo compared to Non Tg Placebo (*** P < 0.001), which was corrected following minocycline treatment (* P < 0.05 compared to Tg Placebo). (B) Specificity of the BACE-1 fluorometric assay: note how the fluorescent units detected from brain homogenates (of a Non Tg Placebo animal) are seven times higher than the background (bg). The signal was completely abolished by co-incubation with a specific BACE inhibitor (BACEi), and was in the range of activity of a recombinant human BACE protein (rhBACE, 2.5 mug). All the data were analyzed with one-way ANOVA followed by Tukey post-hoc test. (C) Specificity of the polyclonal BACE-1 antibody used for western blotting: the specific band (close to 75 kDa) appeared in brain extracts from post natal day 7 (pnd 7) and from adult cortex (ctx), while it was hardly detectable in cerebellum (cereb) sample and was not detectable in a glial preparation from pups. (D) Representative western blots of BACE-1, NFkB and IkB from the same hippocampal samples used for the enzymatic assay (ns = non-specific band recognized by the polyclonal antibody directed against NFkB). (E) Quantification data of BACE-1, NFkB and IkB from
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
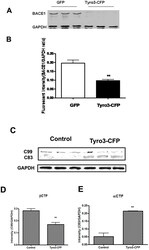
- Figure 3 BACE1 protein level and activity are down-regulated by Tyro3 overexpression in 293APPswe cells. (A, B) Western blot analysis of BACE1 showing that the protein levels of BACE1 in extracts from Tyro3-CFP transfected cells have lower fluorescent intensity than GFP controls. (C, D, E) Increase in C99 (betaCTF) protein level and decrease in C83 (alphaCTF) protein level in extracts from Tyro3-CFP transfected cells were detected by western blot analysis compared to that of GFP control. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
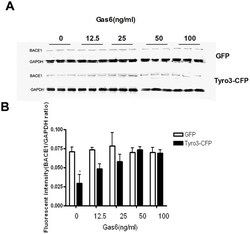
- Figure 4 Gas6 inhibits the decrease in BACE1 protein level induced by Tyro3 overexpression in 293APPswe cells. (A, B) Western blot statistical analysis showed that BACE1 levels were significantly lower in Tyro 3-CFP transfected cells than in GFP controls. Gas6 treatment was found to reverse this effect. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
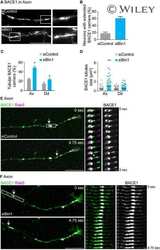
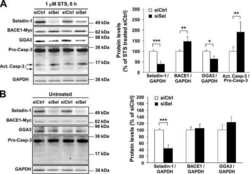
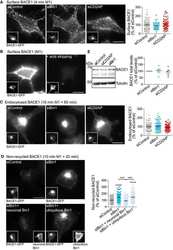
- Fig. 2. Calsyntenin-1/APP/BACE1 transport packages are found along axons and in growth cones. Immunofluorescence staining of dissociated hippocampal neurons. Black-and-white masks (a4-d4) were generated to emphasize the colocalization in axons ( A ) and growth cones ( B ). Inserts (a4-d4) show stainings of the axonal marker Tau-1. Note that BACE1 (a1-d1) colocalizes with calsyntenin-1 (a2,a3), APP (b2,b3), and Stx13 (c2,c3), but not with Rip11 (d2,d3). ( C ) Schematic representation of the C- and P-region of the growth cone. ( D ) Fractions of BACE1 puncta that are colocalized with calsyntenin-1, APP, syntaxin13, and Rip11 puncta. Note that BACE1 colocalizes with calsyntenin-1, APP, and syntaxin13 along axons and in the C-domain of growth cones. ( E ) Fractions of calsyntenin-1, APP, syntaxin13, and Rip11 puncta that are colocalized with BACE1 puncta. Note that the colocalization with BACE1 is confined to axons and the C-domain of growth cones. Values represent mean+-s.e.m. (*, P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
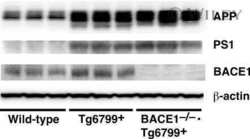
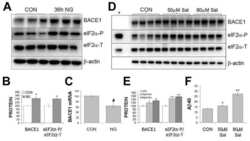
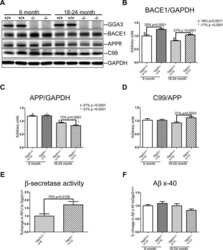
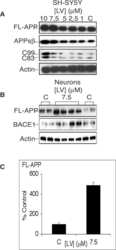
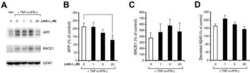
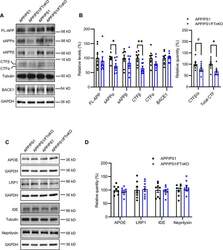
- Fig. 7 Reduced APP proteolytic processing in APP/PS1/FTnKO mice. A , B Immunoblots and densitometric analysis of full-length APP (FL-APP), and APP cleavage products, carboxyl-terminal fragments (CTFalpha and CTFbeta), amino-terminal fragments of APP (sAPPalpha and sAPPbeta), and beta-secretase 1 (BACE1). Protein levels were normalized by the amount of tubulin with the levels in the APP/PS1 control group set as 100%. C , D Immunoblots and densitometric analysis of proteins involved in Abeta clearance pathways including APOE, LRP1, IDE, and neprilysin. Protein levels were normalized by the amount of tubulin or GAPDH, and the levels in the control group set as 100%. n = 8/genotype; Student's t-test, 2-tailed; # p = 0.08; * p < 0.05
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
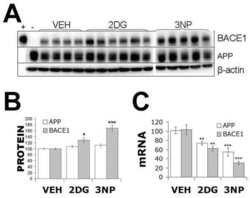
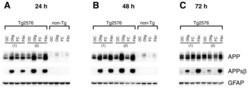
- Figure 8 Oligomeric and fibrillar Abeta42 increase levels of APPsbetasw in Tg2576 transgenic mouse primary astrocytes . Tg2576 or non-transgenic (non-Tg) littermate mouse primary astrocyte cultures were treated with 10 muM oligomeric or fibrillar Abeta42. Following treatment, cells were harvested for APP and APPsbetasw immunoblots. Treatment times are indicated. Olig., Abeta42 oligomer treatment; Fibr., Abeta42 fibril treatment; OC, oligomer vehicle control; FC, fibril vehicle control. The numbers in parentheses indicate primary astrocytes cultured from different Tg2576 mouse pups. Immunosignals identified by the anti-APP antibody 22C11 clearly demonstrated that Tg2576 astrocytes overexpress transgene-derived APPsw (A-C, upper row). Note that Tg2576 APPsw migrates faster than non-Tg astrocytic APP on SDS-PAGE because the Tg2576 transgene expresses the 695 amino acid form of APP, while endogenous APP expressed by astrocytes is the 751 amino acid form. Abeta42 oligomers and fibrils caused both transgenic APPsw and endogenous astrocytic APP to become elevated at 24 and 48 h (A, B). The APPsw increase largely returned to control levels by 72 h for Tg2576 astrocytes (C). Most importantly, oligomeric and fibrillar Abeta42 treatment dramatically induced beta-secretase processing of APPsw to generate robust levels of APPsbetasw in Tg2576 astrocytes (A-C, center row), as indicated by immunosignals produced by an antibody that recognizes the BACE1-cleaved C-terminal neo-epitope of A
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
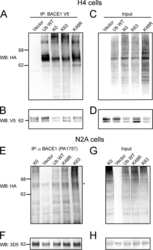
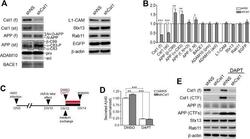
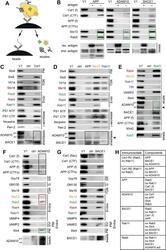
- Fig. 1. Calsyntenin-1/APP vesicles contain BACE1, but no ADAM10. ( A ) Schematic representation of vesicle immunoisolation. Vesicle immunoisolations were performed with antibodies against cytosolic epitopes of vesicle-associated proteins and Dynabeads coated with protein A. Captured vesicles were isolated and washed. Vesicular proteins were eluted by lysis of the captured vesicles with a mild detergent. Note that the vesicular component bearing the antigen targeted for immunoisolation is not or only partially found in the eluate, because of its strong binding to the antibody/protein A/bead complex. Its complete elution required harsher conditions, e.g. boiling of the beads in SDS. Therefore, the antigen used for vesicle immunoisolation is not shown on the Western blots of the (mild) eluates. Examples of antigen elutions under harsh condition from the beads are shown in the panels labeled beads. ( B ) Specificity tests of anti-APP, anti-ADAM10, and anti-BACE1 antibodies, performed by the addition of ~25 ug of recombinant antigen (GST-APPcyto, GST-ADAM10cyto, BACE1[485-501]). Saturation with recombinant antigen prevented vesicle capture. ( C ) Calsyntenin-1 organelles contained BACE1, but no ADAM10 (black box). ( D ) APP and syntaxin13 immunoisolates contain a low level of active ADAM10 and abundant BACE1. Neither BACE1 nor ADAM10 were found in Rab11 immunoisolates. ( E ) Rab5 immunoisolates contain a small amount of active ADAM10 (arrowhead) and abundant BACE1 (green box). ( F
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
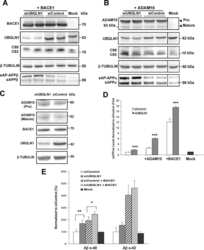
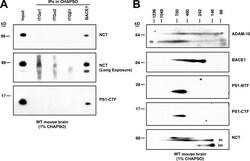
- Figure 1. The beta-secretase BACE1 interacts with gamma-secretase at endogenous levels in WT mouse brain microsomal lysate. (A) 1% CHAPSO-solubilized lysates of WT mouse brain microsomes were immunoprecipitated for BACE1 or ITGalpha1, ITGalpha2, and ITGbeta1 as controls. IPs were blotted to probe for coIP of the gamma-components NCT and PS1-NTF. (B) 1% CHAPSO-solubilized lysates of WT mouse brain microsomes were loaded onto BN-PAGE followed by second-dimension SDS-PAGE and blotted with antibodies to the indicated proteins. *, nonspecific signal; m and im, mature and immature forms.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
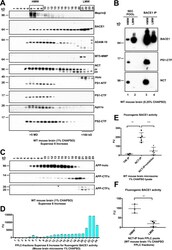
- Figure 2. BACE1 exists with gamma-secretase in an HMW complex isolated from mouse brain by FPLC and has proteolytic activity. (A) 1% CHAPSO-solubilized WT mouse brain microsomes were fractionated on a Superose 6 Increase SEC column by FPLC and blotted with antibodies to the indicated proteins. (B) FPLC fractions were pooled into HMW (6-9) or LMW (21-24) fractions and immunoprecipitated for BACE1 and then blotted with antibodies to the indicated proteins. Note that the amount of BACE1 that was immunoprecipitated varies and may not necessarily reflect the amount in the input. (C) FPLC fractions were blotted with certain antibodies to APP (top two panels, C7; bottom panel, 1G6). (D) FPLC fractions were incubated with a fluorogenic BACE1 peptide substrate. After incubation for 2 h at 37degC, fluorescence was read as BACE1 activity ( n = 3; error bars are SD). (E) 0.25% CHAPSO-solubilized WT mouse brain microsomes were immunoprecipitated for gamma-secretase (anti-NCT) in the absence or presence of two different beta-secretase inhibitors (AZD3293 and inhibitor IV, each at 10 uM), or else anti-TfR as a control. beta-Secretase activity assays were performed on the eluates extracted from the IP resin. n = 5 for the NCT IP, n = 2 for the TfR IP; error bars are SD. Unpaired Student's t test: **, P < 0.01; ***, P < 0.001. (F) Pooled HMW and LMW FPLC fractions from WT mouse brains were immunoprecipitated for gamma-secretase (anti-NCT). beta-Secretase activity assays were performed on the
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry