Antibody data
- Antibody Data
- Antigen structure
- References [3]
- Comments [0]
- Validations
- Immunocytochemistry [4]
- Flow cytometry [1]
- Other assay [2]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA3-069 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CXCR7 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Other
- Description
- PA3-069 detects CXC Chemokine Receptor Type 7 from human and mouse samples. PA3-069 has been successfully used in Western blot and immunocytochemistry procedures. By Western blot, this antibody detects a 42 kDa protein corresponding to mouse CXC Chemokine Receptor Type 7. The PA3-069 antigen is the COOH-terminal amino acids of mouse CXCR7 (347362) SRVSETEYSALEQNTK coupled to KLH.
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- Conc. Not Determined
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Novel insights into neuroinflammation: bacterial lipopolysaccharide, tumor necrosis factor α, and Ureaplasma species differentially modulate atypical chemokine receptor 3 responses in human brain microvascular endothelial cells.
MicroRNA-126/stromal cell-derived factor 1/C-X-C chemokine receptor type 7 signaling pathway promotes post-stroke angiogenesis of endothelial progenitor cell transplantation.
Mechanisms of CXCR7 induction in malignant melanoma development.
Silwedel C, Speer CP, Haarmann A, Fehrholz M, Claus H, Buttmann M, Glaser K
Journal of neuroinflammation 2018 May 23;15(1):156
Journal of neuroinflammation 2018 May 23;15(1):156
MicroRNA-126/stromal cell-derived factor 1/C-X-C chemokine receptor type 7 signaling pathway promotes post-stroke angiogenesis of endothelial progenitor cell transplantation.
Shan C, Ma Y
Molecular medicine reports 2018 Apr;17(4):5300-5305
Molecular medicine reports 2018 Apr;17(4):5300-5305
Mechanisms of CXCR7 induction in malignant melanoma development.
Li XJ, Liu P, Tian WW, Li ZF, Liu BG, Sun JF
Oncology letters 2017 Oct;14(4):4106-4114
Oncology letters 2017 Oct;14(4):4106-4114
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
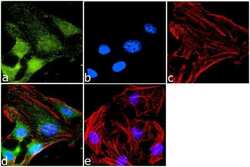
- Experimental details
- Immunofluorescence analysis of CXCR7 was done on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with CXCR7 Rabbit Polyclonal Antibody (Product # PA3-069) at 1:1000 dilution in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing Cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
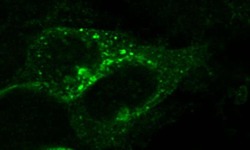
- Experimental details
- Immunocytochemistry of mouse CXCR7 in HEK293 cells
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
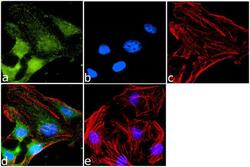
- Experimental details
- Immunofluorescence analysis of CXCR7 was done on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with CXCR7 Rabbit Polyclonal Antibody (Product # PA3-069) at 1:1000 dilution in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing Cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
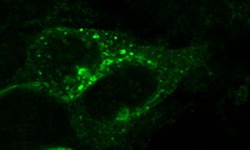
- Experimental details
- Immunocytochemistry of mouse CXCR7 in HEK293 cells
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
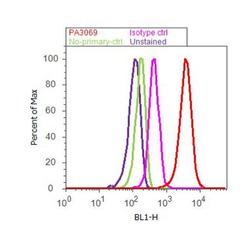
- Experimental details
- Flow cytometry analysis of CXCR7 was done on HeLa cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with CXCR7 Rabbit Polyclonal Antibody (PA3069, red histogram) or with rabbit isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
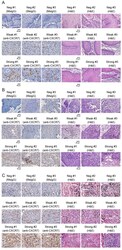
- Experimental details
- Figure 1. Immunohistochemistry staining of CXCR7 protein in skin cancer tissues. Three selected images of negative (MsIgG) control, weak and strong CXCR7 staining, and their H&E staining were presented for each cancer category. Representative images of staining with MsIgG, anti-CXCR7 or H&E for (A) squamous cell carcinoma, (B) basal cell carcinoma and (C) malignant melanoma tissues. Positive CXCR7 staining appears as a brown color in the cytoplasm. Scale bar, 20 um. MsIgG, mouse immunoglobulin G; CXCR7, C-X-C chemokine receptor type 7; H&E, hematoxylin and eosin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
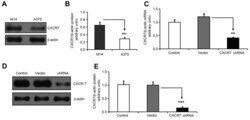
- Experimental details
- Figure 2. CXCR7 shRNA knocks down CXCR7 mRNA and protein expression levels in melanoma M14 cells. (A) Western blot analysis of CXCR7 protein expression levels in melanoma M14 and A375 cells. beta-actin was used as a protein level control. (B) M14 cells had significantly higher CXCR7 protein expression compared with that of A375 cells. The CXCR7/beta-actin protein ratio in M14 and A375 cells was 0.649+-0.080 and 0.288+-0.040, respectively (**P=0.006). (C) CXCR7 shRNA significantly reduced CXCR7 mRNA expression in M14 cells. CXCR7 shRNA knock down was performed in M14 cells. Empty vector-transfected and non-transfected M14 cells were used as controls. Polymerase chain reaction was conducted to monitor CXCR7 mRNA expression. beta-actin was used as an internal mRNA level control. The CXCR7/beta-actin mRNA ratio was 1.000+-0.102 (control), 1.211+-0.117 (vector-transfected) and 0.412+-0.023 (CXCR7 shRNA-transfected) in M14 cells. (**P=0.007 was obtained for the comparison between vector-transfected and CXCR7 shRNA-transfected cells). (D) Western blot images of CXCR7 and beta-actin protein expression levels. (E) CXCR7 shRNA significantly inhibited CXCR7 protein expression in M14 cells. The CXCR7/beta-actin protein ratio was 1.000+-0.002 (control), 1.016+-0.004 (vector) and 0.144+-0.005 (CXCR7 shRNA) in the different groups, which confirmed the knockdown of CXCR7 protein expression in M14 cells (***P
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry