Antibody data
- Antibody Data
- Antigen structure
- References [19]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Flow cytometry [1]
- Other assay [9]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 71-7600 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CDH11 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Recombinant full-length protein
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 0.25 mg/mL
- Storage
- -20°C
Submitted references Human Mesenchymal Stem Cell-Derived Miniature Joint System for Disease Modeling and Drug Testing.
Potassium channel-driven bioelectric signalling regulates metastasis in triple-negative breast cancer.
Side-specific valvular endothelial-interstitial cell mechano-communication via cadherin-11.
Sensitization of knee-innervating sensory neurons by tumor necrosis factor-α-activated fibroblast-like synoviocytes: an in vitro, coculture model of inflammatory pain.
Cadherin 11 Inhibition Downregulates β-catenin, Deactivates the Canonical WNT Signalling Pathway and Suppresses the Cancer Stem Cell-Like Phenotype of Triple Negative Breast Cancer.
The cell-cell junctions of mammalian testes: II. The lamellar smooth muscle monolayer cells of the peritubular wall are laterally connected by vertical adherens junctions-a novel architectonic cell-cell junction system.
Sheep-Specific Immunohistochemical Panel for the Evaluation of Regenerative and Inflammatory Processes in Tissue-Engineered Heart Valves.
Analysis of chromatin accessibility uncovers TEAD1 as a regulator of migration in human glioblastoma.
Genomic Locus Modulating IOP in the BXD RI Mouse Strains.
PLEKHA7 defines an apical junctional complex with cytoskeletal associations and miRNA-mediated growth implications.
Fibrosis and hypoxia-inducible factor-1α-dependent tumors of the soft tissue on loss of von Hippel-Lindau in mesenchymal progenitors.
Evidence for cadherin-11 cleavage in the synovium and partial characterization of its mechanism.
Regulation of cranial morphogenesis and cell fate at the neural crest-mesoderm boundary by engrailed 1.
p120 catenin is required for normal renal tubulogenesis and glomerulogenesis.
Cancer-associated fibroblasts and epithelial-mesenchymal transition in metastatic oral tongue squamous cell carcinoma.
Induction of corneal myofibroblasts by lens-derived transforming growth factor beta1 (TGFbeta1): a transgenic mouse model.
Transcriptome analysis reveals an osteoblast-like phenotype for human osteotropic breast cancer cells.
Gene expression profiling of human colon xenograft tumors following treatment with SU11248, a multitargeted tyrosine kinase inhibitor.
A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells.
Li Z, Lin Z, Liu S, Yagi H, Zhang X, Yocum L, Romero-Lopez M, Rhee C, Makarcyzk MJ, Yu I, Li EN, Fritch MR, Gao Q, Goh KB, O'Donnell B, Hao T, Alexander PG, Mahadik B, Fisher JP, Goodman SB, Bunnell BA, Tuan RS, Lin H
Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2022 Jul;9(21):e2105909
Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2022 Jul;9(21):e2105909
Potassium channel-driven bioelectric signalling regulates metastasis in triple-negative breast cancer.
Payne SL, Ram P, Srinivasan DH, Le TT, Levin M, Oudin MJ
EBioMedicine 2022 Jan;75:103767
EBioMedicine 2022 Jan;75:103767
Side-specific valvular endothelial-interstitial cell mechano-communication via cadherin-11.
Johnson CL, Merryman WD
Journal of biomechanics 2021 Apr 15;119:110253
Journal of biomechanics 2021 Apr 15;119:110253
Sensitization of knee-innervating sensory neurons by tumor necrosis factor-α-activated fibroblast-like synoviocytes: an in vitro, coculture model of inflammatory pain.
Chakrabarti S, Hore Z, Pattison LA, Lalnunhlimi S, Bhebhe CN, Callejo G, Bulmer DC, Taams LS, Denk F, Smith ESJ
Pain 2020 Sep 1;161(9):2129-2141
Pain 2020 Sep 1;161(9):2129-2141
Cadherin 11 Inhibition Downregulates β-catenin, Deactivates the Canonical WNT Signalling Pathway and Suppresses the Cancer Stem Cell-Like Phenotype of Triple Negative Breast Cancer.
Satriyo PB, Bamodu OA, Chen JH, Aryandono T, Haryana SM, Yeh CT, Chao TY
Journal of clinical medicine 2019 Jan 27;8(2)
Journal of clinical medicine 2019 Jan 27;8(2)
The cell-cell junctions of mammalian testes: II. The lamellar smooth muscle monolayer cells of the peritubular wall are laterally connected by vertical adherens junctions-a novel architectonic cell-cell junction system.
Domke LM, Franke WW
Cell and tissue research 2019 Feb;375(2):451-482
Cell and tissue research 2019 Feb;375(2):451-482
Sheep-Specific Immunohistochemical Panel for the Evaluation of Regenerative and Inflammatory Processes in Tissue-Engineered Heart Valves.
Dekker S, van Geemen D, van den Bogaerdt AJ, Driessen-Mol A, Aikawa E, Smits AIPM
Frontiers in cardiovascular medicine 2018;5:105
Frontiers in cardiovascular medicine 2018;5:105
Analysis of chromatin accessibility uncovers TEAD1 as a regulator of migration in human glioblastoma.
Tome-Garcia J, Erfani P, Nudelman G, Tsankov AM, Katsyv I, Tejero R, Bin Zhang, Walsh M, Friedel RH, Zaslavsky E, Tsankova NM
Nature communications 2018 Oct 1;9(1):4020
Nature communications 2018 Oct 1;9(1):4020
Genomic Locus Modulating IOP in the BXD RI Mouse Strains.
King R, Li Y, Wang J, Struebing FL, Geisert EE
G3 (Bethesda, Md.) 2018 May 4;8(5):1571-1578
G3 (Bethesda, Md.) 2018 May 4;8(5):1571-1578
PLEKHA7 defines an apical junctional complex with cytoskeletal associations and miRNA-mediated growth implications.
Kourtidis A, Anastasiadis PZ
Cell cycle (Georgetown, Tex.) 2016;15(4):498-505
Cell cycle (Georgetown, Tex.) 2016;15(4):498-505
Fibrosis and hypoxia-inducible factor-1α-dependent tumors of the soft tissue on loss of von Hippel-Lindau in mesenchymal progenitors.
Mangiavini L, Merceron C, Araldi E, Khatri R, Gerard-O'Riley R, Wilson TL, Sandusky G, Abadie J, Lyons KM, Giaccia AJ, Schipani E
The American journal of pathology 2015 Nov;185(11):3090-101
The American journal of pathology 2015 Nov;185(11):3090-101
Evidence for cadherin-11 cleavage in the synovium and partial characterization of its mechanism.
Noss EH, Watts GF, Zocco D, Keller TL, Whitman M, Blobel CP, Lee DM, Brenner MB
Arthritis research & therapy 2015 May 15;17(1):126
Arthritis research & therapy 2015 May 15;17(1):126
Regulation of cranial morphogenesis and cell fate at the neural crest-mesoderm boundary by engrailed 1.
Deckelbaum RA, Holmes G, Zhao Z, Tong C, Basilico C, Loomis CA
Development (Cambridge, England) 2012 Apr;139(7):1346-58
Development (Cambridge, England) 2012 Apr;139(7):1346-58
p120 catenin is required for normal renal tubulogenesis and glomerulogenesis.
Marciano DK, Brakeman PR, Lee CZ, Spivak N, Eastburn DJ, Bryant DM, Beaudoin GM 3rd, Hofmann I, Mostov KE, Reichardt LF
Development (Cambridge, England) 2011 May;138(10):2099-109
Development (Cambridge, England) 2011 May;138(10):2099-109
Cancer-associated fibroblasts and epithelial-mesenchymal transition in metastatic oral tongue squamous cell carcinoma.
Vered M, Dayan D, Yahalom R, Dobriyan A, Barshack I, Bello IO, Kantola S, Salo T
International journal of cancer 2010 Sep 1;127(6):1356-62
International journal of cancer 2010 Sep 1;127(6):1356-62
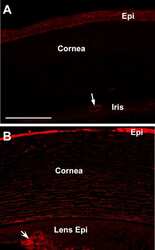
Induction of corneal myofibroblasts by lens-derived transforming growth factor beta1 (TGFbeta1): a transgenic mouse model.
Reneker LW, Bloch A, Xie L, Overbeek PA, Ash JD
Brain research bulletin 2010 Feb 15;81(2-3):287-96
Brain research bulletin 2010 Feb 15;81(2-3):287-96
Transcriptome analysis reveals an osteoblast-like phenotype for human osteotropic breast cancer cells.
Bellahcène A, Bachelier R, Detry C, Lidereau R, Clézardin P, Castronovo V
Breast cancer research and treatment 2007 Jan;101(2):135-48
Breast cancer research and treatment 2007 Jan;101(2):135-48
Gene expression profiling of human colon xenograft tumors following treatment with SU11248, a multitargeted tyrosine kinase inhibitor.
Morimoto AM, Tan N, West K, McArthur G, Toner GC, Manning WC, Smolich BD, Cherrington JM
Oncogene 2004 Feb 26;23(8):1618-26
Oncogene 2004 Feb 26;23(8):1618-26
A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells.
Straub BK, Boda J, Kuhn C, Schnoelzer M, Korf U, Kempf T, Spring H, Hatzfeld M, Franke WW
Journal of cell science 2003 Dec 15;116(Pt 24):4985-95
Journal of cell science 2003 Dec 15;116(Pt 24):4985-95
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
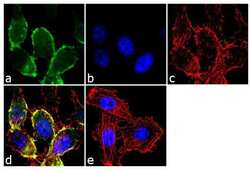
- Experimental details
- Immunofluorescence analysis of CDH11/Cadherin OB was performed using 70% confluent log phase PC-3 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with CDH11/Cadherin OB Rabbit Polyclonal antibody (Product # 71-7600) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing membranous localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
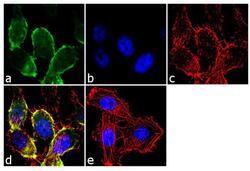
- Experimental details
- Immunofluorescence analysis of CDH11/Cadherin OB was performed using 70% confluent log phase PC-3 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with CDH11/Cadherin OB Rabbit Polyclonal antibody (Product # 71-7600) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing membranous localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
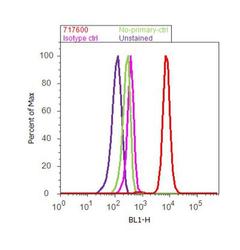
- Experimental details
- Flow cytometry analysis of CDH11 / Cadherin OB was done on PC-3 cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with CDH11 Rabbit Polyclonal Antibody (717600, red histogram) or with rabbit isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
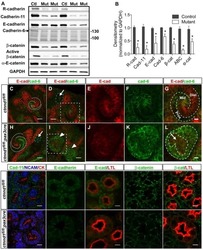
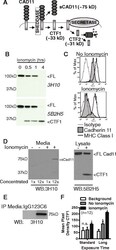
- Fig. 2 Cadherin-11 cleavage stimulation by ionomycin. ( a ) Proposed cadherin-11 cleavage model shows that cadherin-11 is first cleaved extracellularly at the plasma membrane by a cell sheddase, generating a C-terminal fragment 1 (CTF1) and an extracellular domain fragment (sCad11). Then CTF1 is cleaved near the intracellular plasma membrane, releasing C-terminal fragment 2 (CTF2) into the cytosol. CTF2 is likely rapidly degraded by the proteasome but may also effect gene transcription. ( b ) Lysates from RA synovial fibroblasts stimulated with 5 muM ionomycin as indicated were analyzed by western blot using antibodies directed against cadherin-11 extracellular (3H10) or intracellular (5B2H5) epitopes (FL, full length cadherin-11). ( c ) Surface staining for cadherin-11 (3H10), MHC class I (W632) or isotype control on RA synovial fibroblasts before or after 1 hour ionomycin stimulation was determined by flow cytometry (representative, 2 experiments). ( d ) Culture media and cell lysates harvested from RA synovial fibroblasts treated with or without ionomycin for 1 hour were analyzed by western blot for extracellular (3H10) and intracellular (5B2H5) cadherin-11 epitopes. Culture media was left unconcentrated or concentrated approximately 12-fold (representative 3 experiments). ( e ) Culture media from unstimulated cells was immunoprecipitated with anti-cadherin-11 antibody 23C6 or appropriate isotype control prior to western blot analysis (representative 3 experiments). ( f )
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
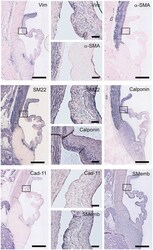
- Figure 5 Valvular interstitial cell (VIC) markers. Tile scans of sheep aortic valve sections (left and right columns), with high magnification zooms (middle column) as indicated. Valves were stained with antibodies against vimentin, alpha-smooth muscle actin (alpha-SMA), SM22, calponin, cadherin-11 (Cad-11), and the embryonic form of myosin heavy chain (SMemb). Scale bars, 1 mm (tile scans) or 100 mum (zooms).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
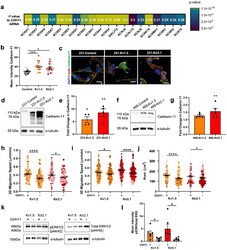
- Fig. 6 Cadherin-11 mediates the pro-migratory effects of K + channel-driven hyperpolarization. (a) Heatmap demonstrating significant correlation of CDH11 mRNA expression with K + channel genes in TNBC patients from TCGA dataset. Pearson's coefficient values for each gene are listed and colour scale indicates degree of statistical significance. (b) Quantification of cadherin-11 expression in 231 cell lines (* p = 0.0168, **** p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
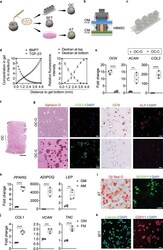
- Engineering and characterization of individual miniJoint tissue components. a) Experimental schematic showing the fabrication of different miniJoint tissue components. OC, osteochondral tissue; SFT, synovial-like fibrous tissue; AT, adipose tissue. b,c) A dual-flow chip, allowing perfusion of osteogenic medium (OM) above the MSC-laden gel insert and chondrogenic medium (CM) below the gel insert b), was 3D printed c) and used to generate the biphasic OC microtissues. d) Simulated and measured biomolecule diffusion within the osteochondral tissue. Assuming a partitioning coefficient of 0.1, equilibrated distribution profile of BMP7, an osteogenic growth factor in OM, and TGF beta 3, a chondrogenic growth factor in CM, in the OC microtissue was calculated (left). For validation purposes, dextran (molecular weight, 20 kDa) labeled with fluorescein isothiocyanate was added to either the top or bottom stream of a dual-flow bioreactor to simulate the diffusion of BMP7 and TGF beta 3, respectively (right). The relative fluorescence intensity was normalized and set in the range of 0-10. N = 4 for the dextran diffusion test. e) Box plots showing the expression of OCN , COL2 , and ACAN in the bone (OC-O) and cartilage (OC-C) components of the OC microtissues. OCN data were normalized to values in OC-C, and COL2 and ACAN data to values in OC-O. Data were analyzed by the Student's t -test ( N = 4 biological replicates). **, p < 0.01; ****, p < 0.0001. f) Safranin O staining of the entire
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
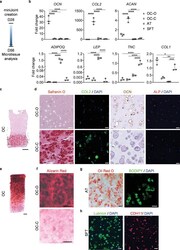
- The microtissues maintained their individual tissue phenotypes after four weeks of co-culture in the miniJoint chip. a) Timeline of generating a normal miniJoint chip with tissue-specific phenotypes maintained. b) Box plots showing the expression of key marker genes in all four tissue components. OCN data were normalized to those in OC-C, COL2 , and ACAN data to values in OC-O, ADIPOQ and LEP data to values in AT, and TNC and COL1 data to values in SFT. Data were analyzed by one-way ANOVA ( N = 3 biological replicates). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. c) Safranin O staining of the biphasic OC unit. Scale bar = 1 mm. d) Histological staining and immunostaining images confirming the presence of bone- and cartilage-specific markers in the corresponding component of the OC unit. Scale bar = 50 um. e) Alizarin Red staining of the OC unit showing the presence of calcium deposits primarily in the OC-O. Scale bar = 500 um. f) Magnified views of the Alizarin Red staining image in e). Scale bar = 200 um. g) Oil Red O and BODIPY staining images showing the retention of lipid droplets in AT. Scale bar = 50 um. h) Immunostaining images showing the expression of lubricin and CDH11 by the synovial-like fibrous tissue (SFT). Scale bar = 50 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
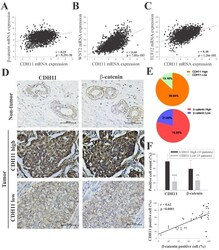
- Figure 2 CDH11 positively modulates beta-catenin expression and activates the canonical WNT signalling pathway. Statistical analyses of the TNBC sub-group of the METABRIC Breast Cancer cohort ( n = 1904) show positive correlation between expression of CDH11 and ( A ) beta-catenin, ( B ) WNT2, and ( C ) TCF12 mRNA. ( D and E ) Differential expression of CDH11 and beta-catenin in non-tumor and TNBC tissue samples demonstrated by representative IHC images and percentages of patients. Scale bar: 50 mum. ( F ) Histogram showing positive correlation between CDH11 and beta-catenin protein expression level in our TNBC cohort ( n = 38). * p < 0.05, ** p < 0.01, *** p < 0.001
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
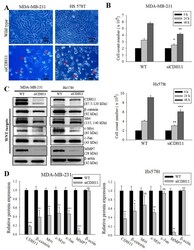
- Figure 3 Loss of CDH11 function using siRNA changes cell morphology and inhibits activation of the canonical WNT signalling pathway in TNBC cells. ( A ) The cell morphology of MDA-MB-231 and Hs578t changed from spindle-like to polygonal after loss of CDH11 expression. ( B ) Graphical representation of the effect of siCDH11 on the doubling time of MDA-MB-231 and Hs578t cells as determined by trypan-blue exclusion. ( C ) Western blot results show CDH11 knockdown decreased beta-catenin, Met, c-Myc, c-Jun, and MMP7 protein expression level in MDA-MB-231 and Hs578t cell lines. ( D ) Graphical representation of C . Results expressed as mean +- SD of assays done 3 times in triplicate. * p < 0.05, ** p < 0.01, *** p < 0.001; NS, not significant; Red arrow, floating non-viable cells.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry