Antibody data
- Antibody Data
- Antigen structure
- References [64]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [2]
- Other assay [67]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 51-2800 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Connexin 26 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 50 μg
- Concentration
- 0.25 mg/mL
- Storage
- -20°C
Submitted references The prognostic value and biological significance of gap junction beta protein 2 (GJB2 or Cx26) in cervical cancer.
The protective effects of systemic dexamethasone on sensory epithelial damage and hearing loss in targeted Cx26-null mice.
Connexin30-Deficiency Causes Mild Hearing Loss With the Reduction of Endocochlear Potential and ATP Release.
Connexin-Based Channel Activity Is Not Specifically Altered by Hepatocarcinogenic Chemicals.
PUPIL enables mapping and stamping of transient electrical connectivity in developing nervous systems.
Connexin Hemichannel Activation by S-Nitrosoglutathione Synergizes Strongly with Photodynamic Therapy Potentiating Anti-Tumor Bystander Killing.
Expression and Functionality of Connexin-Based Channels in Human Liver Cancer Cell Lines.
Gap Junction-Mediated Intercellular Communication of cAMP Prevents CDDP-Induced Ototoxicity via cAMP/PKA/CREB Pathway.
Functional Evaluation of a Rare Variant c.516G>C (p.Trp172Cys) in the GJB2 (Connexin 26) Gene Associated with Nonsyndromic Hearing Loss.
Cholestasis Differentially Affects Liver Connexins.
Comparative Analysis of Cx31 and Cx43 in Differentiation-Competent Rodent Keratinocytes.
Polydisperse molecular architecture of connexin 26/30 heteromeric hemichannels revealed by atomic force microscopy imaging.
Single cell transcriptomics of human epidermis identifies basal stem cell transition states.
Developmental abnormalities in supporting cell phalangeal processes and cytoskeleton in the Gjb2 knockdown mouse model.
The spatial distribution pattern of Connexin26 expression in supporting cells and its role in outer hair cell survival.
Mouse Panx1 Is Dispensable for Hearing Acquisition and Auditory Function.
Immunofluorescence reveals unusual patterns of labelling for connexin43 localized to calbindin-D28K-positive interstitial cells in the pineal gland.
Disease-linked connexin26 S17F promotes volar skin abnormalities and mild wound healing defects in mice.
In vivo genetic manipulation of inner ear connexin expression by bovine adeno-associated viral vectors.
Loss of Panx1 Impairs Mammary Gland Development at Lactation: Implications for Breast Tumorigenesis.
Connexin gap junction channels and chronic rhinosinusitis.
Cell coupling mediated by connexin 26 selectively contributes to reduced adhesivity and increased migration.
Ototoxicity-induced loss of hearing and inner hair cells is attenuated by HSP70 gene transfer.
Mice with conditional deletion of Cx26 exhibit no vestibular phenotype despite secondary loss of Cx30 in the vestibular end organs.
Cx26 knockout predisposes the mammary gland to primary mammary tumors in a DMBA-induced mouse model of breast cancer.
Cx30 exhibits unique characteristics including a long half-life when assembled into gap junctions.
Asymmetric expression of connexins between luminal epithelial- and myoepithelial- cells is essential for contractile function of the mammary gland.
Timed conditional null of connexin26 in mice reveals temporary requirements of connexin26 in key cochlear developmental events before the onset of hearing.
Mammary gland specific knockdown of the physiological surge in Cx26 during lactation retains normal mammary gland development and function.
Re-evaluation of connexins associated with motoneurons in rodent spinal cord, sexually dimorphic motor nuclei and trigeminal motor nucleus.
Connexin 26 null mice exhibit spiral ganglion degeneration that can be blocked by BDNF gene therapy.
Connexin43 reduces melanoma growth within a keratinocyte microenvironment and during tumorigenesis in vivo.
The liver connexin32 interactome is a novel plasma membrane-mitochondrial signaling nexus.
The D50N mutation and syndromic deafness: altered Cx26 hemichannel properties caused by effects on the pore and intersubunit interactions.
Cerebral ischemic injury is enhanced in a model of oculodentodigital dysplasia.
BAAV mediated GJB2 gene transfer restores gap junction coupling in cochlear organotypic cultures from deaf Cx26Sox10Cre mice.
Dominant Cx26 mutants associated with hearing loss have dominant-negative effects on wild type Cx26.
Connexin26 expression in brain parenchymal cells demonstrated by targeted connexin ablation in transgenic mice.
Reduced gap junctional communication among astrocytes in experimental diabetes: contributions of altered connexin protein levels and oxidative-nitrosative modifications.
Ablation of connexin30 in transgenic mice alters expression patterns of connexin26 and connexin32 in glial cells and leptomeninges.
Focal adhesion kinase modulates radial glia-dependent neuronal migration through connexin-26.
Dominant connexin26 mutants associated with human hearing loss have trans-dominant effects on connexin30.
Hyperglycaemia and diabetes impair gap junctional communication among astrocytes.
ATP-mediated cell-cell signaling in the organ of Corti: the role of connexin channels.
The human deafness-associated connexin 30 T5M mutation causes mild hearing loss and reduces biochemical coupling among cochlear non-sensory cells in knock-in mice.
Immunocytochemical traits of type IV fibrocytes and their possible relations to cochlear function and pathology.
Tissue-specific cross-reactivity of connexin32 antibodies: problems and solutions unique to the central nervous system.
Limited intravascular coupling in the rodent brainstem and retina supports a role for glia in regional blood flow.
Human connexin26 and connexin30 form functional heteromeric and heterotypic channels.
Expression of connexins in embryonic mouse neocortical development.
Identification of connexin36 in gap junctions between neurons in rodent locus coeruleus.
Modulation of connexin expression and gap junction communication in astrocytes by the gram-positive bacterium S. aureus.
In vitro optimization of antisense oligodeoxynucleotide design: an example using the connexin gene family.
Connexin43 is overexpressed in Apc(Min/+)-mice adenomas and colocalises with COX-2 in myofibroblasts.
Restoration of functional gap junctions through internal ribosome entry site-dependent synthesis of endogenous connexins in density-inhibited cancer cells.
Freeze-fracture and immunogold analysis of aquaporin-4 (AQP4) square arrays, with models of AQP4 lattice assembly.
Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential.
Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice.
Exocrine specific expression of Connexin32 is dependent on the basic helix-loop-helix transcription factor Mist1.
Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: roles in stem cell proliferation and morphological plasticity?
Localization of connexin26 and connexin32 in putative CO(2)-chemosensitive brainstem regions in rat.
Localization of connexin26 and connexin32 in putative CO(2)-chemosensitive brainstem regions in rat.
Connexin26 in adult rodent central nervous system: demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43.
Connexin26 in adult rodent central nervous system: demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43.
Meng S, Liu Y, Wang X, Wu X, Xie W, Kang X, Liu X, Guo L, Wang C
Frontiers in oncology 2022;12:907960
Frontiers in oncology 2022;12:907960
The protective effects of systemic dexamethasone on sensory epithelial damage and hearing loss in targeted Cx26-null mice.
Xu K, Chen S, Xie L, Qiu Y, Liu XZ, Bai X, Jin Y, Wang XH, Sun Y
Cell death & disease 2022 Jun 10;13(6):545
Cell death & disease 2022 Jun 10;13(6):545
Connexin30-Deficiency Causes Mild Hearing Loss With the Reduction of Endocochlear Potential and ATP Release.
Chen J, Chen P, He B, Gong T, Li Y, Zhang J, Lv J, Mammano F, Hou S, Yang J
Frontiers in cellular neuroscience 2021;15:819194
Frontiers in cellular neuroscience 2021;15:819194
Connexin-Based Channel Activity Is Not Specifically Altered by Hepatocarcinogenic Chemicals.
Leroy K, Pieters A, Cooreman A, Van Campenhout R, Cogliati B, Vinken M
International journal of molecular sciences 2021 Oct 29;22(21)
International journal of molecular sciences 2021 Oct 29;22(21)
PUPIL enables mapping and stamping of transient electrical connectivity in developing nervous systems.
Xie S, Li H, Yao F, Huang J, Yang X, Chen X, Liu Q, Zhuang M, He S
Cell reports 2021 Oct 19;37(3):109853
Cell reports 2021 Oct 19;37(3):109853
Connexin Hemichannel Activation by S-Nitrosoglutathione Synergizes Strongly with Photodynamic Therapy Potentiating Anti-Tumor Bystander Killing.
Nardin C, Peres C, Putti S, Orsini T, Colussi C, Mazzarda F, Raspa M, Scavizzi F, Salvatore AM, Chiani F, Tettey-Matey A, Kuang Y, Yang G, Retamal MA, Mammano F
Cancers 2021 Oct 10;13(20)
Cancers 2021 Oct 10;13(20)
Expression and Functionality of Connexin-Based Channels in Human Liver Cancer Cell Lines.
Leroy K, Silva Costa CJ, Pieters A, Dos Santos Rodrigues B, Van Campenhout R, Cooreman A, Tabernilla A, Cogliati B, Vinken M
International journal of molecular sciences 2021 Nov 10;22(22)
International journal of molecular sciences 2021 Nov 10;22(22)
Gap Junction-Mediated Intercellular Communication of cAMP Prevents CDDP-Induced Ototoxicity via cAMP/PKA/CREB Pathway.
Kim YJ, Lee JS, Kim H, Jang JH, Choung YH
International journal of molecular sciences 2021 Jun 13;22(12)
International journal of molecular sciences 2021 Jun 13;22(12)
Functional Evaluation of a Rare Variant c.516G>C (p.Trp172Cys) in the GJB2 (Connexin 26) Gene Associated with Nonsyndromic Hearing Loss.
Maslova EA, Orishchenko KE, Posukh OL
Biomolecules 2021 Jan 5;11(1)
Biomolecules 2021 Jan 5;11(1)
Cholestasis Differentially Affects Liver Connexins.
Cooreman A, Van Campenhout R, Crespo Yanguas S, Gijbels E, Leroy K, Pieters A, Tabernilla A, Van Brantegem P, Annaert P, Cogliati B, Vinken M
International journal of molecular sciences 2020 Sep 7;21(18)
International journal of molecular sciences 2020 Sep 7;21(18)
Comparative Analysis of Cx31 and Cx43 in Differentiation-Competent Rodent Keratinocytes.
Au A, Shao Q, White KK, Lucaciu SA, Esseltine JL, Barr K, Laird DW
Biomolecules 2020 Oct 14;10(10)
Biomolecules 2020 Oct 14;10(10)
Polydisperse molecular architecture of connexin 26/30 heteromeric hemichannels revealed by atomic force microscopy imaging.
Naulin PA, Lozano B, Fuentes C, Liu Y, Schmidt C, Contreras JE, Barrera NP
The Journal of biological chemistry 2020 Dec 4;295(49):16499-16509
The Journal of biological chemistry 2020 Dec 4;295(49):16499-16509
Single cell transcriptomics of human epidermis identifies basal stem cell transition states.
Wang S, Drummond ML, Guerrero-Juarez CF, Tarapore E, MacLean AL, Stabell AR, Wu SC, Gutierrez G, That BT, Benavente CA, Nie Q, Atwood SX
Nature communications 2020 Aug 25;11(1):4239
Nature communications 2020 Aug 25;11(1):4239
Developmental abnormalities in supporting cell phalangeal processes and cytoskeleton in the Gjb2 knockdown mouse model.
Chen S, Xie L, Xu K, Cao HY, Wu X, Xu XX, Sun Y, Kong WJ
Disease models & mechanisms 2018 Feb 26;11(2)
Disease models & mechanisms 2018 Feb 26;11(2)
The spatial distribution pattern of Connexin26 expression in supporting cells and its role in outer hair cell survival.
Chen S, Xu K, Xie L, Cao HY, Wu X, Du AN, He ZH, Lin X, Sun Y, Kong WJ
Cell death & disease 2018 Dec 5;9(12):1180
Cell death & disease 2018 Dec 5;9(12):1180
Mouse Panx1 Is Dispensable for Hearing Acquisition and Auditory Function.
Zorzi V, Paciello F, Ziraldo G, Peres C, Mazzarda F, Nardin C, Pasquini M, Chiani F, Raspa M, Scavizzi F, Carrer A, Crispino G, Ciubotaru CD, Monyer H, Fetoni AR, M Salvatore A, Mammano F
Frontiers in molecular neuroscience 2017;10:379
Frontiers in molecular neuroscience 2017;10:379
Immunofluorescence reveals unusual patterns of labelling for connexin43 localized to calbindin-D28K-positive interstitial cells in the pineal gland.
Tsao DD, Wang SG, Lynn BD, Nagy JI
The European journal of neuroscience 2017 Jun;45(12):1553-1569
The European journal of neuroscience 2017 Jun;45(12):1553-1569
Disease-linked connexin26 S17F promotes volar skin abnormalities and mild wound healing defects in mice.
Press E, Alaga KC, Barr K, Shao Q, Bosen F, Willecke K, Laird DW
Cell death & disease 2017 Jun 1;8(6):e2845
Cell death & disease 2017 Jun 1;8(6):e2845
In vivo genetic manipulation of inner ear connexin expression by bovine adeno-associated viral vectors.
Crispino G, Galindo Ramirez F, Campioni M, Zorzi V, Praetorius M, Di Pasquale G, Chiorini JA, Mammano F
Scientific reports 2017 Aug 4;7(1):6567
Scientific reports 2017 Aug 4;7(1):6567
Loss of Panx1 Impairs Mammary Gland Development at Lactation: Implications for Breast Tumorigenesis.
Stewart MK, Plante I, Penuela S, Laird DW
PloS one 2016;11(4):e0154162
PloS one 2016;11(4):e0154162
Connexin gap junction channels and chronic rhinosinusitis.
Kim R, Chang G, Hu R, Phillips A, Douglas R
International forum of allergy & rhinology 2016 Jun;6(6):611-7
International forum of allergy & rhinology 2016 Jun;6(6):611-7
Cell coupling mediated by connexin 26 selectively contributes to reduced adhesivity and increased migration.
Polusani SR, Kalmykov EA, Chandrasekhar A, Zucker SN, Nicholson BJ
Journal of cell science 2016 Dec 1;129(23):4399-4410
Journal of cell science 2016 Dec 1;129(23):4399-4410
Ototoxicity-induced loss of hearing and inner hair cells is attenuated by HSP70 gene transfer.
Takada Y, Takada T, Lee MY, Swiderski DL, Kabara LL, Dolan DF, Raphael Y
Molecular therapy. Methods & clinical development 2015;2:15019
Molecular therapy. Methods & clinical development 2015;2:15019
Mice with conditional deletion of Cx26 exhibit no vestibular phenotype despite secondary loss of Cx30 in the vestibular end organs.
Lee MY, Takada T, Takada Y, Kappy MD, Beyer LA, Swiderski DL, Godin AL, Brewer S, King WM, Raphael Y
Hearing research 2015 Oct;328:102-12
Hearing research 2015 Oct;328:102-12
Cx26 knockout predisposes the mammary gland to primary mammary tumors in a DMBA-induced mouse model of breast cancer.
Stewart MK, Bechberger JF, Welch I, Naus CC, Laird DW
Oncotarget 2015 Nov 10;6(35):37185-99
Oncotarget 2015 Nov 10;6(35):37185-99
Cx30 exhibits unique characteristics including a long half-life when assembled into gap junctions.
Kelly JJ, Shao Q, Jagger DJ, Laird DW
Journal of cell science 2015 Nov 1;128(21):3947-60
Journal of cell science 2015 Nov 1;128(21):3947-60
Asymmetric expression of connexins between luminal epithelial- and myoepithelial- cells is essential for contractile function of the mammary gland.
Mroue R, Inman J, Mott J, Budunova I, Bissell MJ
Developmental biology 2015 Mar 1;399(1):15-26
Developmental biology 2015 Mar 1;399(1):15-26
Timed conditional null of connexin26 in mice reveals temporary requirements of connexin26 in key cochlear developmental events before the onset of hearing.
Chang Q, Tang W, Kim Y, Lin X
Neurobiology of disease 2015 Jan;73:418-27
Neurobiology of disease 2015 Jan;73:418-27
Mammary gland specific knockdown of the physiological surge in Cx26 during lactation retains normal mammary gland development and function.
Stewart MK, Plante I, Bechberger JF, Naus CC, Laird DW
PloS one 2014;9(7):e101546
PloS one 2014;9(7):e101546
Re-evaluation of connexins associated with motoneurons in rodent spinal cord, sexually dimorphic motor nuclei and trigeminal motor nucleus.
Bautista W, Rash JE, Vanderpool KG, Yasumura T, Nagy JI
The European journal of neuroscience 2014 Mar;39(5):757-70
The European journal of neuroscience 2014 Mar;39(5):757-70
Connexin 26 null mice exhibit spiral ganglion degeneration that can be blocked by BDNF gene therapy.
Takada Y, Beyer LA, Swiderski DL, O'Neal AL, Prieskorn DM, Shivatzki S, Avraham KB, Raphael Y
Hearing research 2014 Mar;309:124-35
Hearing research 2014 Mar;309:124-35
Connexin43 reduces melanoma growth within a keratinocyte microenvironment and during tumorigenesis in vivo.
Ableser MJ, Penuela S, Lee J, Shao Q, Laird DW
The Journal of biological chemistry 2014 Jan 17;289(3):1592-603
The Journal of biological chemistry 2014 Jan 17;289(3):1592-603
The liver connexin32 interactome is a novel plasma membrane-mitochondrial signaling nexus.
Fowler SL, Akins M, Zhou H, Figeys D, Bennett SA
Journal of proteome research 2013 Jun 7;12(6):2597-610
Journal of proteome research 2013 Jun 7;12(6):2597-610
The D50N mutation and syndromic deafness: altered Cx26 hemichannel properties caused by effects on the pore and intersubunit interactions.
Sanchez HA, Villone K, Srinivas M, Verselis VK
The Journal of general physiology 2013 Jul;142(1):3-22
The Journal of general physiology 2013 Jul;142(1):3-22
Cerebral ischemic injury is enhanced in a model of oculodentodigital dysplasia.
Kozoriz MG, Lai S, Vega JL, Sáez JC, Sin WC, Bechberger JF, Naus CC
Neuropharmacology 2013 Dec;75:549-56
Neuropharmacology 2013 Dec;75:549-56
BAAV mediated GJB2 gene transfer restores gap junction coupling in cochlear organotypic cultures from deaf Cx26Sox10Cre mice.
Crispino G, Di Pasquale G, Scimemi P, Rodriguez L, Galindo Ramirez F, De Siati RD, Santarelli RM, Arslan E, Bortolozzi M, Chiorini JA, Mammano F
PloS one 2011;6(8):e23279
PloS one 2011;6(8):e23279
Dominant Cx26 mutants associated with hearing loss have dominant-negative effects on wild type Cx26.
Zhang J, Scherer SS, Yum SW
Molecular and cellular neurosciences 2011 Jun;47(2):71-8
Molecular and cellular neurosciences 2011 Jun;47(2):71-8
Connexin26 expression in brain parenchymal cells demonstrated by targeted connexin ablation in transgenic mice.
Nagy JI, Lynn BD, Tress O, Willecke K, Rash JE
The European journal of neuroscience 2011 Jul;34(2):263-71
The European journal of neuroscience 2011 Jul;34(2):263-71
Reduced gap junctional communication among astrocytes in experimental diabetes: contributions of altered connexin protein levels and oxidative-nitrosative modifications.
Ball KK, Harik L, Gandhi GK, Cruz NF, Dienel GA
Journal of neuroscience research 2011 Dec;89(12):2052-67
Journal of neuroscience research 2011 Dec;89(12):2052-67
Ablation of connexin30 in transgenic mice alters expression patterns of connexin26 and connexin32 in glial cells and leptomeninges.
Lynn BD, Tress O, May D, Willecke K, Nagy JI
The European journal of neuroscience 2011 Dec;34(11):1783-93
The European journal of neuroscience 2011 Dec;34(11):1783-93
Focal adhesion kinase modulates radial glia-dependent neuronal migration through connexin-26.
Valiente M, Ciceri G, Rico B, Marín O
The Journal of neuroscience : the official journal of the Society for Neuroscience 2011 Aug 10;31(32):11678-91
The Journal of neuroscience : the official journal of the Society for Neuroscience 2011 Aug 10;31(32):11678-91
Dominant connexin26 mutants associated with human hearing loss have trans-dominant effects on connexin30.
Yum SW, Zhang J, Scherer SS
Neurobiology of disease 2010 May;38(2):226-36
Neurobiology of disease 2010 May;38(2):226-36
Hyperglycaemia and diabetes impair gap junctional communication among astrocytes.
Gandhi GK, Ball KK, Cruz NF, Dienel GA
ASN neuro 2010 Mar 15;2(2):e00030
ASN neuro 2010 Mar 15;2(2):e00030
ATP-mediated cell-cell signaling in the organ of Corti: the role of connexin channels.
Majumder P, Crispino G, Rodriguez L, Ciubotaru CD, Anselmi F, Piazza V, Bortolozzi M, Mammano F
Purinergic signalling 2010 Jun;6(2):167-87
Purinergic signalling 2010 Jun;6(2):167-87
The human deafness-associated connexin 30 T5M mutation causes mild hearing loss and reduces biochemical coupling among cochlear non-sensory cells in knock-in mice.
Schütz M, Scimemi P, Majumder P, De Siati RD, Crispino G, Rodriguez L, Bortolozzi M, Santarelli R, Seydel A, Sonntag S, Ingham N, Steel KP, Willecke K, Mammano F
Human molecular genetics 2010 Dec 15;19(24):4759-73
Human molecular genetics 2010 Dec 15;19(24):4759-73
Immunocytochemical traits of type IV fibrocytes and their possible relations to cochlear function and pathology.
Adams JC
Journal of the Association for Research in Otolaryngology : JARO 2009 Sep;10(3):369-82
Journal of the Association for Research in Otolaryngology : JARO 2009 Sep;10(3):369-82
Tissue-specific cross-reactivity of connexin32 antibodies: problems and solutions unique to the central nervous system.
Fowler SL, McLean AC, Bennett SA
Cell communication & adhesion 2009 Dec;16(5-6):117-30
Cell communication & adhesion 2009 Dec;16(5-6):117-30
Limited intravascular coupling in the rodent brainstem and retina supports a role for glia in regional blood flow.
Kuo IY, Chan-Ling T, Wojcikiewicz RJ, Hill CE
The Journal of comparative neurology 2008 Dec 20;511(6):773-87
The Journal of comparative neurology 2008 Dec 20;511(6):773-87
Human connexin26 and connexin30 form functional heteromeric and heterotypic channels.
Yum SW, Zhang J, Valiunas V, Kanaporis G, Brink PR, White TW, Scherer SS
American journal of physiology. Cell physiology 2007 Sep;293(3):C1032-48
American journal of physiology. Cell physiology 2007 Sep;293(3):C1032-48
Expression of connexins in embryonic mouse neocortical development.
Cina C, Bechberger JF, Ozog MA, Naus CC
The Journal of comparative neurology 2007 Sep 20;504(3):298-313
The Journal of comparative neurology 2007 Sep 20;504(3):298-313
Identification of connexin36 in gap junctions between neurons in rodent locus coeruleus.
Rash JE, Olson CO, Davidson KG, Yasumura T, Kamasawa N, Nagy JI
Neuroscience 2007 Jul 29;147(4):938-56
Neuroscience 2007 Jul 29;147(4):938-56
Modulation of connexin expression and gap junction communication in astrocytes by the gram-positive bacterium S. aureus.
Esen N, Shuffield D, Syed MM, Kielian T
Glia 2007 Jan 1;55(1):104-17
Glia 2007 Jan 1;55(1):104-17
In vitro optimization of antisense oligodeoxynucleotide design: an example using the connexin gene family.
Law LY, Zhang WV, Stott NS, Becker DL, Green CR
Journal of biomolecular techniques : JBT 2006 Sep;17(4):270-82
Journal of biomolecular techniques : JBT 2006 Sep;17(4):270-82
Connexin43 is overexpressed in Apc(Min/+)-mice adenomas and colocalises with COX-2 in myofibroblasts.
Husøy T, Knutsen HK, Cruciani V, Olstørn HB, Mikalsen SO, Løberg EM, Alexander J
International journal of cancer 2005 Sep 1;116(3):351-8
International journal of cancer 2005 Sep 1;116(3):351-8
Restoration of functional gap junctions through internal ribosome entry site-dependent synthesis of endogenous connexins in density-inhibited cancer cells.
Lahlou H, Fanjul M, Pradayrol L, Susini C, Pyronnet S
Molecular and cellular biology 2005 May;25(10):4034-45
Molecular and cellular biology 2005 May;25(10):4034-45
Freeze-fracture and immunogold analysis of aquaporin-4 (AQP4) square arrays, with models of AQP4 lattice assembly.
Rash JE, Davidson KG, Yasumura T, Furman CS
Neuroscience 2004;129(4):915-34
Neuroscience 2004;129(4):915-34
Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential.
Teubner B, Michel V, Pesch J, Lautermann J, Cohen-Salmon M, Söhl G, Jahnke K, Winterhager E, Herberhold C, Hardelin JP, Petit C, Willecke K
Human molecular genetics 2003 Jan 1;12(1):13-21
Human molecular genetics 2003 Jan 1;12(1):13-21
Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice.
Nagy JI, Ionescu AV, Lynn BD, Rash JE
Glia 2003 Dec;44(3):205-18
Glia 2003 Dec;44(3):205-18
Exocrine specific expression of Connexin32 is dependent on the basic helix-loop-helix transcription factor Mist1.
Rukstalis JM, Kowalik A, Zhu L, Lidington D, Pin CL, Konieczny SF
Journal of cell science 2003 Aug 15;116(Pt 16):3315-25
Journal of cell science 2003 Aug 15;116(Pt 16):3315-25
Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: roles in stem cell proliferation and morphological plasticity?
Mercier F, Hatton GI
The Journal of comparative neurology 2001 Feb 26;431(1):88-104
The Journal of comparative neurology 2001 Feb 26;431(1):88-104
Localization of connexin26 and connexin32 in putative CO(2)-chemosensitive brainstem regions in rat.
Solomon IC, Halat TJ, El-Maghrabi MR, O'Neal MH 3rd
Respiration physiology 2001 Dec;129(1-2):101-21
Respiration physiology 2001 Dec;129(1-2):101-21
Localization of connexin26 and connexin32 in putative CO(2)-chemosensitive brainstem regions in rat.
Solomon IC, Halat TJ, El-Maghrabi MR, O'Neal MH 3rd
Respiration physiology 2001 Dec;129(1-2):101-21
Respiration physiology 2001 Dec;129(1-2):101-21
Connexin26 in adult rodent central nervous system: demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43.
Nagy JI, Li X, Rempel J, Stelmack G, Patel D, Staines WA, Yasumura T, Rash JE
The Journal of comparative neurology 2001 Dec 24;441(4):302-23
The Journal of comparative neurology 2001 Dec 24;441(4):302-23
Connexin26 in adult rodent central nervous system: demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43.
Nagy JI, Li X, Rempel J, Stelmack G, Patel D, Staines WA, Yasumura T, Rash JE
The Journal of comparative neurology 2001 Dec 24;441(4):302-23
The Journal of comparative neurology 2001 Dec 24;441(4):302-23
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
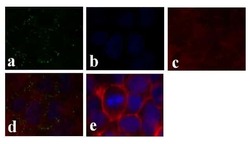
- Experimental details
- Immunofluorescence analysis of Connexin 26/GJB2 was done on 70% confluent log phase Caco-2 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with Connexin 26/GJB2 Rabbit polyclonal Antibody (Product # 51-2800) at 2 µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing junctional localization. Panel e shows no primary antibody control. The images were captured at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
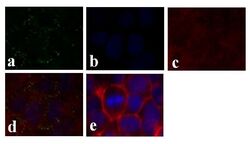
- Experimental details
- Immunofluorescence analysis of Connexin 26/GJB2 was done on 70% confluent log phase Caco-2 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with Connexin 26/GJB2 Rabbit polyclonal Antibody (Product # 51-2800) at 2 µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing junctional localization. Panel e shows no primary antibody control. The images were captured at 20X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
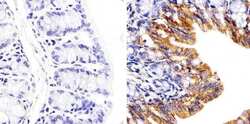
- Experimental details
- Immunohistochemistry analysis of Connexin 26/GJB2 showing staining in the cytoplasm of paraffin-embedded mouse colon tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Connexin 26/GJB2 polyclonal antibody (Product # 51-2800) diluted in 3% BSA-PBS at a dilution of 1:20 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
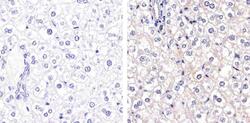
- Experimental details
- Immunohistochemistry analysis of Connexin 26 showing staining in the membrane of paraffin-embedded mouse liver tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Connexin 26 Polyclonal antibody (Product # 51-2800) diluted in 3% BSA-PBS at a dilution of 1:20 overnight at 4ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
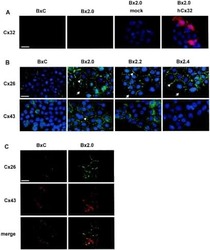
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
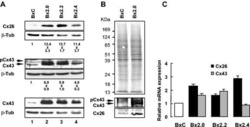
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
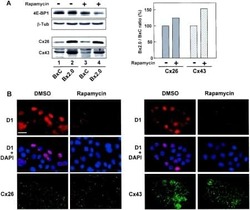
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
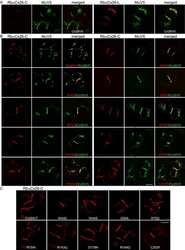
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
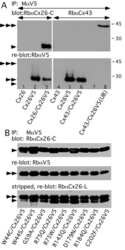
- Figure 5 Recovery of Cx26 expression in Cx26 Sox10Cre organotypic cultures transduced with BAAVCx26CFP. Top, immunoreactivity to Cx26 antibodies in a representative Cx26 loxP/loxP culture. Middle, lack of Cx26 immunoreactivity in a representative Cx26 Sox10Cre culture. Bottom, immunoreactivity to GFP antibodies, which also recognize CFP, in a representative Cx26 Sox10Cre culture transduced with BAAVCx26CFP. In all panels, actin filaments were stained with Texas red conjugated phalloidin (red). Scale bar, 10 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
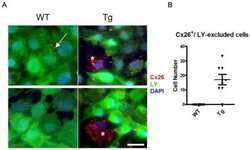
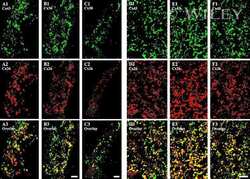
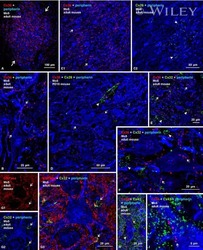
- Figure 5 Mammary tumors from Cx26 knockout mice express similar epithelial protein markers as control mice A. Representative images of paraffin-embedded primary mammary tumor sections immunolabelled with luminal and myoepithelial markers that included; Cx26 (i, ii, iii, red), Cx43 (iv, v, vi, red), keratin 14 (vi, viii green), keratin 8 (vii, green), keratin 10 (ix, red), alpha-smooth muscle actin (x, red), E-cadherin (xi, green) and beta-catenin (xii, red). Hoechst denotes nuclei. Scale bars=50 mum. B. Table indicates relative number of cells that are positive for the luminal and myoepithelial markers based on immunofluorescent labelling. +++50-100%, ++11-49%, +1-10%, -0%.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
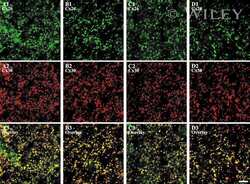
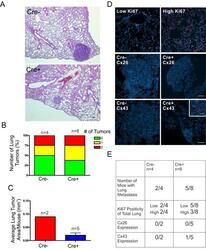
- Figure 6 DMBA-treated Cx26 knockout mice exhibit similar incidence of metastases to the lungs A. , B. Hematoxylin and eosin stained lung sections were evaluated for evidence of lung tumors revealing a similar proportion of mice that developed lung tumors in Cre+ and Cre- mice. C. Evaluation of average lung tumor area per mouse between Cre+ and Cre- mice revealed the likelihood of Cre- mice having larger lung tumor areas compared to Cre+ mice. D. Representative images of paraffin-embedded lung sections immunolabelled for Ki67 (Red), Cx26 (Red) and Cx43 (Red) revealed a similar percentage of lung tissue expressing high levels of Ki67 positivity between Cre- and Cre+ mice and tumors mostly negative, but not always (insert), for Cx26 and Cx43 expression. Hoechst denotes Nuclei. Scale bars = 50 mum. E. Quantification of Ki67, Cx26 and Cx43 immunofluorescent analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
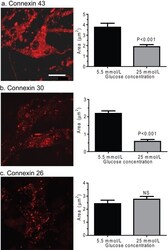
- Figure 8 Effect of hyperglycaemia on staining of immunoreactive Cx proteins in cultured astrocytes Composite z-stacks of confocal images (left column) of immunostained astrocytes showed a low-intensity background and prominent staining of punctate or vesicular immunoreactive material that appeared to be mainly intracellular. This morphological appearance of immunostaining was evident for Cx43 ( A ), Cx30 ( B ) and Cx26 ( C ) protein in astrocytes grown on coverslips for 14 days in a medium containing 5.5 mmol/l glucose; the scale bar is 12.5 mum and applies to all panels. Areas of these immunostained punctate/vesicular objects (right columns) are means (vertical bars represent 1 S.D.) from the following numbers of objects per group: Cx43, 5.5 mmol/l: n = 752 objects in cells on five coverslips; 25 mmol/l: n = 884 objects in cells on five coverslips; Cx30, 5.5 mmol/l: n = 1099 objects, five coverslips; 25 mmol/l: n = 1177 objects, five coverslips; Cx26, 5.5 mmol/l: n = 514 objects, four coverslips; and 25 mmol/l: n = 974 objects, five coverslips.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
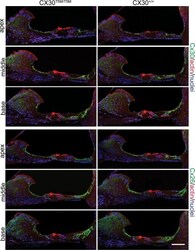
- Figure 3. Connexin immunoreactity in the adult organ of Corti. Maximal projection rendering of two consecutive midmodiolar confocal optical sections, taken at 1 mum intervals in the indicated cochlear turns of Cx30 T5M/T5M (left) and Cx30 +/+ mice (right) on P30. Cx30 and Cx26 expression was analysed with selective antibodies (green), nuclei were stained with DAPI (blue) and actin filaments with Texas red conjugated phalloidin (red). Scale bar, 50 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
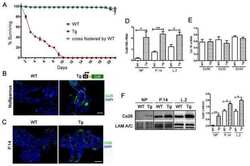
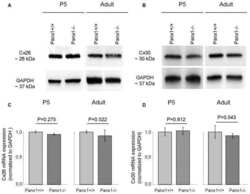
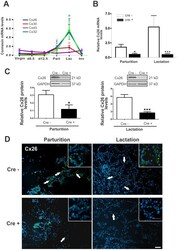
- Figure 1 BLG-Cre; Cx26 fl/fl mice exhibit a dramatic reduction in Cx26. (A) Real-time PCR analysis of wild-type mice revealed that Cx26, Cx32 and Cx30 are upregulated at parturition and lactation. (B, C) Real-time PCR and Western blot analysis of mammary glands from control (open columns) and Cre-treated (solid columns) mice revealed a dramatic reduction in Cx26 mRNA and protein levels at partition and lactation. *p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
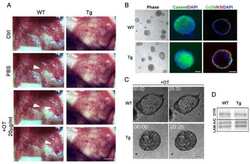
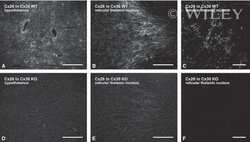
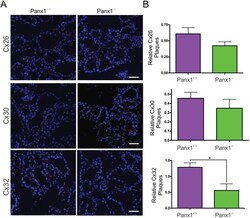
- Fig 7 Lactating Panx1 -/- mice have fewer Cx32 gap junctions in the mammary gland. (A) Immunofluorescent analysis of mammary gland cryosections during early lactation for Cx26, Cx30 and Cx32 (red) and cytokeratin14 (green) revealed no change in Cx26 and Cx30 gap junctions in knockout mice, while fewer Cx32 gap junctions were observed compared to control mice. Hoescht (blue) denotes nuclei. Scale bar = 50 um. (B) Values represent the mean number of connexin plaques (red) relative to the pixel area of the nuclei (blue), multiplied by a factor of 1x10 2 , per 0.3mm 2 +- S.E.M. N = 5.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
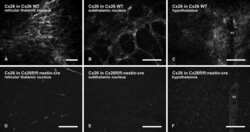
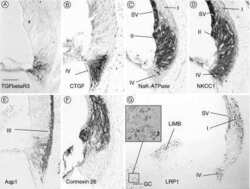
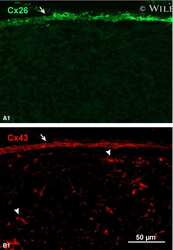
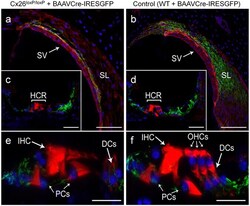
- Figure 3 Confocal immunofluorescence imaging of cochlear cross-sections from mice injected at P25 with BAAVCre-IRESGFP viral vectors. Color code: Cx26, green; actin filaments, red; nuclei, blue. ( a,b ) Stria vascularis (SV) and spiral ligament (SL). ( c,d ) organ of Corti; HCR, hair cell region. ( e,f ) close-up view of the HCR from c,d, respectively. DCs: Deiters' cells; IHC: inner hair cell; OHCs: outer hair cells; PCs: pillar cells. Scale bars: 50 mum in ( a-d ); 25 mum in ( e,f ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 5 Recovery of Cx26 expression in Cx26 Sox10Cre organotypic cultures transduced with BAAVCx26CFP. Top, immunoreactivity to Cx26 antibodies in a representative Cx26 loxP/loxP culture. Middle, lack of Cx26 immunoreactivity in a representative Cx26 Sox10Cre culture. Bottom, immunoreactivity to GFP antibodies, which also recognize CFP, in a representative Cx26 Sox10Cre culture transduced with BAAVCx26CFP. In all panels, actin filaments were stained with Texas red conjugated phalloidin (red). Scale bar, 10 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
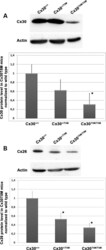
- Figure 4. Quantitative immunoblot analyses of connexin expression in the adult cochlea. Protein levels were normalized to their corresponding actin bands for quantification. Asterisk in ( A ) indicates a significant reduction in Cx30 in Cx30 T5M/T5M mice compared with Cx30 protein level in wild-type mice. Asterisks in ( B ) indicate a significant reduction in Cx26 in Cx30 T5M/T5M mice compared with Cx26 protein level in wild-type mice.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
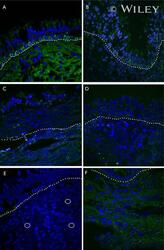
- Experimental details
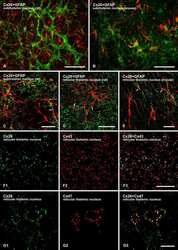
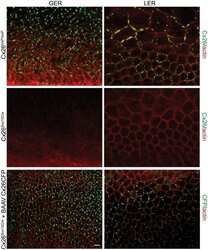
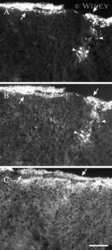
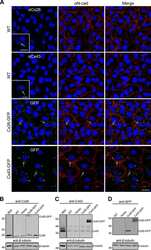
- Representative immunohistochemistry of Cx26, Cx30, and Cx43 in sinus mucosa of control and CRS patients. The white dotted line demarcates the epithelium and stroma. Nuclei were stained with DAPI (blue). (A, B) Paired control and CRS patient sections with Cx26 staining (green) around the basal epithelial nuclei. (C, D) Paired control and CRS patient sections with Cx30 staining (green) through the epithelial layers, with some green autofluorescence within the stroma. (E) Minimal Cx43 in the stroma (white circles) of a control patient. (F) CRS patient showed massive elevation of Cx43 in the stroma. CRS = chronic rhinosinusitis; DAPI = 4',6-diamidino-2-phenylindole.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
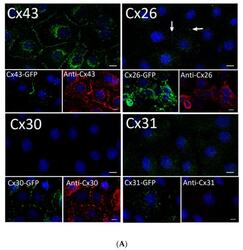
- Experimental details
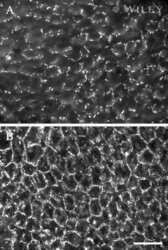
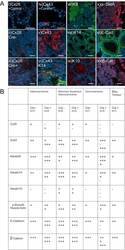
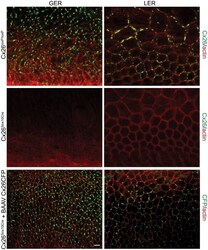
- Figure 2 Rat epidermal keratinocytes (REKs) express endogenous Cx43 and retain the capacity to assemble GFP-tagged Cx26, Cx30 and Cx31 into gap junctions. ( A ) Basal keratinocyte-like REKs express endogenous Cx43, trace amounts of Cx26 (arrows) but no detectable levels of Cx30 or Cx31 protein. REKs engineered to express GFP-tagged connexins were used to demonstrate that the anti-connexin antibodies were efficient at detecting connexins with the exception of the anti-Cx31 antibody. ( B ) REKs engineered to express GFP-tagged Cx43, Cx26, Cx30 and Cx31 all assembled prototypical gap junctions characterized by small punctate structures at sites of cell-cell interface (arrows). In addition, an array of intracellular fluorescent structures could be identified that reflect organelles and vesicles involved in connexin transport. Nuclei were stained with Hoechst dye. Bars = 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
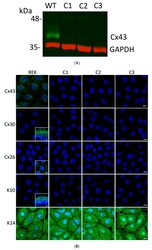
- Experimental details
- Figure 3 Ablation of Cx43 did not act to stimulate connexin reprogramming or differentiation of REKs. ( A ) CRISPR-Cas9 was effectively used to ablate Cx43 from REKs as revealed by immunoblotting wild-type (WT) and three separate clones (C1, C2, C3) for Cx43. Immunoblotting for GAPDH was used as a loading control. ( B ) Immunofluorescent labeling for Cx43, Cx26 and Cx30 revealed that Cx43 was ablated from all three CRISPR-Cas9 treated clones and neither Cx26 nor Cx30 exhibited increased expression to compensate for the loss of Cx43. Immunolabeling for keratin 10 (K10) and keratin 14 (K14) revealed that REKs retained their basal cell characteristics upon Cx43 ablation. Inserts represent transverse or en face sections of mouse skin as positive controls for anti-Cx30 and anti-K10 antibodies while normal rat kidney cells engineered to express Cx26-GFP were used as a positive control for the anti-Cx26 antibody. Nuclei were stained with Hoechst dye. Bars = 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
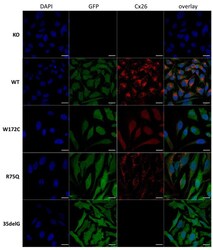
- Experimental details
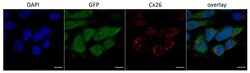
- Figure 1 Localization of Cx26 in several newly established HeLa cell lines (confocal micrographs). The panels of Cx26 variants are designated (top down) as KO, WT, W172C, R75Q, and 35delG for cell lines HeLa Cx26-KO, HeLa-Cx26wt, HeLa-p.W172C, HeLa-p.R75Q, and HeLa-c.35delG, respectively. Nuclei were visualized with DAPI (blue), transgenic HeLa cells were visualized with Green Fluorescent Protein (GFP) and Cx26 was immunostained with antibodies against C-terminus of Cx26 (red). Scale bar = 20 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 2 A fraction of HeLa-p.W172C cells with reduced number of protein granules (confocal micrographs). Blue color indicates DAPI nuclei staining, green color corresponds to GFP signal, red color corresponds to Cx26 signal. Scale bar = 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
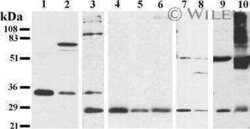
- Figure 3 Analysis of the Cx26 protein expression in transgenic HeLa cell lines. The total lysates of cells were subjected to Western blot analysis with antibodies against Cx26 and the loading control, alpha-tubulin. The panels of Cx26 variants are designated as KO, WT, W172C, R75Q, and 35delG for cell lines HeLa Cx26-KO, HeLa-Cx26wt, HeLa-p.W172C, HeLa-p.R75Q, and HeLa-c.35delG, respectively.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
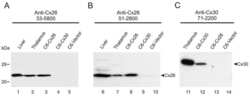
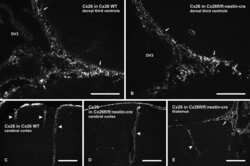
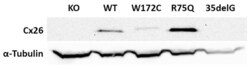
- 4 Distribution of Cx26 in subependymal and subpial zones. A: Intense Cx26-ir punctation (antibody 71-0500; green) in the subependymal layer (SEL) of the third ventricle (3V). Note the decreasing gradient of intensity from the ependymal borderline. Ependymocytes (Ep) and blood vessels (arrows) appear pink due to immunoreactivity for both S100beta (red) and vimentin (blue). B: Intense immunoreactivity for basic fibroblast growth factor (bFGF) in the nuclei of astrocytes in the SEL of 3V. C: The presence of numerous Cx26-ir puncta (antibody 51-2800) in meningeal projections (M) of the lateral ventricle (LV). Puncta were small in the SEL but large on the periphery of blood vessels (BV). Note the lines of small, Cx26-ir puncta along the arrows. D: Immunoreactivity for Cx26 (antibody 51-2800; green) and Cx43 (antibody 13-8300; red) was detected either as double-labeled puncta (arrows) or as single puncta (arrowheads) in the SEL of 3V. The ependyma was primarily Cx43 immunoreactive. E: Distribution of Cx26 immunoreactivity (antibody 13-8100) in the supraoptic nucleus (SON) and underlying meninges. Note the similar concentration of Cx26-ir puncta in the meninges and ventral glial limitans (vgl). White dashed lines indicate the brain surface. Cx26 immunoreactivity was intense in proximity to BV. OT, optic tract; Amyg, amygdala. Scale bars = 20 mum in A; 25 mum in B-D; 50 mum in E.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
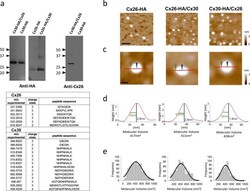
- Figure 1 Molecular volumes obtained for homomeric Cx26-HA, heteromeric Cx26-HA/Cx30, and Cx30-HA/Cx26 hemichannels from AFM imaging. a , identification of purified hemichannels. Upper , detection of Cx30-HA/Cx26, Cx26-HA/Cx30, and Cx26-HA protein in eluates from HA-agarose columns. Samples were analyzed by SDS-PAGE and immunoblotting, using mono and polyclonal anti-HA and anti-Cx26 primary Ab, respectively, followed by a horseradish peroxidase-conjugated goat anti-mouse and rabbit secondary Ab, respectively. Lower , peptide sequences identified by MS analysis after tryptic digestion from heteromeric Cx30-HA/Cx26 hemichannels, corresponding to sequences found for both Cx30 and Cx26 isoforms. b , low-magnification image ( scale bar , 50 nm). c , high-magnification images of single proteins. Sections through particles are shown as red lines including two points, height and radius at half height ( blue and green arrows , respectively) ( scale bar , 20 nm). d , particle height analysis of the indicated section. e , frequency distribution of molecular volumes. Black curves indicate fitted Gaussian functions.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
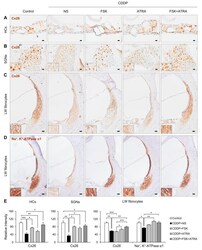
- Figure 5 Immunohistochemical staining of Cx26 and Na + /K + -ATPase in rats treated with NS, FSK, ATRA, or FSK + ATRA under CDDP exposure. Representative images of Cx26 (brown, DAB) in the OC ( A ), SGNs ( B ), and LW fibrocytes ( C ) obtained from the middle turn of the cochlea (n = 5 per group). ( D ) Representative image of Na + /K + -ATPase in LW fibrocytes. The cells were counterstained with hematoxylin (blue). The inset shows a magnified image of the square box. ( E ) Quantification of the chromogenic intensity in the OC, SGNs, and LW fibrocytes in the middle turn of the cochlea in each group. Scale bar = 20 mum * p < 0.05, ** p < 0.01, *** p < 0.001. NS, normal saline; CDDP, cisplatin; FSK, forskolin; ATRA, all-trans retinoic acid; OC, organ of Corti; SGNs, spiral ganglion neurons; LW, lateral wall; Cx26, connexin26.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
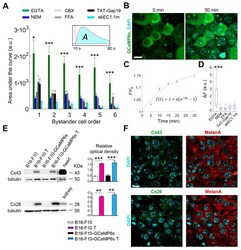
- Figure 2 Connexin (Cx) hemichannels (HCs) expressed in melanoma cells mediate the propagation of calcium (Ca 2+ ) waves induced by focal photodynamic therapy (fPDT) in vivo. ( A ) Pooled results of fPDT trials in GCaMP6s-expressing dorsal skinfold chamber (DSC) tumors in the following conditions: Ca 2+ -free extracellular medium (CFEM) supplemented with ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA, 5 mM); normal extracellular medium containing 2 mM of Ca 2+ (NEM, control); CFEM supplemented with EGTA (5 mM) plus carbenoxolone (CBX, 100 uM) or flufenamic acid (FFA, 100 uM) or TAT-Gap19 (150 uM) or abEC1.1m (1 muM). The histogram shows the area under GCaMP6s Delta F / F 0 traces ( A , inset) computed between the onset of fPDT ( t = 10 s) and the end of the observation time window ( t = 80 s) for each bystander cell order (abscissa); pooled data [mean +- standard error of the mean (s.e.m.)] for n >= 6 experiments in at least 2 different tumors for each condition. a.u., arbitrary units; *, p < 0.05; ***, p < 0.001, the Kruskal-Wallis test (for post hoc pairwise comparisons, see Table S1 ). Data for EGTA and NEM conditions are also shown in Figure 1 C; ( B - D ) In vivo 4',6-Diamidine-2'-phenylindole dihydrochloride (DAPI) uptake experiments: GCaMP6s-expressing DSC melanomas were incubated with DAPI (5 muM) dissolved in: CFEM supplemented with 5 mM of EGTA; NEM; CFEM supplemented with 5 mM of EGTA plus FFA (100 uM) or TAT-Gap19 (150 uM) or abEC1.1m (
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
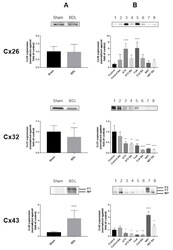
- Figure 2 Connexin protein expression in cholestasis. Semi-quantitative immunoblot analysis of Cx26, Cx32 and Cx43 species in livers of cholestatic mice ( A ) and in human hepatoma HepaRG cells cultured in cholestatic conditions ( B ) was performed. For Cx43, both the phosphorylated (P) and non-phosphorylated (NP) variant could be detected. Signals of the three connexins were normalized against total protein loading and expressed as relative alterations compared to sham-operated animals or to control samples, respectively. ( A ) Liver sections were obtained from male mice following bile duct ligation (BDL) for 20 days. Data were processed by a parametric student t-test with Welch's correction or a non-parametric Mann-Whitney test. Data are expressed as means +/- SD with * p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
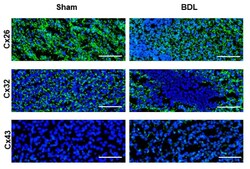
- Figure 5 Connexin protein localization in livers of cholestatic mice. Liver sections were obtained from male mice following bile duct ligation (BDL) for 20 days. Cellular localization of Cx26, Cx32 and Cx43 (green) was revealed by immunohistochemistry analysis with nuclear counterstaining using DAPI (blue). Scale bar, 100 um (Sham n = 3; BDL n = 3) (N = 1).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
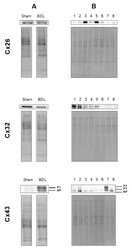
- Experimental details
- Figure 8 Total protein loading of samples used in immunoblot analysis. Semi-quantitative immunoblot analysis of Cx26, Cx32 and Cx43 in liver of cholestatic mice ( A ) and in human hepatoma HepaRG cells cultured in cholestatic conditions ( B ) was performed. For Cx43, both the phosphorylated (P) and non-phosphorylated (NP) variant could be detected. Signals of the three connexins were normalized against total protein loading, which are shown for two representative samples. ( A ) Liver sections were obtained from male mice following bile duct ligation (BDL) for 20 days (Sham n = 12; BDL n = 18) (N = 1). ( B ) Human hepatoma HepaRG cells were exposed to cholestatic drugs either in the absence or presence of a 50x concentrated mixture of bile acids (BA) for 72 h and compared to untreated human hepatoma HepaRG cells, indicated in the figure as control. The different experimental conditions are presented in the figure as: 1 = control ( n = 3); 2 = control BA ( n = 3); 3 = atazanavir (ATV) ( n = 3); 4 = ATV BA ( n = 3); 5 = cyclosporine A (CsA) ( n = 3); 6 = CsA BA ( n = 3); 7 = nefazodone (NEF) ( n = 3); 8 = NEF BA) ( n = 3) (N = 1).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
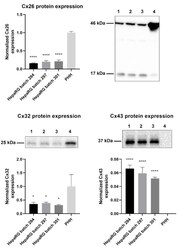
- Experimental details
- Figure 1 Cx26, Cx32, and Cx43 protein expression in human hepatoma HepaRG cells compared to primary human hepatocytes (PHH). Proteins were extracted from human hepatoma HepaRG cells ( n = 3 and N = 3) and PHH ( n = 1 and N = 3) and immunoblotting was performed. Densiometric quantitative data were obtained via Image Lab 6.0.1 software (Bio-Rad, Hercules, CA, USA). Data were normalized to the total protein loading ( Figure S1 ) and expressed as a ratio to PHH (if the target was expressed in PHH). Significant differences compared to PHH were calculated with a parametric one-way analysis of variance (ANOVA) followed by a Dunnett's post-hoc test to correct for multiple comparisons. Data are expressed as mean +- standard deviation with * p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
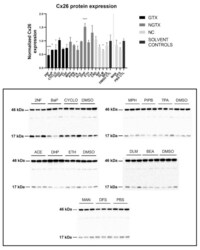
- Experimental details
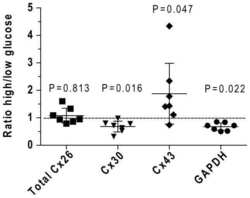
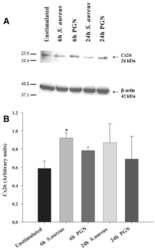
- Cx26 protein expression in human hepatoma HepaRG cells exposed to GTX, NGTX, and NC chemicals. Human hepatoma HepaRG cells ( n = 3 and N = 1) were exposed to (non)-carcinogenic chemicals for 72 h. Densiometric quantitative data were obtained via Image Lab 6.0.1 software (Bio-Rad, Hercules, CA, USA). Data were normalized to the total protein loading ( Figure S2 ) according to Bio-Rad's instructions [] and expressed as a ratio to their respective solvent control (DMSO CTL or PBS CTL). Significant difference compared to the solvent control (DMSO CTL or PBS CTL) was calculated with a parametric one-way ANOVA followed by a Dunnett's post-hoc test to correct for multiple comparisons. Data are expressed as mean +- standard deviation with * p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
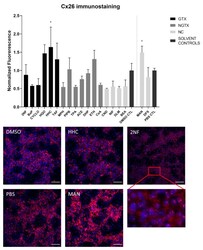
- Experimental details
- Cx26 protein localization in human hepatoma HepaRG cells exposed to GTX, NGTX, and NC chemicals. Human hepatoma HepaRG cells ( n = 1 and N = 1; at least three images per well) were exposed to GTX, NGTX, and NC chemicals for 72 h. The area and intensity of the fluorescent signal was quantified via ImageJ software (version 1.52p, Bethesda, MD, USA) and normalized to the fluorescent signal of the respective solvent control (DMSO CTL or PBS CTL). Significant difference compared to the solvent control was calculated with a parametric one-way ANOVA followed by a Dunnett's post-hoc test to correct for multiple comparisons. Data are expressed as mean +- standard deviation with * p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
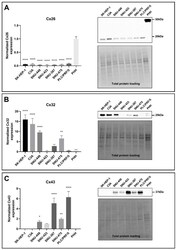
- Figure 3 Cx26 ( A ), Cx32 ( B ) and Cx43 ( C ) protein expression in liver cancer cell lines and primary human hepatocytes (PHH). Cancer cell lines ( n = 1, N = 3) were grown to 100% confluence, while PHH were used in suspension for protein extraction. Immunoblotting and visualization were done with the Pierce(tm) ECL Western Blotting Substrate kit (Thermo Fisher Scientific, Waltham, MA, USA) on a ChemiDoc TM MP imaging system. All signals were divided by their respective total protein loading signal and normalized by the sum of all data points in a replicate []. Unlike Cx43, which was not expressed by PHH, Cx26 and Cx32 are expressed relative to their expression in PHH. Data are expressed as mean +- standard deviation with * p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
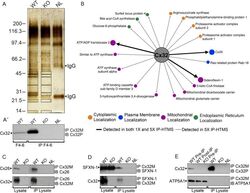
- Figure 2 Identification of novel Cx32 interactors by IP-HTMS ofCx32-enrichedliver membrane fractions and validation by co-IP of tissue lysates.(A) Silver stain of proteins found in sucrose gradient fractions 4-6(Figure 1 B) following IP with monoclonal anti-Cx32(Table 1 ). Negative controls included IP reactionsfrom KO fractions 4-6 (Supplemental Figure 1, Supporting Information ) and a no lysate (NL) mock IP reaction.Black boxes indicate gel regions excised for tryptic digest. Mobilityof precipitating IgG heavy and light chains are indicated by arrowheads.(A') Western blot of Cx32-enriched fractions 4-6 (F4-6,5 mug) and 1 muL (10%) of the corresponding IP productsfrom these same fractions analyzed in (A). Analysis confirmed presenceof bait (Cx32) in WT samples and lack of cross-reactivity in KO samples.Cx32-M indicates use of monoclonal anti-Cx32; Cx32-P indicates useof polyclonal anti-Cx32 antibody (Table 1 ).(B) IP-HTMS identified one known (Cx26) and 17 novel Cx32 interactingpartners in replicate screens of 1 mg and 5 mg input protein. Interactionnetwork generated by Osprey version 1.2.0 of proteins present in WTbut not KO IP-HTMS. Proteins identified in both the 1 mg and 5 mgscreens are represented with larger icons and thicker interactionlines than proteins identified only in the 5 mg screen. (C) Reciprocalco-IPs of liver lysates for Cx32 and Cx26. (D) Reciprocal co-IPs forCx32 and SFXN-1. Specificity was also verified in independent IPsusing an isotype IgG control
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
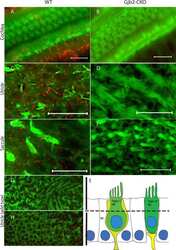
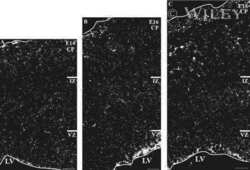
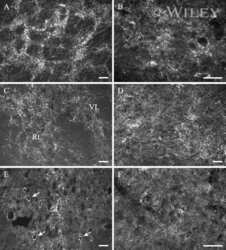
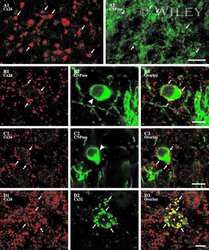
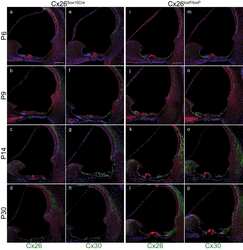
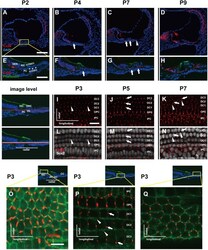
- Fig. 1 Distribution patterns of Cx26 in different SCs of the mouse cochlea at different postnatal time points. a - d Postnatal Cx26 immunolabeling (red) in cochlear radial sections. The yellow box in a indicates the location of the organ of Corti. e - h Postnatal Cx26 immunolabeling (red) in radial sections of the organ of Corti. Asterisks indicate the different rows of DCs. D1:DC1, the first row of DCs; O1:OHC1, the first row of OHCs. White arrows and arrowheads indicate different SCs that show Cx26 expression. i - k Postnatal Cx26 immunolabeling (red) in flattened preparations. l - n Postnatal Cx26 (red) and Sox2 (white) immunolabeling in corresponding flattened preparations. Images of the radial section (left) are given to show the levels of the images. The white arrowheads indicate Cx26 expression on the radial sides of DCs. The white arrows indicate longitudinal Cx26 expression in DCs. o Cx26 immunolabeling in ISCs at P3. p Cx26 immunolabeling in different rows of DCs at P3. q Cx26 immunolabeling in CCs at P3. Images of the radial section are shown on top of o , p , and q , and the yellow boxes in the images are the areas of magnification in o , p , and q . GER great epithelial ridge, ISC inner sulcus cell, PC pillar cell, IHC inner hair cell, OHC outer hair cell, CC Claudius cell. The scales in panels a , e , i , and o represent 100, 40, 30, and 10 um, respectively
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
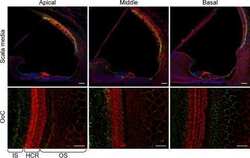
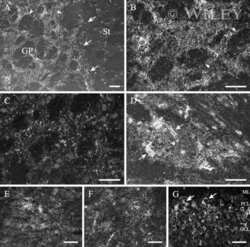
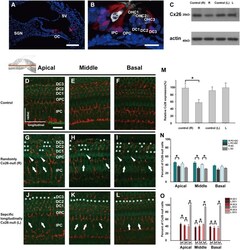
- Fig. 2 Cx26 expression patterns in different experimental groups. a , b The tdTomato staining (red) shows that Cre was activated in cells in the cochlea ( a ) and the organ of Corti (OC) ( b ). c Representative western blot results show Cx26 expression in the control groups, the randomly Cx26- group (R group, R), and the specific longitudinally Cx26- group (L group, L) at P7. d - f Cx26 immunolabeling in the apical, middle, and basal turns of the control group, respectively. g - i Cx26 immunolabeling in the apical, middle, and basal turns of the R group, respectively. j - l Cx26 immunolabeling in the apical, middle, and basal turns of the L group, respectively. Asterisks indicate the Cx26- DCs. The white arrowheads indicate the region of Cx26- OPCs, whereas the white arrows indicate the region of Cx26- IPCs. m Relative expression of cochlear Cx26 at P7 ( n = 4 in each group) in the control and experimental groups. n Cx26- cell counts at P7 in different turns of the cochlea from different experimental groups ( n = 3 in each group). o Cx26- DC counts at P7 ( n = 3 in each group). *Significant difference between two groups ( P < 0.05). SV striae vascularis, SGN spiral ganglion neuron. The scales in panels a , b , and d represent 100, 30, and 30 um, respectively
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
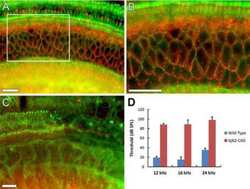
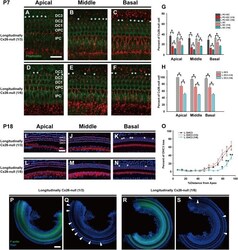
- Fig. 5 Cx26 expression patterns and OHC loss patterns in different longitudinally Cx26- groups. a - c Cx26 immunolabeling (red) in the apical, middle, and basal turns of the longitudinally Cx26- group (1/3), respectively. d - f Cx26 immunolabeling in the apical, middle, and basal turns of the longitudinally Cx26- group (1/6), respectively. Asterisks indicate Cx26- DCs. g Cx26- cell counts at P7 in different turns of the organ of Corti ( n = 3 in each group). h Cx26- DC3 counts at P7 ( n = 3 in each group). * and # indicate significant differences between two groups ( P < 0.05). i - k Representative images of HCs in the apical ( i ), middle ( j ), and basal turns ( k ) from the longitudinally Cx26- group (1/3). l - n Representative images of HCs in the different turns from the longitudinally Cx26- group (1/6). o Quantifications of OHC3 loss at specific cochlear locations in different groups at P18. Asterisk indicates a significant difference compared with the specific longitudinally Cx26- group. The hash symbol indicates a significant difference compared with the longitudinally Cx26- group (1/3). p - s Representative images of the middle-basal parts of flattened cochlear preparations in different groups. White arrowheads indicated the regions of OHC3 loss. The scales in panels a , i , and p represent 30, 40, and 200 um, respectively
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry