Antibody data
- Antibody Data
- Antigen structure
- References [76]
- Comments [0]
- Validations
- Immunocytochemistry [4]
- Other assay [50]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 34-9100 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Claudin 7 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Reactivity
- Human, Mouse, Canine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 0.25 mg/mL
- Storage
- -20°C
Submitted references MarvelD3 Is Upregulated in Ulcerative Colitis and Has Attenuating Effects during Colitis Indirectly Stabilizing the Intestinal Barrier.
The dual GLP-1 and GLP-2 receptor agonist dapiglutide promotes barrier function in murine short bowel.
Organic osmolytes increase expression of specific tight junction proteins in skin and alter barrier function in keratinocytes.
Local and downstream actions of proximal tubule angiotensin II signaling on Na(+) transporters in the mouse nephron.
Citrus limon Peel Powder Reduces Intestinal Barrier Defects and Inflammation in a Colitic Murine Experimental Model.
APC regulation of ESRP1 and p120-catenin isoforms in colorectal cancer cells.
Tumor Necrosis Factor Alpha Effects on the Porcine Intestinal Epithelial Barrier Include Enhanced Expression of TNF Receptor 1.
Amyloid precursor-like protein 2 interacts with claudin-7 and affects ovarian cancer cell survival.
Mucin-2 knockout is a model of intercellular junction defects, mitochondrial damage and ATP depletion in the intestinal epithelium.
Adverse Effects of Coumestrol and Genistein on Mammary Morphogenesis and Future Milk Production Ability of Mammary Epithelial Cells.
Matriptase Cleaves EpCAM and TROP2 in Keratinocytes, Destabilizing Both Proteins and Associated Claudins.
Deficiency of Stomach-Type Claudin-18 in Mice Induces Gastric Tumor Formation Independent of H pylori Infection.
Syncytiotrophoblast of Placentae from Women with Zika Virus Infection Has Altered Tight Junction Protein Expression and Increased Paracellular Permeability.
Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity.
Probiotics Prevents Sensitization to Oral Antigen and Subsequent Increases in Intestinal Tight Junction Permeability in Juvenile-Young Adult Rats.
In vitro and in vivo effects of a mycotoxin, deoxynivalenol, and a trace metal, cadmium, alone or in a mixture on the intestinal barrier.
Tracking adiponectin biodistribution via fluorescence molecular tomography indicates increased vascular permeability after streptozotocin-induced diabetes.
Association of Cytokeratin 5 and Claudin 3 expression with BRCA1 and BRCA2 germline mutations in women with early breast cancer.
Early life nutrition influences susceptibility to chronic inflammatory colitis in later life.
Vitamin D Receptor Deletion Leads to the Destruction of Tight and Adherens Junctions in Lungs.
Regulation of epithelial migration by epithelial cell adhesion molecule requires its Claudin-7 interaction domain.
TGF-β1 alters esophageal epithelial barrier function by attenuation of claudin-7 in eosinophilic esophagitis.
Altered Cytokine Expression and Barrier Properties after In Vitro Infection of Porcine Epithelial Cells with Enterotoxigenic Escherichia coli and Probiotic Enterococcus faecium.
Claudin-4 knockout by TALEN-mediated gene targeting in MDCK cells: Claudin-4 is dispensable for the permeability properties of tight junctions in wild-type MDCK cells.
Contractile forces at tricellular contacts modulate epithelial organization and monolayer integrity.
Claudin-6 enhances cell invasiveness through claudin-1 in AGS human adenocarcinoma gastric cancer cells.
Matriptase-mediated cleavage of EpCAM destabilizes claudins and dysregulates intestinal epithelial homeostasis.
Downregulation of lipolysis-stimulated lipoprotein receptor promotes cell invasion via claudin-1-mediated matrix metalloproteinases in human endometrial cancer.
Region Specific Differences of Claudin-5 Expression in Pediatric Intracranial Ependymomas: Potential Prognostic Role in Supratentorial Cases.
HOXA5 determines cell fate transition and impedes tumor initiation and progression in breast cancer through regulation of E-cadherin and CD24.
A microwave antigen retrieval method using two heating steps for enhanced immunostaining on aldehyde-fixed paraffin-embedded tissue sections.
SPROUTY-2 represses the epithelial phenotype of colon carcinoma cells via upregulation of ZEB1 mediated by ETS1 and miR-200/miR-150.
Salmonella enteritidis Effector AvrA Stabilizes Intestinal Tight Junctions via the JNK Pathway.
Connexins, E-cadherin, Claudin-7 and β-catenin transiently form junctional nexuses during the post-natal mammary gland development.
Claudin-2 knockout by TALEN-mediated gene targeting in MDCK cells: claudin-2 independently determines the leaky property of tight junctions in MDCK cells.
Autocrine CSF1R signaling mediates switching between invasion and proliferation downstream of TGFβ in claudin-low breast tumor cells.
ZO-1 knockout by TALEN-mediated gene targeting in MDCK cells: involvement of ZO-1 in the regulation of cytoskeleton and cell shape.
Disrupted tight junctions in the small intestine of cystic fibrosis mice.
Claudins 1, 2, 3, 4, 5 and 7 in solar keratosis and squamocellular carcinoma of the skin.
Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis.
Claudin 1 expression characterizes human uterine cervical reserve cells.
EpCAM contributes to formation of functional tight junction in the intestinal epithelium by recruiting claudin proteins.
Cingulin is dispensable for epithelial barrier function and tight junction structure, and plays a role in the control of claudin-2 expression and response to duodenal mucosa injury.
Prostaglandin-induced cervical remodelling in humans in the first trimester is associated with increased expression of specific tight junction, but not gap junction proteins.
Functional characterization and localization of a gill-specific claudin isoform in Atlantic salmon.
Transforming growth factor beta 1 induces tight junction disruptions and loss of transepithelial resistance across porcine vas deferens epithelial cells.
Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function.
Enhanced immunohistochemical resolution of claudin proteins in glycolmethacrylate-embedded tissue biopsies.
[The clinical significance of Claudin-7 and slug expression in lung squamous cell carcinoma and adenocarcinoma].
The level of claudin-7 is reduced as an early event in colorectal carcinogenesis.
Identification of a claudin-4 and E-cadherin score to predict prognosis in breast cancer.
beta-catenin expression and claudin expression pattern as prognostic factors of prostatic cancer progression.
Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells.
Heterogeneity for stem cell-related markers according to tumor subtype and histologic stage in breast cancer.
Novel snail1 target proteins in human colon cancer identified by proteomic analysis.
Claudin-1, -2, -3, -4, -7, -8, and -10 protein expression in biliary tract cancers.
Hepatocyte nuclear factor 4alpha, a key factor for homeostasis, cell architecture, and barrier function of the adult intestinal epithelium.
Altered gene expression in breast cancer liver metastases.
Inducible overexpression of cingulin in stably transfected MDCK cells does not affect tight junction organization and gene expression.
Differentiation potential of urothelium from patients with benign bladder dysfunction.
Differences in claudin synthesis in primary cultures of acinar cells from rat salivary gland are correlated with the specific three-dimensional organization of the cells.
Loss of claudins-1 and -7 and expression of claudins-3 and -4 correlate with prognostic variables in prostatic adenocarcinomas.
The tight junction proteins claudin-7 and -8 display a different subcellular localization at Henle's loops and collecting ducts of rabbit kidney.
Epithelial barrier characteristics and expression of cell adhesion molecules in proximal tubule-derived cell lines commonly used for in vitro toxicity studies.
Claudin 1 differentiates endometrioid and serous papillary endometrial adenocarcinoma.
Endothelia of term human placentae display diminished expression of tight junction proteins during preeclampsia.
Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase RhoA.
Changes of cell adhesion and extracellular matrix (ECM) components in cervical intraepithelial neoplasia.
Inducible expression of Snail selectively increases paracellular ion permeability and differentially modulates tight junction proteins.
Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells.
Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells.
Expression of claudin-7 and -8 along the mouse nephron.
Expression and function of tight junctions in the crypt epithelium of human palatine tonsils.
Effect of formalin, acetone, and RNAlater fixatives on tissue preservation and different size amplicons by real-time PCR from paraffin-embedded tissue.
Effect of formalin, acetone, and RNAlater fixatives on tissue preservation and different size amplicons by real-time PCR from paraffin-embedded tissue.
Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast.
Weiß F, Czichos C, Knobe L, Voges L, Bojarski C, Michel G, Fromm M, Krug SM
Cells 2022 May 4;11(9)
Cells 2022 May 4;11(9)
The dual GLP-1 and GLP-2 receptor agonist dapiglutide promotes barrier function in murine short bowel.
Reiner J, Thiery J, Held J, Berlin P, Skarbaliene J, Vollmar B, Jaster R, Eriksson PO, Lamprecht G, Witte M
Annals of the New York Academy of Sciences 2022 Aug;1514(1):132-141
Annals of the New York Academy of Sciences 2022 Aug;1514(1):132-141
Organic osmolytes increase expression of specific tight junction proteins in skin and alter barrier function in keratinocytes.
El-Chami C, Foster AR, Johnson C, Clausen RP, Cornwell P, Haslam IS, Steward MC, Watson REB, Young HS, O'Neill CA
The British journal of dermatology 2021 Mar;184(3):482-494
The British journal of dermatology 2021 Mar;184(3):482-494
Local and downstream actions of proximal tubule angiotensin II signaling on Na(+) transporters in the mouse nephron.
Nelson JW, McDonough AA, Xiang Z, Ralph DL, Robertson JA, Giani JF, Bernstein KE, Gurley SB
American journal of physiology. Renal physiology 2021 Jul 1;321(1):F69-F81
American journal of physiology. Renal physiology 2021 Jul 1;321(1):F69-F81
Citrus limon Peel Powder Reduces Intestinal Barrier Defects and Inflammation in a Colitic Murine Experimental Model.
Tinh NTT, Sitolo GC, Yamamoto Y, Suzuki T
Foods (Basel, Switzerland) 2021 Jan 25;10(2)
Foods (Basel, Switzerland) 2021 Jan 25;10(2)
APC regulation of ESRP1 and p120-catenin isoforms in colorectal cancer cells.
Faux MC, King LE, Kane SR, Love C, Sieber OM, Burgess AW
Molecular biology of the cell 2021 Jan 15;32(2):120-130
Molecular biology of the cell 2021 Jan 15;32(2):120-130
Tumor Necrosis Factor Alpha Effects on the Porcine Intestinal Epithelial Barrier Include Enhanced Expression of TNF Receptor 1.
Droessler L, Cornelius V, Markov AG, Amasheh S
International journal of molecular sciences 2021 Aug 14;22(16)
International journal of molecular sciences 2021 Aug 14;22(16)
Amyloid precursor-like protein 2 interacts with claudin-7 and affects ovarian cancer cell survival.
Dahiya N
Future science OA 2020 Mar 16;6(4):FSO457
Future science OA 2020 Mar 16;6(4):FSO457
Mucin-2 knockout is a model of intercellular junction defects, mitochondrial damage and ATP depletion in the intestinal epithelium.
Borisova MA, Achasova KM, Morozova KN, Andreyeva EN, Litvinova EA, Ogienko AA, Morozova MV, Berkaeva MB, Kiseleva E, Kozhevnikova EN
Scientific reports 2020 Dec 3;10(1):21135
Scientific reports 2020 Dec 3;10(1):21135
Adverse Effects of Coumestrol and Genistein on Mammary Morphogenesis and Future Milk Production Ability of Mammary Epithelial Cells.
Kumai A, Tsugami Y, Wakasa H, Suzuki N, Suzuki T, Nishimura T, Kobayashi K
Advanced biosystems 2020 Apr;4(4):e1900187
Advanced biosystems 2020 Apr;4(4):e1900187
Matriptase Cleaves EpCAM and TROP2 in Keratinocytes, Destabilizing Both Proteins and Associated Claudins.
Wu CJ, Lu M, Feng X, Nakato G, Udey MC
Cells 2020 Apr 21;9(4)
Cells 2020 Apr 21;9(4)
Deficiency of Stomach-Type Claudin-18 in Mice Induces Gastric Tumor Formation Independent of H pylori Infection.
Suzuki K, Sentani K, Tanaka H, Yano T, Suzuki K, Oshima M, Yasui W, Tamura A, Tsukita S
Cellular and molecular gastroenterology and hepatology 2019;8(1):119-142
Cellular and molecular gastroenterology and hepatology 2019;8(1):119-142
Syncytiotrophoblast of Placentae from Women with Zika Virus Infection Has Altered Tight Junction Protein Expression and Increased Paracellular Permeability.
Miranda J, Martín-Tapia D, Valdespino-Vázquez Y, Alarcón L, Espejel-Nuñez A, Guzmán-Huerta M, Muñoz-Medina JE, Shibayama M, Chávez-Munguía B, Estrada-Gutiérrez G, Lievano S, Ludert JE, González-Mariscal L
Cells 2019 Sep 29;8(10)
Cells 2019 Sep 29;8(10)
Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity.
Otani T, Nguyen TP, Tokuda S, Sugihara K, Sugawara T, Furuse K, Miura T, Ebnet K, Furuse M
The Journal of cell biology 2019 Oct 7;218(10):3372-3396
The Journal of cell biology 2019 Oct 7;218(10):3372-3396
Probiotics Prevents Sensitization to Oral Antigen and Subsequent Increases in Intestinal Tight Junction Permeability in Juvenile-Young Adult Rats.
Tulyeu J, Kumagai H, Jimbo E, Watanabe S, Yokoyama K, Cui L, Osaka H, Mieno M, Yamagata T
Microorganisms 2019 Oct 16;7(10)
Microorganisms 2019 Oct 16;7(10)
In vitro and in vivo effects of a mycotoxin, deoxynivalenol, and a trace metal, cadmium, alone or in a mixture on the intestinal barrier.
Luo S, Terciolo C, Bracarense APFL, Payros D, Pinton P, Oswald IP
Environment international 2019 Nov;132:105082
Environment international 2019 Nov;132:105082
Tracking adiponectin biodistribution via fluorescence molecular tomography indicates increased vascular permeability after streptozotocin-induced diabetes.
Yoon N, Dadson K, Dang T, Chu T, Noskovicova N, Hinz B, Raignault A, Thorin E, Kim S, Jeon JS, Jonkman J, McKee TD, Grant J, Peterson JD, Kelly SP, Sweeney G
American journal of physiology. Endocrinology and metabolism 2019 Nov 1;317(5):E760-E772
American journal of physiology. Endocrinology and metabolism 2019 Nov 1;317(5):E760-E772
Association of Cytokeratin 5 and Claudin 3 expression with BRCA1 and BRCA2 germline mutations in women with early breast cancer.
Danzinger S, Tan YY, Rudas M, Kastner MT, Weingartshofer S, Muhr D, Singer CF
BMC cancer 2019 Jul 15;19(1):695
BMC cancer 2019 Jul 15;19(1):695
Early life nutrition influences susceptibility to chronic inflammatory colitis in later life.
Ley D, Desseyn JL, Gouyer V, Plet S, Tims S, Renes I, Mischke M, Gottrand F
Scientific reports 2019 Dec 2;9(1):18111
Scientific reports 2019 Dec 2;9(1):18111
Vitamin D Receptor Deletion Leads to the Destruction of Tight and Adherens Junctions in Lungs.
Chen H, Lu R, Zhang YG, Sun J
Tissue barriers 2018;6(4):1-13
Tissue barriers 2018;6(4):1-13
Regulation of epithelial migration by epithelial cell adhesion molecule requires its Claudin-7 interaction domain.
Barth AIM, Kim H, Riedel-Kruse IH
PloS one 2018;13(10):e0204957
PloS one 2018;13(10):e0204957
TGF-β1 alters esophageal epithelial barrier function by attenuation of claudin-7 in eosinophilic esophagitis.
Nguyen N, Fernando SD, Biette KA, Hammer JA, Capocelli KE, Kitzenberg DA, Glover LE, Colgan SP, Furuta GT, Masterson JC
Mucosal immunology 2018 Mar;11(2):415-426
Mucosal immunology 2018 Mar;11(2):415-426
Altered Cytokine Expression and Barrier Properties after In Vitro Infection of Porcine Epithelial Cells with Enterotoxigenic Escherichia coli and Probiotic Enterococcus faecium.
Kern M, Günzel D, Aschenbach JR, Tedin K, Bondzio A, Lodemann U
Mediators of inflammation 2017;2017:2748192
Mediators of inflammation 2017;2017:2748192
Claudin-4 knockout by TALEN-mediated gene targeting in MDCK cells: Claudin-4 is dispensable for the permeability properties of tight junctions in wild-type MDCK cells.
Tokuda S, Hirai T, Furuse M
PloS one 2017;12(8):e0182521
PloS one 2017;12(8):e0182521
Contractile forces at tricellular contacts modulate epithelial organization and monolayer integrity.
Salomon J, Gaston C, Magescas J, Duvauchelle B, Canioni D, Sengmanivong L, Mayeux A, Michaux G, Campeotto F, Lemale J, Viala J, Poirier F, Minc N, Schmitz J, Brousse N, Ladoux B, Goulet O, Delacour D
Nature communications 2017 Jan 13;8:13998
Nature communications 2017 Jan 13;8:13998
Claudin-6 enhances cell invasiveness through claudin-1 in AGS human adenocarcinoma gastric cancer cells.
Torres-Martínez AC, Gallardo-Vera JF, Lara-Holguin AN, Montaño LF, Rendón-Huerta EP
Experimental cell research 2017 Jan 1;350(1):226-235
Experimental cell research 2017 Jan 1;350(1):226-235
Matriptase-mediated cleavage of EpCAM destabilizes claudins and dysregulates intestinal epithelial homeostasis.
Wu CJ, Feng X, Lu M, Morimura S, Udey MC
The Journal of clinical investigation 2017 Feb 1;127(2):623-634
The Journal of clinical investigation 2017 Feb 1;127(2):623-634
Downregulation of lipolysis-stimulated lipoprotein receptor promotes cell invasion via claudin-1-mediated matrix metalloproteinases in human endometrial cancer.
Shimada H, Satohisa S, Kohno T, Konno T, Takano KI, Takahashi S, Hatakeyama T, Arimoto C, Saito T, Kojima T
Oncology letters 2017 Dec;14(6):6776-6782
Oncology letters 2017 Dec;14(6):6776-6782
Region Specific Differences of Claudin-5 Expression in Pediatric Intracranial Ependymomas: Potential Prognostic Role in Supratentorial Cases.
Virág J, Haberler C, Baksa G, Piurkó V, Hegedüs Z, Reiniger L, Bálint K, Chocholous M, Kiss A, Lotz G, Glasz T, Schaff Z, Garami M, Hegedűs B
Pathology oncology research : POR 2017 Apr;23(2):245-252
Pathology oncology research : POR 2017 Apr;23(2):245-252
HOXA5 determines cell fate transition and impedes tumor initiation and progression in breast cancer through regulation of E-cadherin and CD24.
Teo WW, Merino VF, Cho S, Korangath P, Liang X, Wu RC, Neumann NM, Ewald AJ, Sukumar S
Oncogene 2016 Oct 20;35(42):5539-5551
Oncogene 2016 Oct 20;35(42):5539-5551
A microwave antigen retrieval method using two heating steps for enhanced immunostaining on aldehyde-fixed paraffin-embedded tissue sections.
Gu L, Cong J, Zhang J, Tian YY, Zhai XY
Histochemistry and cell biology 2016 Jun;145(6):675-80
Histochemistry and cell biology 2016 Jun;145(6):675-80
SPROUTY-2 represses the epithelial phenotype of colon carcinoma cells via upregulation of ZEB1 mediated by ETS1 and miR-200/miR-150.
Barbáchano A, Fernández-Barral A, Pereira F, Segura MF, Ordóñez-Morán P, Carrillo-de Santa Pau E, González-Sancho JM, Hanniford D, Martínez N, Costales-Carrera A, Real FX, Pálmer HG, Rojas JM, Hernando E, Muñoz A
Oncogene 2016 Jun 9;35(23):2991-3003
Oncogene 2016 Jun 9;35(23):2991-3003
Salmonella enteritidis Effector AvrA Stabilizes Intestinal Tight Junctions via the JNK Pathway.
Lin Z, Zhang YG, Xia Y, Xu X, Jiao X, Sun J
The Journal of biological chemistry 2016 Dec 23;291(52):26837-26849
The Journal of biological chemistry 2016 Dec 23;291(52):26837-26849
Connexins, E-cadherin, Claudin-7 and β-catenin transiently form junctional nexuses during the post-natal mammary gland development.
Dianati E, Poiraud J, Weber-Ouellette A, Plante I
Developmental biology 2016 Aug 1;416(1):52-68
Developmental biology 2016 Aug 1;416(1):52-68
Claudin-2 knockout by TALEN-mediated gene targeting in MDCK cells: claudin-2 independently determines the leaky property of tight junctions in MDCK cells.
Tokuda S, Furuse M
PloS one 2015;10(3):e0119869
PloS one 2015;10(3):e0119869
Autocrine CSF1R signaling mediates switching between invasion and proliferation downstream of TGFβ in claudin-low breast tumor cells.
Patsialou A, Wang Y, Pignatelli J, Chen X, Entenberg D, Oktay M, Condeelis JS
Oncogene 2015 May 21;34(21):2721-31
Oncogene 2015 May 21;34(21):2721-31
ZO-1 knockout by TALEN-mediated gene targeting in MDCK cells: involvement of ZO-1 in the regulation of cytoskeleton and cell shape.
Tokuda S, Higashi T, Furuse M
PloS one 2014;9(8):e104994
PloS one 2014;9(8):e104994
Disrupted tight junctions in the small intestine of cystic fibrosis mice.
De Lisle RC
Cell and tissue research 2014 Jan;355(1):131-42
Cell and tissue research 2014 Jan;355(1):131-42
Claudins 1, 2, 3, 4, 5 and 7 in solar keratosis and squamocellular carcinoma of the skin.
Hintsala HR, Siponen M, Haapasaari KM, Karihtala P, Soini Y
International journal of clinical and experimental pathology 2013;6(12):2855-63
International journal of clinical and experimental pathology 2013;6(12):2855-63
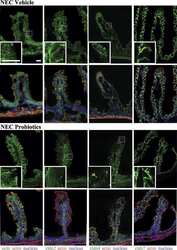
Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis.
Bergmann KR, Liu SX, Tian R, Kushnir A, Turner JR, Li HL, Chou PM, Weber CR, De Plaen IG
The American journal of pathology 2013 May;182(5):1595-606
The American journal of pathology 2013 May;182(5):1595-606
Claudin 1 expression characterizes human uterine cervical reserve cells.
Zinner B, Gyöngyösi B, Babarczi E, Kiss A, Sobel G
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2013 Dec;61(12):880-8
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2013 Dec;61(12):880-8
EpCAM contributes to formation of functional tight junction in the intestinal epithelium by recruiting claudin proteins.
Lei Z, Maeda T, Tamura A, Nakamura T, Yamazaki Y, Shiratori H, Yashiro K, Tsukita S, Hamada H
Developmental biology 2012 Nov 15;371(2):136-45
Developmental biology 2012 Nov 15;371(2):136-45
Cingulin is dispensable for epithelial barrier function and tight junction structure, and plays a role in the control of claudin-2 expression and response to duodenal mucosa injury.
Guillemot L, Schneider Y, Brun P, Castagliuolo I, Pizzuti D, Martines D, Jond L, Bongiovanni M, Citi S
Journal of cell science 2012 Nov 1;125(Pt 21):5005-14
Journal of cell science 2012 Nov 1;125(Pt 21):5005-14
Prostaglandin-induced cervical remodelling in humans in the first trimester is associated with increased expression of specific tight junction, but not gap junction proteins.
Ghulé VV, Gray C, Galimberti A, Anumba DO
Journal of translational medicine 2012 Mar 7;10:40
Journal of translational medicine 2012 Mar 7;10:40
Functional characterization and localization of a gill-specific claudin isoform in Atlantic salmon.
Engelund MB, Yu AS, Li J, Madsen SS, Færgeman NJ, Tipsmark CK
American journal of physiology. Regulatory, integrative and comparative physiology 2012 Jan 15;302(2):R300-11
American journal of physiology. Regulatory, integrative and comparative physiology 2012 Jan 15;302(2):R300-11
Transforming growth factor beta 1 induces tight junction disruptions and loss of transepithelial resistance across porcine vas deferens epithelial cells.
Pierucci-Alves F, Yi S, Schultz BD
Biology of reproduction 2012 Feb;86(2):36
Biology of reproduction 2012 Feb;86(2):36
Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function.
Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW
The American journal of pathology 2012 Feb;180(2):626-35
The American journal of pathology 2012 Feb;180(2):626-35
Enhanced immunohistochemical resolution of claudin proteins in glycolmethacrylate-embedded tissue biopsies.
Collins JE, Kirk A, Campbell SK, Mason J, Wilson SJ
Methods in molecular biology (Clifton, N.J.) 2011;762:371-82
Methods in molecular biology (Clifton, N.J.) 2011;762:371-82
[The clinical significance of Claudin-7 and slug expression in lung squamous cell carcinoma and adenocarcinoma].
Li R, Zhang D, Cai C, Dong J
Zhongguo fei ai za zhi = Chinese journal of lung cancer 2011 Jun;14(6):492-6
Zhongguo fei ai za zhi = Chinese journal of lung cancer 2011 Jun;14(6):492-6
The level of claudin-7 is reduced as an early event in colorectal carcinogenesis.
Bornholdt J, Friis S, Godiksen S, Poulsen SS, Santoni-Rugiu E, Bisgaard HC, Lothe IM, Ikdahl T, Tveit KM, Johnson E, Kure EH, Vogel LK
BMC cancer 2011 Feb 10;11:65
BMC cancer 2011 Feb 10;11:65
Identification of a claudin-4 and E-cadherin score to predict prognosis in breast cancer.
Szasz AM, Nemeth Z, Gyorffy B, Micsinai M, Krenacs T, Baranyai Z, Harsanyi L, Kiss A, Schaff Z, Tokes AM, Kulka J
Cancer science 2011 Dec;102(12):2248-54
Cancer science 2011 Dec;102(12):2248-54
beta-catenin expression and claudin expression pattern as prognostic factors of prostatic cancer progression.
Szász AM, Nyirády P, Majoros A, Szendrõi A, Szûcs M, Székely E, Tõkés AM, Romics I, Kulka J
BJU international 2010 Mar;105(5):716-22
BJU international 2010 Mar;105(5):716-22
Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells.
Chang TL, Ito K, Ko TK, Liu Q, Salto-Tellez M, Yeoh KG, Fukamachi H, Ito Y
Gastroenterology 2010 Jan;138(1):255-65.e1-3
Gastroenterology 2010 Jan;138(1):255-65.e1-3
Heterogeneity for stem cell-related markers according to tumor subtype and histologic stage in breast cancer.
Park SY, Lee HE, Li H, Shipitsin M, Gelman R, Polyak K
Clinical cancer research : an official journal of the American Association for Cancer Research 2010 Feb 1;16(3):876-87
Clinical cancer research : an official journal of the American Association for Cancer Research 2010 Feb 1;16(3):876-87
Novel snail1 target proteins in human colon cancer identified by proteomic analysis.
Larriba MJ, Casado-Vela J, Pendás-Franco N, Peña R, García de Herreros A, Berciano MT, Lafarga M, Casal JI, Muñoz A
PloS one 2010 Apr 20;5(4):e10221
PloS one 2010 Apr 20;5(4):e10221
Claudin-1, -2, -3, -4, -7, -8, and -10 protein expression in biliary tract cancers.
Németh Z, Szász AM, Tátrai P, Németh J, Gyorffy H, Somorácz A, Szíjártó A, Kupcsulik P, Kiss A, Schaff Z
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2009 Feb;57(2):113-21
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2009 Feb;57(2):113-21
Hepatocyte nuclear factor 4alpha, a key factor for homeostasis, cell architecture, and barrier function of the adult intestinal epithelium.
Cattin AL, Le Beyec J, Barreau F, Saint-Just S, Houllier A, Gonzalez FJ, Robine S, Pinçon-Raymond M, Cardot P, Lacasa M, Ribeiro A
Molecular and cellular biology 2009 Dec;29(23):6294-308
Molecular and cellular biology 2009 Dec;29(23):6294-308
Altered gene expression in breast cancer liver metastases.
Erin N, Wang N, Xin P, Bui V, Weisz J, Barkan GA, Zhao W, Shearer D, Clawson GA
International journal of cancer 2009 Apr 1;124(7):1503-16
International journal of cancer 2009 Apr 1;124(7):1503-16
Inducible overexpression of cingulin in stably transfected MDCK cells does not affect tight junction organization and gene expression.
Paschoud S, Citi S
Molecular membrane biology 2008 Jan;25(1):1-13
Molecular membrane biology 2008 Jan;25(1):1-13
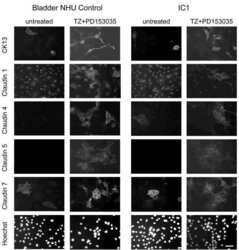
Differentiation potential of urothelium from patients with benign bladder dysfunction.
Southgate J, Varley CL, Garthwaite MA, Hinley J, Marsh F, Stahlschmidt J, Trejdosiewicz LK, Eardley I
BJU international 2007 Jun;99(6):1506-16
BJU international 2007 Jun;99(6):1506-16
Differences in claudin synthesis in primary cultures of acinar cells from rat salivary gland are correlated with the specific three-dimensional organization of the cells.
Qi B, Fujita-Yoshigaki J, Michikawa H, Satoh K, Katsumata O, Sugiya H
Cell and tissue research 2007 Jul;329(1):59-70
Cell and tissue research 2007 Jul;329(1):59-70
Loss of claudins-1 and -7 and expression of claudins-3 and -4 correlate with prognostic variables in prostatic adenocarcinomas.
Sheehan GM, Kallakury BV, Sheehan CE, Fisher HA, Kaufman RP Jr, Ross JS
Human pathology 2007 Apr;38(4):564-9
Human pathology 2007 Apr;38(4):564-9
The tight junction proteins claudin-7 and -8 display a different subcellular localization at Henle's loops and collecting ducts of rabbit kidney.
Gonzalez-Mariscal L, Namorado Mdel C, Martin D, Sierra G, Reyes JL
Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2006 Sep;21(9):2391-8
Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2006 Sep;21(9):2391-8
Epithelial barrier characteristics and expression of cell adhesion molecules in proximal tubule-derived cell lines commonly used for in vitro toxicity studies.
Prozialeck WC, Edwards JR, Lamar PC, Smith CS
Toxicology in vitro : an international journal published in association with BIBRA 2006 Sep;20(6):942-53
Toxicology in vitro : an international journal published in association with BIBRA 2006 Sep;20(6):942-53
Claudin 1 differentiates endometrioid and serous papillary endometrial adenocarcinoma.
Sobel G, Németh J, Kiss A, Lotz G, Szabó I, Udvarhelyi N, Schaff Z, Páska C
Gynecologic oncology 2006 Nov;103(2):591-8
Gynecologic oncology 2006 Nov;103(2):591-8
Endothelia of term human placentae display diminished expression of tight junction proteins during preeclampsia.
Liévano S, Alarcón L, Chávez-Munguía B, González-Mariscal L
Cell and tissue research 2006 Jun;324(3):433-48
Cell and tissue research 2006 Jun;324(3):433-48
Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase RhoA.
Guillemot L, Citi S
Molecular biology of the cell 2006 Aug;17(8):3569-77
Molecular biology of the cell 2006 Aug;17(8):3569-77
Changes of cell adhesion and extracellular matrix (ECM) components in cervical intraepithelial neoplasia.
Sobel G, Szabó I, Páska C, Kiss A, Kovalszky I, Kádár A, Paulin F, Schaff Z
Pathology oncology research : POR 2005;11(1):26-31
Pathology oncology research : POR 2005;11(1):26-31
Inducible expression of Snail selectively increases paracellular ion permeability and differentially modulates tight junction proteins.
Carrozzino F, Soulié P, Huber D, Mensi N, Orci L, Cano A, Féraille E, Montesano R
American journal of physiology. Cell physiology 2005 Oct;289(4):C1002-14
American journal of physiology. Cell physiology 2005 Oct;289(4):C1002-14
Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells.
Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE
American journal of physiology. Cell physiology 2005 Jun;288(6):C1231-41
American journal of physiology. Cell physiology 2005 Jun;288(6):C1231-41
Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells.
Lipschutz JH, Li S, Arisco A, Balkovetz DF
The Journal of biological chemistry 2005 Feb 4;280(5):3780-8
The Journal of biological chemistry 2005 Feb 4;280(5):3780-8
Expression of claudin-7 and -8 along the mouse nephron.
Li WY, Huey CL, Yu AS
American journal of physiology. Renal physiology 2004 Jun;286(6):F1063-71
American journal of physiology. Renal physiology 2004 Jun;286(6):F1063-71
Expression and function of tight junctions in the crypt epithelium of human palatine tonsils.
Go M, Kojima T, Takano K, Murata M, Ichimiya S, Tsubota H, Himi T, Sawada N
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2004 Dec;52(12):1627-38
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2004 Dec;52(12):1627-38
Effect of formalin, acetone, and RNAlater fixatives on tissue preservation and different size amplicons by real-time PCR from paraffin-embedded tissue.
Páska C, Bögi K, Szilák L, Tokés A, Szabó E, Sziller I, Rigó J Jr, Sobel G, Szabó I, Kaposi-Novák P, Kiss A, Schaff Z
Diagnostic molecular pathology : the American journal of surgical pathology, part B 2004 Dec;13(4):234-40
Diagnostic molecular pathology : the American journal of surgical pathology, part B 2004 Dec;13(4):234-40
Effect of formalin, acetone, and RNAlater fixatives on tissue preservation and different size amplicons by real-time PCR from paraffin-embedded tissue.
Páska C, Bögi K, Szilák L, Tokés A, Szabó E, Sziller I, Rigó J Jr, Sobel G, Szabó I, Kaposi-Novák P, Kiss A, Schaff Z
Diagnostic molecular pathology : the American journal of surgical pathology, part B 2004 Dec;13(4):234-40
Diagnostic molecular pathology : the American journal of surgical pathology, part B 2004 Dec;13(4):234-40
Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast.
Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S
Oncogene 2003 Apr 3;22(13):2021-33
Oncogene 2003 Apr 3;22(13):2021-33
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
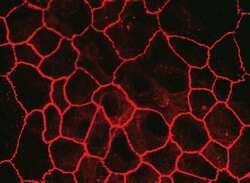
- Experimental details
- Immunofluorescent staining of MDCK cells using Rabbit anti-Claudin-7. Image courtesy of Dr. Saraswati Sukumar, Johns Hopkins School of Medicine, Baltimore, MD. (Product # 34-9100)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
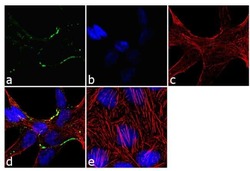
- Experimental details
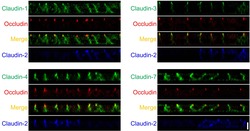
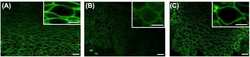
- Immunofluorescence analysis of Claudin-7 was done on 90% confluent log phase Caco2 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with Claudin-7 Rabbit Polyclonal Antibody (Product # 34-9100) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cell junction localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
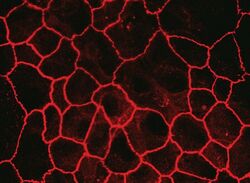
- Experimental details
- Immunofluorescent staining of MDCK cells using Rabbit anti-Claudin-7. Image courtesy of Dr. Saraswati Sukumar, Johns Hopkins School of Medicine, Baltimore, MD. (Product # 34-9100)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
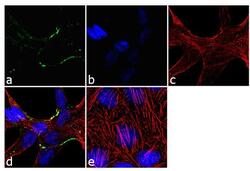
- Experimental details
- Immunofluorescence analysis of Claudin-7 was done on 90% confluent log phase Caco2 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with Claudin-7 Rabbit Polyclonal Antibody (Product # 34-9100) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cell junction localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
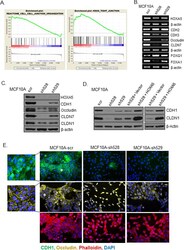
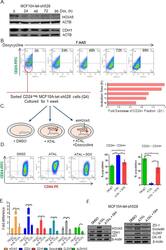
- Figure 2 Loss of HOXA5 disrupts cell-cell adhesion molecules that govern epithelial integrity A . Gene set enrichment analysis of MCF10A-scr and HOXA5 depleted cells indicates that cell-cell junction genes are enriched for expression in MCF10A-scr cells. B . RT-PCR analysis shows reduced expression of EMT markers (CDH2 and CDH3), tight junction molecules (Occludin and CLDN7) and increased expression of luminal fate marker, FOXA1 and reduced expression of FOXQ1 in MCF10A-scr, HOXA5 depleted MCF10A-sh528 and MCF10A-sh529 cells. C . Western blot analysis of HOXA5 and HOXA5-regulated genes CDH1, Occludin, CLDN7 and CLDN1 in MCF10A-scr, -sh528 and -sh529 cells. beta-actin serves as the loading control. D . Western blot analysis shows decrease in CDH1 and CLDN1 protein upon HOXA5 depletion in MCF10A cells; and reversal by ectopic expression of HOXA5 in the knock-down clones. E . Representative immunofluorescence images of CDH1 (Green), Occludin (Yellow), actin stress fiber, Phalloidin (Red) and nuclear marker, DAPI (Blue) with expanded views (2x) of CDH1 and Occludin in MCF10A-scr cells to clearly visualize localization.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 6 Retinal-induced cell differentiation in MCF10A is compromised when HOXA5 is repressed A . Western blot analysis of HOXA5 and CDH1 expression in MCF10A-tet-sh528 cells with dox-induced depletion of HOXA5 over 96 hours. beta-actin serves as the loading control. B . Flow cytometry analysis of CD24 expression in MCF10A-tet-HOXA5 with doxycycline-induced depletion of HOXA5 shows decrease of CD24+ population (7-AAD negative) from 47% to 24% over 96 hours. C . Flow sorted CD24 - population was treated with DMSO, 1 uM retinal (ATAL) or 1 uM ATAL + 100 nM doxycycline (DOX) and cultured for 1 week. Flow cytometry analysis of CD24 (x-axis) and CD44 (y-axis). Quantitative analysis is shown in the bar graph. D . Quantitative RT-PCR analysis of HOXA5, CD24, CDH1, Occludin, CLDN7 and ALDH1A3 in MCF10A-tet-sh528 cells treated with DMSO, 1 uM retinal (ATAL) or 1 uM ATAL + 100 nM doxycycline (DOX) and cultured for 1 week. E . Western blot analysis of HOXA5, CDH1, p21, ZO-1, Occludin, CLDN7 and CK18 in cells treated with DMSO, ATAL and ATAL+ doxycycline as in (D). beta-actin serves as the loading control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
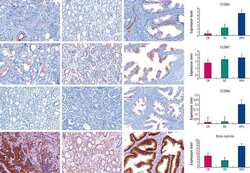
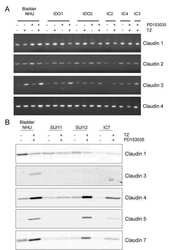
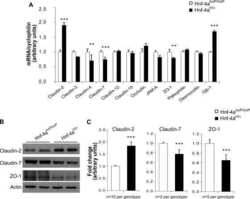
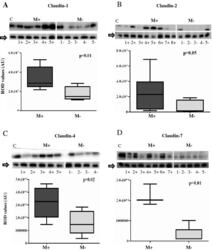
- Figure 1 Comparison of expression levels of claudins- 1 (1A), 2 (1B), 4 (1C) and 7(1D) in Misoprostol treated (M+) (samples 1+ to 7+) versus untreated (M-) (samples 1- to 5-) cervix . Representative immunoblots in the top panels whilst box plots, lower panel, represent mean (SE) relative optical densities (ROD) for all studied tissues: M+ (n = 10) vs. M- (n = 5). Block arrows showing Gbetaeta as internal loading control at 35 kDa. C-positive control in the first lane. C-1 = claudin-1; C-2 = claudin-2; C-4 = claudin-4; C-7 = claudin-7. ROD- relative optical density in Arbitrary Units (AU).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
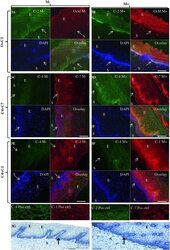
- Figure 3 Representative confocal photomicrographs comparing localization patterns of claudins-1 (C1), -2(C2), -4(C4) and -7(C7) and occludin (Ocld) in Misoprostol treated (M+, right panel) and untreated (M-, left panel) cervix . The FIT-c (green) and Cyanine-3 (Cy-3-red) labelled secondary antibodies were used; DAPI (blue) for nuclear staining with overlays of red, green and blue (RGB) channels. In the untreated cervix, occludin (red) shifted to intermediate and more superficial layers (3B-red). In the untreated cervix, occludin (3A-red) and claudin-2 (3A green) were expressed in basal layers only (3A-overaly) but following Misoprostol treatment, de novo expression of claudin-2 (3B-green) in the intermediate layer resulted in both now localizing in the basal and intermediate layers (3B-overlay). Compared to diffuse lattice pattern in basal to intermediate layers in the untreated cervix (3C red), Misoprostol induced expression of claudin-7(3D red) in basal and intermediate layers. Claudin-4 was expressed mainly in the nuclei throughout the ectothelium (3C green) in the untreated cervix, with de novo cytoplasmic expression in Misoprostol-treated tissue (3D & 3F green). Claudins 1 and 4 exhibit nuclear and plasma membrane expressions in Misoprostol-treated cohort (3F). Arrows at basement membranes; S-stroma, E-epithelium. 3A- 2 x 2 tile; x 20air objective. NA-0.8, Bar = 8 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
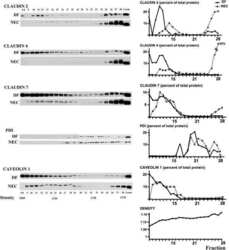
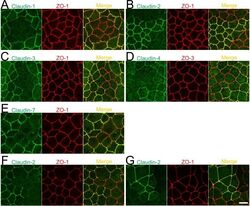
- Fig 11 Localization of claudins at TJs between control and their respective knockout cells. (A-E) Immunofluorescence analysis of claudins and ZO-1 or ZO-3 in MDCK II cells transfected with the TALEN constructs for claudin-1, -2, -3, -4, or-7 gene knockouts. After transfection, cells were subcultured on filter inserts for 4 days before the analysis of claudin-2 and -3 (B and C), and for 2 days before the analysis of claudin-1, -4, and -7 (A, D, and E). (F) Immunofluorescence analysis of claudin-2 and ZO-1. TALEN constructs for the claudin-2 gene knockout were transfected into MDCK II cells, which were subcultured on filter inserts for 2 days before analysis. (G) Immunofluorescence analysis of claudin-2 and ZO-1 in a co-culture of wild-type and claudin-2 expressing MDCK I cells. Cells were cultured on filter inserts for 4 days before analysis. Scale bar = 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
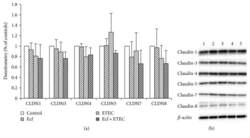
- Figure 3 Protein expression (Western blot) of claudin-1, claudin-3, claudin-4, claudin-5, claudin-7, and claudin-8 after treatment with bacterial strains (Ecf, ETEC, and Ecf + ETEC). (a) Protein expression relative to respective controls [means +- SEM]. Samples were taken after 8 h. No differences were observed between treatment groups. N = 3 independent experiments per bar. (b) Exemplarily, data of one Western blot is shown. 1 = control (8 h), 2 = Ecf (8 h), 3 = ETEC (8 h), 4 = Ecf (8 h) + ETEC (6 h), and 5 = Ecf (10 h) + ETEC (8 h). Sample 4 was included as a control to rule out effects of longer total bacterial incubation (preincubation) and was not included in the statistical analysis shown in (a).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
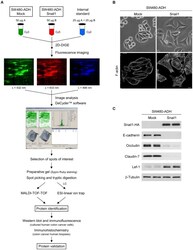
- Figure 1 Experimental workflow and characterization of SW480-ADH Mock and Snail1 cells. (A) Scheme of the workflow followed in this study. (B) Representative phase-contrast micrographs (upper panels) and confocal laser immunofluorescence images showing F-actin staining (lower panels) of SW480-ADH Mock and Snail1 cells. Scale bar, 20 um (upper panels) and 10 um (lower panels). (C) Western blot analysis of whole-cell extracts from SW480-ADH Mock and Snail1 cells showing Snail1 overexpression and its effect on the expression of epithelial (E-cadherin, Occludin and Claudin-7) and mesenchymal (Lef-1) markers. beta-Tubulin was used as a loading control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
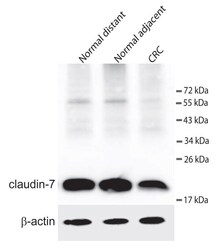
- Figure 3 Western blot detecting claudin-7 and ss-actin . The upper panel shows claudin-7 in samples from a colorectal cancer patient. Normal distant is a sample taken as far away from the tumour as possible in the surgically removed tissue. Normal adjacent is a sample taken just adjacent to the tumour and CRC is from the cancer itself. The lower panel shows the loading control (ss-actin).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
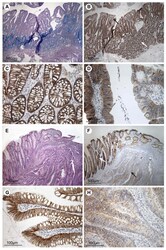
- Figure 4 Immunohistochemical staining for claudin-7 in colorectal adenomas, carcinomas and adjacent normal tissue . The top four pictures are taken from an individual with dysplasia but no record of carcinoma and the bottom four pictures are from a patient with colorectal cancer. A) Tissue section including both normal mucosa and dysplastic tissue, stained with PAS/Alcian. B) Neighbouring tissue section stained for claudin-7. The white arrow indicates mucosa of normal appearance and the black arrow indicates dysplastic tissue. C) High magnification of the normal mucosa from B, showing staining mainly at the basolateral cell membranes of the epithelial cells. D) High magnification of the dysplastic tissue from B showing a patchy staining pattern with areas of low and normal staining. E) Tissue section with cancerous tissue to the right and normal mucosa to the left, stained with Hematoxylin and Eosin. F) Neighbouring tissue section stained for claudin-7. The white arrow indicates the mucosa with normal appearance. The black arrow points out carcinomatous tissue. G) High magnification of histologically normally appearing mucosa from F, showing staining mainly localised to the basolateral cell membranes of the surface epithelial cells. H) High magnification of carcinomatous tissue showing faint, patchy staining of the epithelial strands of tumour tissue. Scale bars: 500 mum (A + B + E + F), 100 mum (C + D + G + H).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
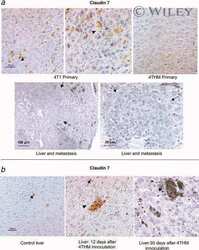
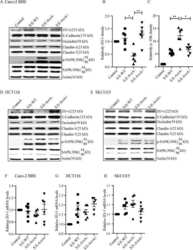
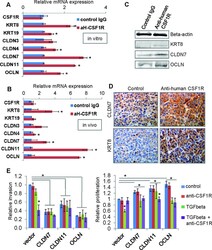
- Figure 7 Autocrine CSF1R signaling maintains low expression of tight junction proteins and luminal keratins in claudin-low breast cancer cells A. MDA-MB-231 cells were treated in vitro with either control or the inhibitory antibody to human CSF1R (aH-CSF1R), in the presence of TGFbeta. After 48hours, mRNA expression of a panel of luminal keratins and tight junction proteins was assessed by real-time PCR. Results are shown as average mRNA expression relative to control treatment. N=3 separate experiments with duplicate plates. B. MDA-MB-231 orthotopic tumors were treated in vivo with either control IgG or the inhibitory antibody to human CSF1R (aH-CSF1R). After 48hours, tumor cells were isolated by FACS (due to GFP stable expression in the MDA-MB-231 cells), and total RNA was extracted. mRNA expression of a panel of luminal keratins and tight junction proteins was then assessed by real-time PCR. Results are shown as average mRNA expression relative to control treatment. N=3 tumors per condition. C. Western blot to assess protein levels after MDA-MB-231 cells treatment in vitro as described in panel A. Beta-actin was used as a positive control. Representative images are shown from two separate experiments. D. Immunohistochemistry of tumor sections for keratin 8 (KRT8) and claudin 7 (CLDN7) in the tumors treated as described in panel B. Representative images are shown from three separate tumors per group. E. In vitro invasion was measured in matrigel-coated transwells for contro
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
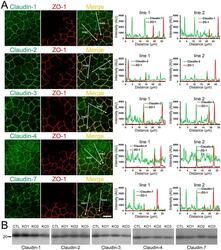
- Figure 12 Effects of ZO-1 knockout on the localization and expression levels of claudins. (A) Effects of ZO-1 knockout on the localization of claudins. Control and ZO-1 knockout cells of clone 1 were co-cultured on filter inserts. Signal intensity of claudins on lines shown in confocal microscopic images ( arrows ) were analyzed. Claudin-2 fluorescent signal at TJs was increased but claudin-1 and -7 signals at TJs were reduced in ZO-1 knockout cells. Scale bar, 10 um. (B) Immunoblots of claudins in control MDCK II cells and ZO-1 knockout clones. Similar expression levels of claudins were observed in control and knockout cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
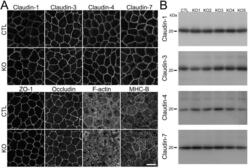
- Fig 3 Effects of claudin-2 knockout on the localization of other TJ proteins and cytoskeleton. (A) Immunofluorescence analysis of claudin-1, -3, -4, -7, ZO-1, occludin, F-actin, and myosin heavy chain II-B (MHC-B) in control and claudin-2 knockout cells. Claudin-1 and -7 showed a tendency to be more clearly localized at TJs in claudin-2 knockout cells. Scale bar = 10 mum. (B) Immunoblots of claudin-1, -3, -4, and -7 in control MDCK II cells and claudin-2 knockout clones. Similar expression levels of claudin-1, -3, -4, and -7 were observed in control cells and knockout clones.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
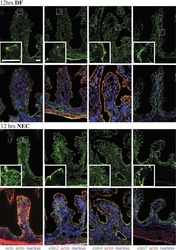
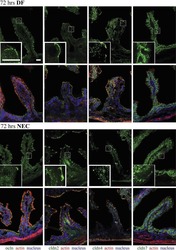
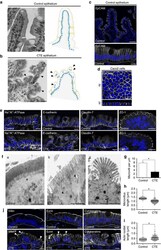
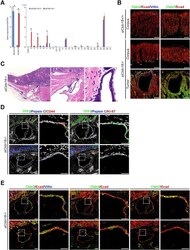
- Figure 1 Cell organization defects occur in the intestinal epithelium of CTE patients. ( a , b ) Histological analysis of hematoxylin-eosin stained paraffin sections of duodenal biopsies from control ( a ) and CTE patients ( b ). Scale bars, 10 mum. For each condition, a corresponding scheme of the epithelial organization is presented. CTE intestinal epithelium displays tufts (black arrowheads). ( c , d ) Confocal microscopy analysis of EpCAM distribution in duodenal control biopsy ( c ) or in Caco2 cells ( d ). Intestinal epithelium baseline is demarcated by dotted white line ( c ). Scale bars, upper panel 50 mum ( c ), lower panel 10 mum ( c ) and 10 mum ( d ). ( e ), Confocal microscopy analyses of Na + /K + -ATPase, E-cadherin, claudin-7 and ZO-1 distribution in control or CTE biopsies. N (Control) =6 biopsies, N (CTE) =6 biopsies. Scale bars, 10 mum. ( f ) Transmission electron microscopy (TEM) ultrastructural analysis of enterocyte apical membranes in control ( a ) and CTE ( b , c ) biopsies, showing actin rootlets ( black arrowheads ). Scale bars, 5 mum. N (Control) =3 biopsies, N (CTE) =3 biopsies. ( g ) Statistical analysis of microvillus density in control and CTE enterocytes. t test, * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
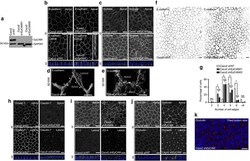
- Experimental details
- Figure 2 EpCAM silencing leads to unusual tight junction positioning defects at tricellular junctions. ( a ) Western blot analysis of the EpCAM expression level in control ( Caco2 shNT ) or EpCAM-deprived ( Caco2 shEpCAM #1 and Caco2 shEpCAM #2 ) clones. GAPDH was used as loading control. ( b , c ) Confocal microscopy analysis of E-cadherin ( b ) and Scribble ( c ) on the apical or lateral sides in control and EpCAM-depleted cells. Scale bars, 5 mum. Nuclei were detected with Hoechst 33342 staining (blue). ( d , e ) 3D-SIM microscopy analysis of E-cadherin localization in control ( d ) and EpCAM-deprived ( e ) cells. Scale bars, 2 mum. ( f ) Cell shape analysis after E-cadherin immunostaining in control or EpCAM-deprived cells. Scale bars, 5 mum. ( g ), Statistical analysis of polygonal shape in control or EpCAM-deprived cells. Three independent experiments were carried out. N (Caco2shNT)=517 cells, N (Caco2shEpCAM#1)=550 cells, N (Caco2shEpCAM#2)=427 cells. Caco2 shNT cells with two cell edges=0%, three cell edges=0.455+-0.787%, four cell edges=8.673+-3.406%, five cell edges =48.154+-11.123%, six cell edges=35.652+-8.888%, more than six cell edges=7.067+-3.851 cells%. Caco2 shEpCAM#1 cells with two cell edges=1.822+-0.733 cells%, three cell edges=22.031+-5.906%, four cell edges=46.061+-3.851%, five cell edges=24.728+-4.344%, six cell edges=5.027+-2.210%, more than six cell edges=0.330+-0.572%. Caco2 shEpCAM#2 cells with two cell edges=0.966+-0.848%, three cell edges=12.542+-
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
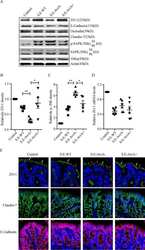
- Figure 3. Expression levels of CLDN7, APLP2 proteins and their localization in OVCA420 cells. (A) Immunoprecipitation of CLDN7 and APLP2. OVCA420 cells were grown for 72 h and cell lysates were immunoprecipitated with anti-CLDN7, anti-APLP2 and anti-GAPDH antibodies. Proteins were visualized by immunoblotting with an anti-APLP2 and anti-CLDN7 antibodies. Total cell lysate serves as a positive control and no primary antibody in immunoprecipitation serves as a negative control. (B) Immunoblotting analysis of CLDN7 and APLP2 after siRNA-mediated knockdown of CLDN7 and APLP2. GAPDH was used as a loading control. (C) Immunofluorescence of OVCA420 cell line shows strong expression of CLDN7 mainly at cell junction and APLP2 expression at cell junctions as well as cytoplasm. CLDN7 and APLP2 also show colocalization at cell junctions. After fixing in methanol, OVCA420 cells were incubated with primary CLDN7 and APLP2 antibodies and visualized with secondary antibodies conjugated to Alexa fluor (Red color represents APLP2 and green color represents CLDN7). Nuclei were counterstained with DAPI.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
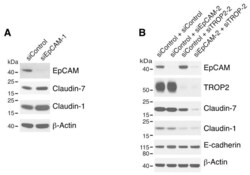
- Figure 2 Both EpCAM and TROP2 regulate claudin expression in keratinocytes. HaCaT cells were transfected with control or EpCAM siRNAs ( A ), control, EpCAM, TROP2, or EpCAM and TROP2 siRNAs ( B ) using electroporation. 72 h after transfection, cell lysates were prepared, resolved using SDS-PAGE and immunoblotted with anti-EpCAM, anti-TROP2, anti-claudin-7 or anti-claudin-1 to assess corresponding protein levels. beta-actin was used as a loading control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
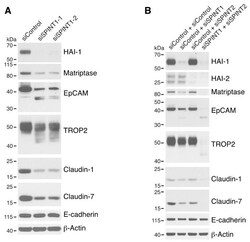
- Figure 5 HAI-1 and HAI-2 regulate EpCAM and TROP2 cleavage and claudin levels. HaCaT cells were transfected using electroporation with control siRNA or one of two different SPINT1 siRNAs (siSPINT1-1 and siSPINT1-2) ( A ), control, SPINT1, SPINT2, or SPINT1, and SPINT2 siRNAs ( B ). After 72 h, cell lysates were prepared, subjected to electrophoresis and analyzed via Western blotting for HA1-1, HAI-2, matriptase, EpCAM, TROP2, claudin-1, claudin-7 and E-cadherin. beta-actin was used as a loading control. Representative data from 1 of 3-4 experiments is shown.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
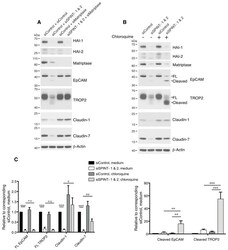
- Figure 6 HAIs act via matriptase to regulate degradation of EpCAM, TROP2, and claudins in lysosomes. HaCaT cells were transfected using electroporation with control or SPINT1 plus SPINT2, matriptase, or SPINT1, SPINT2 plus matriptase siRNAs ( A ), or control or SPINT1 and SPINT2 siRNAs ( B ). Before being harvested at 72 h after transfection ( A , B ), cells were treated with or without 100 muM chloroquine for 20 h ( B ). RIPA cell lysates were resolved using SDS-PAGE and immunoblotted with anti-HAI-1, anti-HAI-2, anti-matriptase, anti-EpCAM anti-TROP2, anti-claudin-1, or anti-claudin-7. beta-actin was used as a loading control. Representative data from 1 of 5 experiments is shown. ( C ) Band intensities corresponding to full-length (FL) EpCAM, FL TROP2, claudin-1 and claudin-7 (left panel) and cleaved EpCAM and cleaved TROP2 (right panel) were quantified and normalized to beta-actin. Data are plotted as ratios (means +- SEM) relative to corresponding untreated siControls ( n = 5). A two-way ANOVA was used to calculate p values for multiple group comparisons to assess mean differences between groups (* p < 0.05, ** p < 0.01, *** p < 0.001 or 0.0001, n.s. p > 0.05).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
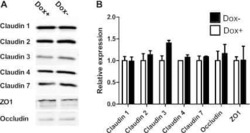
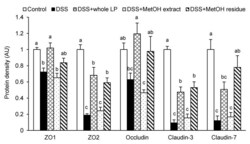
- Figure 4 Effects of whole LP powder, methanol (MetOH) extract, and MetOH extraction residue (MetOH residue) on tight junction protein expression in the colon of dextran sodium sulfate (DSS)-induced colitic mice. Protein expression of zonula occludens (ZO)-1, ZO-2, occludin, claudin-3, and claudin-7 in the colon of mice fed diets with and without whole LP powder, MetOH extract, and MetOH residue, with or without DSS administration, as determined by immunoblot analysis. Values are the mean +- SEM ( n = 7). Means without a common letter differ, p < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
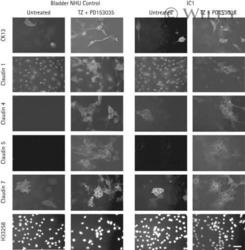
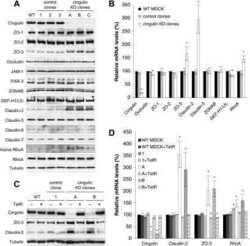
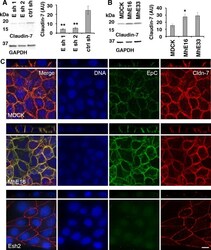
- Fig 5 Levels and localization of tight junction protein Claudin-7 are regulated by EpCAM in MDCK cells. (A) EpCAM-depleted MDCK lines Esh1, Esh2 and control shRNA line ctrl sh were SDS extracted one day after plating at subconfluent cell density. Protein levels of Claudin-7 and of GAPDH as a loading control were analyzed in the same immunoblot. The graphs show quantification of Claudin-7 protein normalized to GAPDH levels in the same sample. Error bars: S.E.M. of four samples for each cell line; p values derived from unpaired Student's t test: ** p = 0.0042 for Esh1 to ctrl sh and ** p = 0.0057 for Esh2 to ctrl sh. (B) Claudin-7 protein levels in MDCK cells and cell lines MhE16, MhE33 overexpressing human EpCAM were analyzed as described in (A). Arbitrary units for protein intensities in Y-axis (AU) x10 3 ; error bars: S.E.M. of three samples for each cell line; p value derived from unpaired Student's t test: * p = 0.042 for MhE16 to MDCK. (C) Confocal images were acquired of cells 24 hours after plating on IBIDI chambers and 0 hours after removal of the stencil. The figure shows maximum intensity projections and orthogonal projections of confocal stacks of MDCK, MhE16 and Esh2 cells stained for nuclei (blue), EpCAM (green), and Claudin-7 (red). In the MDCK and MhE16 lines, both EpCAM and Claudin-7 localize along basolateral membrane. In the Esh2 line, Claudin-7 does not distribute throughout the basolateral membrane, and EpCAM staining is very weak. In Esh2 line, greater con
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
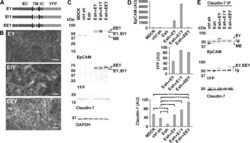
- Fig 6 Rescue of Claudin-7 protein levels by expression of EpCAM-YFP or point mutants of EpCAM-YFP in MDCK cells depleted of EpCAM. (A) Schematic of EpCAM-YFP (EY) and EpCAM-YFP mutant proteins (EIY and EEY). Extracellular domain (EC), transmembrane domain (TM), intracellular domain (IC) and YFP fusion are indicated. Approximate position of amino acids mutated in EIY and EEY are indicated with their one letter codes. (B) EpCAM-depleted Esh2-derived cell lines expressing EY, EIY or EEY were plated at confluent density onto an IBIDI 4 well dish for one day and YFP fluorescence was imaged before well chambers were removed for a migration assay. YFP fluorescence localizes to cell-cell contacts in all three cell lines. Scale bars: 50 mum. (C) Indicated MDCK cell lines were cultured, extracted and protein levels were analyzed as described for Figs 1A , 2A and 5 . EpCAM-YFP fusion proteins EY, EIY and EEY appear as double bands; MDCK-endogenous EpCAM (ME) is a single band. EEY appears slightly larger and is poorly recognized by the EpCAM extracellular domain antibody (top EpCAM blot). Expression of Claudin-7 is reduced in the parental Esh2 line (Esh) compared to MDCK and control shRNA line ctrl sh (Claudin-7 blot; see also Fig 5 ). (D) Graphs show quantifications of indicated proteins normalized to GAPDH levels in the same sample. For EpCAM protein levels, residual MDCK-endogenous EpCAM (ME) and EpCAM-YFP protein double band levels (EY, EIY, EEY) were combined to show total EpCAM lev
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
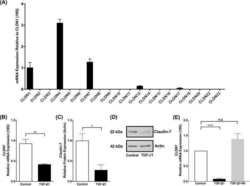
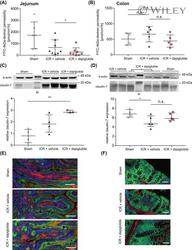
- 2 FIGURE Dapiglutide strengthens barrier function in the jejunum but not in the colon. (A) FITC-4 kDa dextran permeability of stripped jejunum was measured in the Ussing chamber for n = 6-8 mice, mean +- SD, * p < 0.05. (B) FITC-4 kDa dextran permeability of stripped colon was measured in the Ussing chamber for n = 4-5 mice, mean +- SD. (C) Upper panel: representative western blot of claudin-7 in jejunum tissue. Lower panel: corresponding densitometric analysis of relative claudin-7 protein content, n = 3-4 mice, mean +- SEM, ** p < 0.01. (D) Upper panel: representative western blot of claudin-7 in colon tissue. Lower panel: corresponding densitometric analysis of relative claudin-7 protein content, n = 4-5 mice, mean +- SEM, * p < 0.05. (E) Immunofluorescence images of jejunum sections: claudin-7 in green, F-actin in red, and nuclei in blue. Scale bar: 50 mum. (F) Immunofluorescence images of colon sections: claudin-7 in green, F-actin in red, and nuclei in blue. Scale bar: 50 mum
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
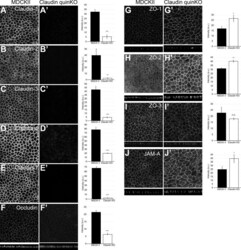
- Figure 2. Localization of TJ proteins in claudin quinKO cells. (A-J) Immunofluorescence analyses of parental MDCK II cells (A-J) and claudin quinKO cells (A'-J'). (A-E) Claudin-1 (A), claudin-2 (B), claudin-3 (C), claudin-4 (D), and claudin-7 (E) expression was abolished in claudin quinKO cells. (F) Occludin localization to apical junctions was reduced. (G-I) ZO-1 (G) and ZO-2 (H) were more concentrated at apical junctions, while ZO-3 (I) localization was not altered in claudin quinKO cells. (J) JAM-A was more concentrated at apical junctions in claudin quinKO cells. Graphs are quantitation of the fluorescence intensity and represent mean +- SD ( n = 3 each). *, P < 0.05; **, P < 0.005; ***, P < 0.0005, compared by t test. Scale bar: 20 um. n.s., not significant.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
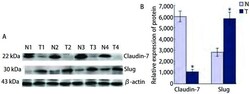
- 2 Western blotJian Ce Fei Yan Zu Zhi Ji Qi Yan Pang Zu Zhi Zhong Claudin-7He SlugDe Biao Da . A:Fei Lin Yan , Xian Yan Zhong Claudin-7He SlugDe Dan Bai Biao Da Qing Kuang ;T1, 2:SCC;T3, 4:ADC;N:Yan Pang Zu Zhi ;B:Claudin-7He SlugDan Bai De Xiang Dui Biao Da Liang . * : P < 0.05. Expression of Claudin-7 and Slug protein in lung can-cer tissues and paracancerous specimens by Western blot. A: Result of Western blot for expressions of Claudin-7 and Slug in lung quamous cell carcinoma and adenocarcinoma; T1, 2: SCC; T3, 4: ADC; N: paracancerous specimens; B: The histogram of relative expression rate of Claudin-7 protein compared to Slug. * : P < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
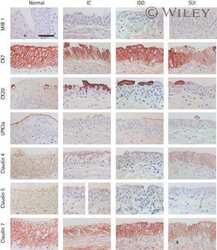
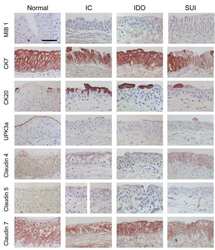
- Figure 7 Development of intestinal metaplasia and ectopic gastric gland in aged stCldn18-/- mice. ( A ) Expression levels of Cldns in the stomach from s tCldn18+/+ (n = 6), and stCldn18-/- (n = 7), mice at 100 w.o. quantified by qRT-PCR. The expression levels of Cldn2 , 4 , 7 , and luCldn18 were significantly increased in the stomach of stCldn18-/- mice at old age. Gene expressions were normalized to GAPDH. Results are expressed as mean +- SD. Comparisons between 2 groups were performed using Student's t test, and differences with P < .05 were considered statistically significant. n.s., not significant. * P < .05. ( B ) Immunofluorescence micrographs for Cldn2 or Cldn7 ( green ) co-stained with E-cadherin ( red ) and with or without villin ( blue ) in the stomach from stCldn18+/+ and stCldn18-/- mice (around 50 w.o.). Representative images from at least 3 independent experiments are shown. Scale bars = 100 mum. ( C ) Hematoxylin and eosin-stained images of the ectopic gastric gland in aged stCldn18-/- mice. Ectopic gastric glands were found in 1 of 3 old stCldn18-/- mice by examining stomach tissue slices. Right panels are magnified images of the boxed regions in the left panels. Representative images from at least 2 independent experiments are shown. Scale bars = 100, 50, 10 mum, left to right panel. ( D ) ( Left ) Immunofluorescence micrographs for TFF2 ( green ) and Pepsin C ( blue ) as SPEM cell markers co-stained with CD44 (red) and DAPI ( white ) in the ectopic gastric
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
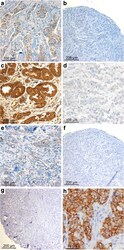
- Fig. 1 Claudin (CLDN) 3. Positive expression in BRCA1 breast cancer (BC) ( a ) and negative control (isotypic antibody) ( b ). Positive CLDN4 expression in BRCA1 -mutated BC ( c ), negative control of CLDN4 (isotypic antibody) ( d ). Positive CLDN7 in BRCA2 BC ( e ), negative CLDN7 in BRCA1 -related tumor ( f ), and negative control of CLDN7 (isotypic antibody) ( g ). Positive CK5 staining in BRCA1 BC ( h )
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry