Antibody data
- Antibody Data
- Antigen structure
- References [8]
- Comments [0]
- Validations
- Other assay [9]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 35-7600 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- PKP3 Monoclonal Antibody (23E3/4)
- Antibody type
- Monoclonal
- Antigen
- Synthetic peptide
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 23E3/4
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- -20°C
Submitted references Proteomic characterization of GSK3β knockout shows altered cell adhesion and metabolic pathway utilisation in colorectal cancer cells.
Increased Cholesterol Biosynthesis Is a Key Characteristic of Breast Cancer Stem Cells Influencing Patient Outcome.
MMP7 is required to mediate cell invasion and tumor formation upon Plakophilin3 loss.
Plakophilin3 loss leads to an increase in PRL3 levels promoting K8 dephosphorylation, which is required for transformation and metastasis.
Unimpaired skin carcinogenesis in Desmoglein 3 knockout mice.
E-cadherin and plakoglobin recruit plakophilin3 to the cell border to initiate desmosome assembly.
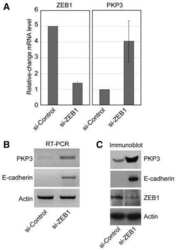
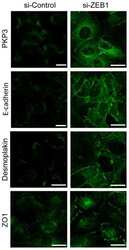
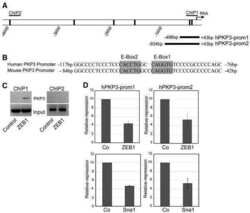
The transcription factor ZEB1 (deltaEF1) represses Plakophilin 3 during human cancer progression.
Plakophilin 3 oncogene as prognostic marker and therapeutic target for lung cancer.
Bowler-Barnett E, Martinez-Garcia FD, Sherwood M, Aleidan A, John S, Weston S, Wang Y, Divecha N, Skipp P, Ewing RM
PloS one 2021;16(11):e0246707
PloS one 2021;16(11):e0246707
Increased Cholesterol Biosynthesis Is a Key Characteristic of Breast Cancer Stem Cells Influencing Patient Outcome.
Ehmsen S, Pedersen MH, Wang G, Terp MG, Arslanagic A, Hood BL, Conrads TP, Leth-Larsen R, Ditzel HJ
Cell reports 2019 Jun 25;27(13):3927-3938.e6
Cell reports 2019 Jun 25;27(13):3927-3938.e6
MMP7 is required to mediate cell invasion and tumor formation upon Plakophilin3 loss.
Basu S, Thorat R, Dalal SN
PloS one 2015;10(4):e0123979
PloS one 2015;10(4):e0123979
Plakophilin3 loss leads to an increase in PRL3 levels promoting K8 dephosphorylation, which is required for transformation and metastasis.
Khapare N, Kundu ST, Sehgal L, Sawant M, Priya R, Gosavi P, Gupta N, Alam H, Karkhanis M, Naik N, Vaidya MM, Dalal SN
PloS one 2012;7(6):e38561
PloS one 2012;7(6):e38561
Unimpaired skin carcinogenesis in Desmoglein 3 knockout mice.
Baron S, Hoang A, Vogel H, Attardi LD
PloS one 2012;7(11):e50024
PloS one 2012;7(11):e50024
E-cadherin and plakoglobin recruit plakophilin3 to the cell border to initiate desmosome assembly.
Gosavi P, Kundu ST, Khapare N, Sehgal L, Karkhanis MS, Dalal SN
Cellular and molecular life sciences : CMLS 2011 Apr;68(8):1439-54
Cellular and molecular life sciences : CMLS 2011 Apr;68(8):1439-54
The transcription factor ZEB1 (deltaEF1) represses Plakophilin 3 during human cancer progression.
Aigner K, Descovich L, Mikula M, Sultan A, Dampier B, Bonné S, van Roy F, Mikulits W, Schreiber M, Brabletz T, Sommergruber W, Schweifer N, Wernitznig A, Beug H, Foisner R, Eger A
FEBS letters 2007 Apr 17;581(8):1617-24
FEBS letters 2007 Apr 17;581(8):1617-24
Plakophilin 3 oncogene as prognostic marker and therapeutic target for lung cancer.
Furukawa C, Daigo Y, Ishikawa N, Kato T, Ito T, Tsuchiya E, Sone S, Nakamura Y
Cancer research 2005 Aug 15;65(16):7102-10
Cancer research 2005 Aug 15;65(16):7102-10
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
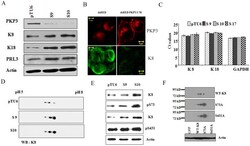
- Figure 1 High K8 levels in the PKP3 knockdown clones are due to a decrease in phosphorylation. A. Protein extracts from the vector control (pTU6) and PKP3 knockdown clones (S9 and S10) were resolved on SDSPAGE gels and Western blots performed with the indicated antibodies. B. S9 cells were transfected with either dsRed or the shRNA resistant dsRed PKP3 3.7R cDNA. 48 hours post transfection cells were stained with antibodies to K8 (green) and visualized by confocal microscopy. Note that dsRed PKP3 3.7R localizes to the border as previously described (indicated by arrow) [28] . Original magnification is 630X with a 2X optical zoom. Scale bar 5 um. C. A real time PCR analysis to determine the mRNA levels of K8 and K18 was performed on RNA isolated from the vector control and PKP3 knockdown clones. GAPDH was used as an internal control for normalization. The Ct values for all samples are shown on the Y-axis. D. Protein extracts from the PKP3 knockdown clones or the vector control were subjected to 2-dimensional gel electrophoresis and Western blots performed with antibodies to K8. E. Protein extracts from the vector control cells or the PKP3 knockdown clones were resolved on SDS-PAGE gels followed by Western blotting with antibodies to K8 or phosphospecific antibodies against S73 (alpha-S73), S431 (alpha-S431) and actin. F. HCT116 cells transfected with GFPK8 or GFPS73A or GFP S431A were resolved on two dimensional gels followed by Western blots with antibodies to K8. MW markers
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
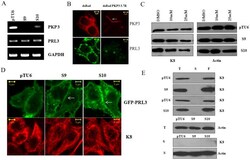
- Figure 2 Plakophilin3 loss leads to an increase in PRL3 levels. A. RNA prepared from the vector control or PKP3 knockdown clones was used as a template in reverse transcriptase coupled PCR reactions to determine the mRNA levels of PRL3 and PKP3. A PCR for GAPDH served as a loading control. B. S9 cells were transfected with either dsRed or the shRNA resistant dsRed PKP3 3.7R cDNA. 48 hours post transfection cells were stained with antibodies to PRL3 (green) and visualized by confocal microscopy. Note that dsRed PKP3 3.7R localizes to the border as previously described (indicated by arrow) [28] . Original magnification is 630X with a 2X optical zoom. Scale bar 5 um. C. The vector control or PKP3 knockdown clones were treated with either the vehicle control (DMSO) or the indicated concentrations of the PRL3 inhibitor. Protein extracts were resolved on gels followed by Western blotting with antibodies to K8 and beta-actin. D. GFP PRL3 was transfected into either vector control (pTU6) or PKP3 knockdown clones (S9 and S10). 48 hours post transfection, the cells were stained with antibodies to K8 (red) and visualized by confocal microscopy. Note that GFP PRL3 shows a marginally enhanced localization to the border in S9 and S10 cells in contrast to pTU6 and doesn't show increased localization on K8 filaments. Original magnification is 630X with a 2X optical zoom. Scale bar 5 um. E. Total cell extracts (T), Soluble fractions (S) and the filament fractions (F) were prepared as des
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
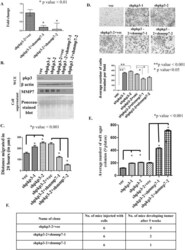
- Fig 2 Loss of MMP7 leads to a decrease in transformation in cells lacking PKP3. (A) mRNA prepared from HCT116 derived PKP3 knockdown cells transfected with the vector control (shpkp3-2 + vec) or the MMP7 knockdown construct (shpkp3-2 + shMMP7-1 and shpkp3-2 + MMP7-2) was used as a substrate for reverse transcriptase followed by real time PCR reactions using oligonucleotides specific for MMP7. All expression was normalized to the levels of GAPDH. The fold change is graphed on the Y-axis and the clone name is on the X-axis. Note that MMP7 levels are lowered in the double knockdown clones as compared to the vector control. The standard errors are plotted and student's t test was performed (* indicates a p value
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
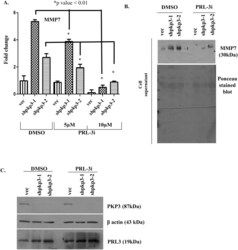
- Fig 3 MMP7 expression decreases upon inhibition of PRL3 activity. (A) The HCT116 derived PKP3 knockdown clones (shpkp3-1 and shpkp3-2) or the vector control (vec) were treated with either the vehicle control (DMSO) or 5 or 10 muM PRL-3 inhibitor-1(PRL-3i) for 24 hours. The mRNA prepared from the treated cells was used as a substrate for reverse transcriptase followed by real time PCR reactions using oligonucleotides specific for MMP7. All expression was normalized to the levels of GAPDH. The fold change is graphed on the Y-axis and the clone name is on the X-axis. The standard errors are plotted and student's t test was performed. Note that MMP7 levels are lowered upon treatment with PRL-3 inhibitor. (B) The HCT116 derived vector control (vec) and PKP3 knockdown clones (shpkp3-1 and shpkp3-2) were treated with either DMSO or 10 muM PRL3 inhibitor-1(PRL3i) for 24 hours or 48 hours. The cell supernatants were collected and a100mug of acetone precipitated protein was resolved on 12% SDS PAGE gels followed by Western blotting with antibodies to MMP7. The same blot was stained with Ponceau stain to indicate equal loading. (C) The whole cell lysates of HCT116 derived vector control and plakophilin3 knockdown clones treated with DMSO or PRL3i for 24 hours were resolved on 12% poly-acrylamide gel. This was followed by Western blotting with antibodies to PKP3, beta actin and PRL3. The molecular weights of these proteins are indicated in brackets.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
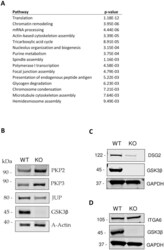
- 10.1371/journal.pone.0246707.g003 Fig 3 A. Enriched pathways represented in differentially abundant proteins. Enriched pathways were identified using Pathway Studio from the set of differential proteins (p
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Other assay
Other assay