PA1-901
antibody from Invitrogen Antibodies
Targeting: ITPR1
ACV, Insp3r1, IP3R1, PPP1R94, SCA15, SCA16, SCA29
 Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [42]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [1]
- Flow cytometry [1]
- Other assay [22]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-901 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- IP3 Receptor 1 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA1-901 detects inositol 1,4,5-trisphosphate receptor type-I (IP3R-I) from canine, mouse and rat tissues. PA1-901 has been successfully used in Western blot, immunohistochemistry, immunofluoresence and immunoprecipitation procedures. By Western blot, this antibody detects an ~240 kDa protein representing IP3R-I from rat and mouse brain extract. Immunohistochemical staining of IP3R-I in rat cerebellum with PA1-901 results in staining of Purkinje cells. PA1-901 immunizing peptide corresponds to amino acid residues 1829-1848 from human IP3R-I. This sequence is completely conserved in human, mouse, and rat IP3R-I. PA1-901 immunizing peptide (Cat. # PEP-019) is available for use in neutralization and control experiments.
- Reactivity
- Human, Mouse, Rat, Canine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- 1.3 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Kctd7 deficiency induces myoclonic seizures associated with Purkinje cell death and microvascular defects.
Alpinumisoflavone Disrupts Endoplasmic Reticulum and Mitochondria Leading to Apoptosis in Human Ovarian Cancer.
iRhom pseudoproteases regulate ER stress-induced cell death through IP(3) receptors and BCL-2.
Dual antibody strategy for high-resolution imaging of murine Purkinje cells and their dendrites across multiple layers.
β2-adrenergic receptor regulates ER-mitochondria contacts.
FUNDC1-dependent mitochondria-associated endoplasmic reticulum membranes are involved in angiogenesis and neoangiogenesis.
Osthole interacts with an ER-mitochondria axis and facilitates tumor suppression in ovarian cancer.
Itpr1 regulates the formation of anterior eye segment tissues derived from neural crest cells.
Stigmasterol Causes Ovarian Cancer Cell Apoptosis by Inducing Endoplasmic Reticulum and Mitochondrial Dysfunction.
Fucoidan Derived from Fucus vesiculosus Inhibits the Development of Human Ovarian Cancer via the Disturbance of Calcium Homeostasis, Endoplasmic Reticulum Stress, and Angiogenesis.
XBP1-KLF9 Axis Acts as a Molecular Rheostat to Control the Transition from Adaptive to Cytotoxic Unfolded Protein Response.
Voltage-dependent Ca(2+) entry into smooth muscle during contraction promotes endothelium-mediated feedback vasodilation in arterioles.
Antibodies to inositol 1,4,5-triphosphate receptor 1 in patients with cerebellar disease.
An optimized surgical approach for obtaining stable extracellular single-unit recordings from the cerebellum of head-fixed behaving mice.
Pumilio1 haploinsufficiency leads to SCA1-like neurodegeneration by increasing wild-type Ataxin1 levels.
Stable expression and function of the inositol 1,4,5-triphosphate receptor requires palmitoylation by a DHHC6/selenoprotein K complex.
TGFβ1 downregulates neurite outgrowth, expression of Ca2+ transporters, and mitochondrial dynamics of in vitro cerebellar granule cells.
Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity.
Triadin/Junctin double null mouse reveals a differential role for Triadin and Junctin in anchoring CASQ to the jSR and regulating Ca(2+) homeostasis.
Increased arterial smooth muscle Ca2+ signaling, vasoconstriction, and myogenic reactivity in Milan hypertensive rats.
Investigation of the role of sigma1-receptors in inositol 1,4,5-trisphosphate dependent calcium signaling in hepatocytes.
Paclitaxel accelerates spontaneous calcium oscillations in cardiomyocytes by interacting with NCS-1 and the InsP3R.
A novel postsynaptic mechanism for heterosynaptic sharing of short-term plasticity.
Regulation of multidrug resistance-associated protein 2 by calcium signaling in mouse liver.
Intracellular Ca2+ regulating proteins in vascular smooth muscle cells are altered with type 1 diabetes due to the direct effects of hyperglycemia.
Efficient generation of mature cerebellar Purkinje cells from mouse embryonic stem cells.
Ablation of skeletal muscle triadin impairs FKBP12/RyR1 channel interactions essential for maintaining resting cytoplasmic Ca2+.
Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry.
Mini-dystrophin expression down-regulates overactivation of G protein-mediated IP3 signaling pathway in dystrophin-deficient muscle cells.
Mini-dystrophin expression down-regulates IP3-mediated calcium release events in resting dystrophin-deficient muscle cells.
The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria.
Plasma and intracellular membrane inositol 1,4,5-trisphosphate receptors mediate the Ca(2+) increase associated with the ATP-induced increase in ciliary beat frequency.
Individual subtypes of enteroendocrine cells in the mouse small intestine exhibit unique patterns of inositol 1,4,5-trisphosphate receptor expression.
InsP3 receptor type 2 and oscillatory and monophasic Ca2+ transients in rat adrenal chromaffin cells.
ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors.
IP3 receptors and associated Ca2+ signals localize to satellite cells and to components of the neuromuscular junction in skeletal muscle.
Type-specific inositol 1,4,5-trisphosphate receptor localization in the vomeronasal organ and its interaction with a transient receptor potential channel, TRPC2.
Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction.
Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction.
IP(3) receptor function and localization in myotubes: an unexplored Ca(2+) signaling pathway in skeletal muscle.
Inhibition of type I and III IP(3)Rs by TGF-beta is associated with impaired calcium release in mesangial cells.
Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux.
Liang JH, Alevy J, Akhanov V, Seo R, Massey CA, Jiang D, Zhou J, Sillitoe RV, Noebels JL, Samuel MA
Disease models & mechanisms 2022 Sep 1;15(9)
Disease models & mechanisms 2022 Sep 1;15(9)
Alpinumisoflavone Disrupts Endoplasmic Reticulum and Mitochondria Leading to Apoptosis in Human Ovarian Cancer.
Hong T, Ham J, Song G, Lim W
Pharmaceutics 2022 Mar 4;14(3)
Pharmaceutics 2022 Mar 4;14(3)
iRhom pseudoproteases regulate ER stress-induced cell death through IP(3) receptors and BCL-2.
Dulloo I, Atakpa-Adaji P, Yeh YC, Levet C, Muliyil S, Lu F, Taylor CW, Freeman M
Nature communications 2022 Mar 10;13(1):1257
Nature communications 2022 Mar 10;13(1):1257
Dual antibody strategy for high-resolution imaging of murine Purkinje cells and their dendrites across multiple layers.
Botta S, Chemiakine A, Gennarino VA
STAR protocols 2022 Jun 17;3(2):101427
STAR protocols 2022 Jun 17;3(2):101427
β2-adrenergic receptor regulates ER-mitochondria contacts.
Lim Y, Cho IT, Rennke HG, Cho G
Scientific reports 2021 Nov 2;11(1):21477
Scientific reports 2021 Nov 2;11(1):21477
FUNDC1-dependent mitochondria-associated endoplasmic reticulum membranes are involved in angiogenesis and neoangiogenesis.
Wang C, Dai X, Wu S, Xu W, Song P, Huang K
Nature communications 2021 May 10;12(1):2616
Nature communications 2021 May 10;12(1):2616
Osthole interacts with an ER-mitochondria axis and facilitates tumor suppression in ovarian cancer.
Bae H, Lee JY, Song J, Song G, Lim W
Journal of cellular physiology 2021 Feb;236(2):1025-1042
Journal of cellular physiology 2021 Feb;236(2):1025-1042
Itpr1 regulates the formation of anterior eye segment tissues derived from neural crest cells.
Kinoshita A, Ohyama K, Tanimura S, Matsuda K, Kishino T, Negishi Y, Asahina N, Shiraishi H, Hosoki K, Tomiwa K, Ishihara N, Mishima H, Mori R, Nakashima M, Saitoh S, Yoshiura KI
Development (Cambridge, England) 2021 Aug 15;148(16)
Development (Cambridge, England) 2021 Aug 15;148(16)
Stigmasterol Causes Ovarian Cancer Cell Apoptosis by Inducing Endoplasmic Reticulum and Mitochondrial Dysfunction.
Bae H, Song G, Lim W
Pharmaceutics 2020 May 28;12(6)
Pharmaceutics 2020 May 28;12(6)
Fucoidan Derived from Fucus vesiculosus Inhibits the Development of Human Ovarian Cancer via the Disturbance of Calcium Homeostasis, Endoplasmic Reticulum Stress, and Angiogenesis.
Bae H, Lee JY, Yang C, Song G, Lim W
Marine drugs 2020 Jan 9;18(1)
Marine drugs 2020 Jan 9;18(1)
XBP1-KLF9 Axis Acts as a Molecular Rheostat to Control the Transition from Adaptive to Cytotoxic Unfolded Protein Response.
Fink EE, Moparthy S, Bagati A, Bianchi-Smiraglia A, Lipchick BC, Wolff DW, Roll MV, Wang J, Liu S, Bakin AV, Kandel ES, Lee AH, Nikiforov MA
Cell reports 2018 Oct 2;25(1):212-223.e4
Cell reports 2018 Oct 2;25(1):212-223.e4
Voltage-dependent Ca(2+) entry into smooth muscle during contraction promotes endothelium-mediated feedback vasodilation in arterioles.
Garland CJ, Bagher P, Powell C, Ye X, Lemmey HAL, Borysova L, Dora KA
Science signaling 2017 Jul 4;10(486)
Science signaling 2017 Jul 4;10(486)
Antibodies to inositol 1,4,5-triphosphate receptor 1 in patients with cerebellar disease.
Fouka P, Alexopoulos H, Chatzi I, Dedos SG, Samiotaki M, Panayotou G, Politis P, Tzioufas A, Dalakas MC
Neurology(R) neuroimmunology & neuroinflammation 2017 Jan;4(1):e306
Neurology(R) neuroimmunology & neuroinflammation 2017 Jan;4(1):e306
An optimized surgical approach for obtaining stable extracellular single-unit recordings from the cerebellum of head-fixed behaving mice.
White JJ, Lin T, Brown AM, Arancillo M, Lackey EP, Stay TL, Sillitoe RV
Journal of neuroscience methods 2016 Mar 15;262:21-31
Journal of neuroscience methods 2016 Mar 15;262:21-31
Pumilio1 haploinsufficiency leads to SCA1-like neurodegeneration by increasing wild-type Ataxin1 levels.
Gennarino VA, Singh RK, White JJ, De Maio A, Han K, Kim JY, Jafar-Nejad P, di Ronza A, Kang H, Sayegh LS, Cooper TA, Orr HT, Sillitoe RV, Zoghbi HY
Cell 2015 Mar 12;160(6):1087-98
Cell 2015 Mar 12;160(6):1087-98
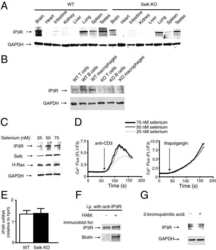
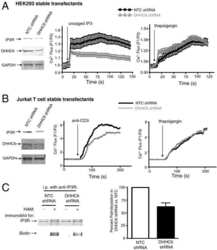
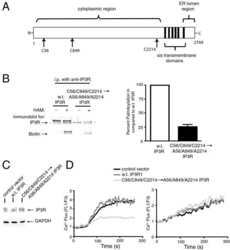
Stable expression and function of the inositol 1,4,5-triphosphate receptor requires palmitoylation by a DHHC6/selenoprotein K complex.
Fredericks GJ, Hoffmann FW, Rose AH, Osterheld HJ, Hess FM, Mercier F, Hoffmann PR
Proceedings of the National Academy of Sciences of the United States of America 2014 Nov 18;111(46):16478-83
Proceedings of the National Academy of Sciences of the United States of America 2014 Nov 18;111(46):16478-83
TGFβ1 downregulates neurite outgrowth, expression of Ca2+ transporters, and mitochondrial dynamics of in vitro cerebellar granule cells.
Jaskova K, Pavlovicova M, Cagalinec M, Lacinova L, Jurkovicova D
Neuroreport 2014 Mar 26;25(5):340-6
Neuroreport 2014 Mar 26;25(5):340-6
Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity.
Arruda AP, Pers BM, Parlakgül G, Güney E, Inouye K, Hotamisligil GS
Nature medicine 2014 Dec;20(12):1427-35
Nature medicine 2014 Dec;20(12):1427-35
Triadin/Junctin double null mouse reveals a differential role for Triadin and Junctin in anchoring CASQ to the jSR and regulating Ca(2+) homeostasis.
Boncompagni S, Thomas M, Lopez JR, Allen PD, Yuan Q, Kranias EG, Franzini-Armstrong C, Perez CF
PloS one 2012;7(7):e39962
PloS one 2012;7(7):e39962
Increased arterial smooth muscle Ca2+ signaling, vasoconstriction, and myogenic reactivity in Milan hypertensive rats.
Linde CI, Karashima E, Raina H, Zulian A, Wier WG, Hamlyn JM, Ferrari P, Blaustein MP, Golovina VA
American journal of physiology. Heart and circulatory physiology 2012 Feb 1;302(3):H611-20
American journal of physiology. Heart and circulatory physiology 2012 Feb 1;302(3):H611-20
Investigation of the role of sigma1-receptors in inositol 1,4,5-trisphosphate dependent calcium signaling in hepatocytes.
Abou-Lovergne A, Collado-Hilly M, Monnet FP, Koukoui O, Prigent S, Coquil JF, Dupont G, Combettes L
Cell calcium 2011 Jul;50(1):62-72
Cell calcium 2011 Jul;50(1):62-72
Paclitaxel accelerates spontaneous calcium oscillations in cardiomyocytes by interacting with NCS-1 and the InsP3R.
Zhang K, Heidrich FM, DeGray B, Boehmerle W, Ehrlich BE
Journal of molecular and cellular cardiology 2010 Nov;49(5):829-35
Journal of molecular and cellular cardiology 2010 Nov;49(5):829-35
A novel postsynaptic mechanism for heterosynaptic sharing of short-term plasticity.
Reissner KJ, Pu L, Schaffhausen JH, Boyle HD, Smith IF, Parker I, Carew TJ
The Journal of neuroscience : the official journal of the Society for Neuroscience 2010 Jun 30;30(26):8797-806
The Journal of neuroscience : the official journal of the Society for Neuroscience 2010 Jun 30;30(26):8797-806
Regulation of multidrug resistance-associated protein 2 by calcium signaling in mouse liver.
Cruz LN, Guerra MT, Kruglov E, Mennone A, Garcia CR, Chen J, Nathanson MH
Hepatology (Baltimore, Md.) 2010 Jul;52(1):327-37
Hepatology (Baltimore, Md.) 2010 Jul;52(1):327-37
Intracellular Ca2+ regulating proteins in vascular smooth muscle cells are altered with type 1 diabetes due to the direct effects of hyperglycemia.
Searls YM, Loganathan R, Smirnova IV, Stehno-Bittel L
Cardiovascular diabetology 2010 Feb 1;9:8
Cardiovascular diabetology 2010 Feb 1;9:8
Efficient generation of mature cerebellar Purkinje cells from mouse embryonic stem cells.
Tao O, Shimazaki T, Okada Y, Naka H, Kohda K, Yuzaki M, Mizusawa H, Okano H
Journal of neuroscience research 2010 Feb 1;88(2):234-47
Journal of neuroscience research 2010 Feb 1;88(2):234-47
Ablation of skeletal muscle triadin impairs FKBP12/RyR1 channel interactions essential for maintaining resting cytoplasmic Ca2+.
Eltit JM, Feng W, Lopez JR, Padilla IT, Pessah IN, Molinski TF, Fruen BR, Allen PD, Perez CF
The Journal of biological chemistry 2010 Dec 3;285(49):38453-62
The Journal of biological chemistry 2010 Dec 3;285(49):38453-62
Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry.
Cheshenko N, Liu W, Satlin LM, Herold BC
Molecular biology of the cell 2007 Aug;18(8):3119-30
Molecular biology of the cell 2007 Aug;18(8):3119-30
Mini-dystrophin expression down-regulates overactivation of G protein-mediated IP3 signaling pathway in dystrophin-deficient muscle cells.
Balghi H, Sebille S, Constantin B, Patri S, Thoreau V, Mondin L, Mok E, Kitzis A, Raymond G, Cognard C
The Journal of general physiology 2006 Feb;127(2):171-82
The Journal of general physiology 2006 Feb;127(2):171-82
Mini-dystrophin expression down-regulates IP3-mediated calcium release events in resting dystrophin-deficient muscle cells.
Balghi H, Sebille S, Mondin L, Cantereau A, Constantin B, Raymond G, Cognard C
The Journal of general physiology 2006 Aug;128(2):219-30
The Journal of general physiology 2006 Aug;128(2):219-30
The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria.
Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, Rodrigues MA, Gomez MV, Nathanson MH, Leite MF
The Journal of biological chemistry 2005 Dec 9;280(49):40892-900
The Journal of biological chemistry 2005 Dec 9;280(49):40892-900
Plasma and intracellular membrane inositol 1,4,5-trisphosphate receptors mediate the Ca(2+) increase associated with the ATP-induced increase in ciliary beat frequency.
Barrera NP, Morales B, Villalón M
American journal of physiology. Cell physiology 2004 Oct;287(4):C1114-24
American journal of physiology. Cell physiology 2004 Oct;287(4):C1114-24
Individual subtypes of enteroendocrine cells in the mouse small intestine exhibit unique patterns of inositol 1,4,5-trisphosphate receptor expression.
Wang S, Liu J, Li L, Wice BM
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2004 Jan;52(1):53-63
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2004 Jan;52(1):53-63
InsP3 receptor type 2 and oscillatory and monophasic Ca2+ transients in rat adrenal chromaffin cells.
Inoue M, lin H, Imanaga I, Ogawa K, Warashina A
Cell calcium 2004 Jan;35(1):59-70
Cell calcium 2004 Jan;35(1):59-70
ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors.
Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS
American journal of physiology. Lung cellular and molecular physiology 2003 Sep;285(3):L680-90
American journal of physiology. Lung cellular and molecular physiology 2003 Sep;285(3):L680-90
IP3 receptors and associated Ca2+ signals localize to satellite cells and to components of the neuromuscular junction in skeletal muscle.
Powell JA, Molgó J, Adams DS, Colasante C, Williams A, Bohlen M, Jaimovich E
The Journal of neuroscience : the official journal of the Society for Neuroscience 2003 Sep 10;23(23):8185-92
The Journal of neuroscience : the official journal of the Society for Neuroscience 2003 Sep 10;23(23):8185-92
Type-specific inositol 1,4,5-trisphosphate receptor localization in the vomeronasal organ and its interaction with a transient receptor potential channel, TRPC2.
Brann JH, Dennis JC, Morrison EE, Fadool DA
Journal of neurochemistry 2002 Dec;83(6):1452-60
Journal of neurochemistry 2002 Dec;83(6):1452-60
Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction.
Clapp TR, Stone LM, Margolskee RF, Kinnamon SC
BMC neuroscience 2001;2:6
BMC neuroscience 2001;2:6
Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction.
Clapp TR, Stone LM, Margolskee RF, Kinnamon SC
BMC neuroscience 2001;2:6
BMC neuroscience 2001;2:6
IP(3) receptor function and localization in myotubes: an unexplored Ca(2+) signaling pathway in skeletal muscle.
Powell JA, Carrasco MA, Adams DS, Drouet B, Rios J, Müller M, Estrada M, Jaimovich E
Journal of cell science 2001 Oct;114(Pt 20):3673-83
Journal of cell science 2001 Oct;114(Pt 20):3673-83
Inhibition of type I and III IP(3)Rs by TGF-beta is associated with impaired calcium release in mesangial cells.
Sharma K, Mc Gowan TA, Wang L, Madesh M, Kaspar V, Szalai G, Thomas AP, Hajnóczky G
American journal of physiology. Renal physiology 2000 Jun;278(6):F1022-9
American journal of physiology. Renal physiology 2000 Jun;278(6):F1022-9
Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux.
Cameron AM, Steiner JP, Roskams AJ, Ali SM, Ronnett GV, Snyder SH
Cell 1995 Nov 3;83(3):463-72
Cell 1995 Nov 3;83(3):463-72
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
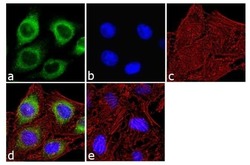
- Experimental details
- Immunofluorescence analysis of IP3 Receptor I was done on 70% confluent log phase SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with IP3 Receptor I Rabbit Polyclonal Antibody (Product # PA1-901) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
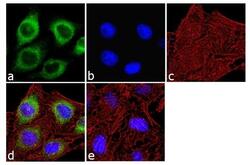
- Experimental details
- Immunofluorescence analysis of IP3 Receptor I was done on 70% confluent log phase SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with IP3 Receptor I Rabbit Polyclonal Antibody (Product # PA1-901) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
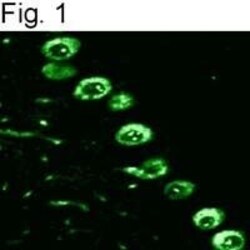
- Experimental details
- Immunolocalization of IP3 receptor type I in rat cerebellum using Product # PA1-901.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
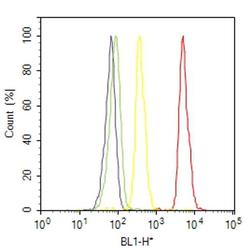
- Experimental details
- Flow cytometry analysis of IP3 Receptor I was done on SH-SY5Y cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with IP3 Receptor I Rabbit Polyclonal Antibody (PA1901, red histogram) or with rabbit isotype control (yellow histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
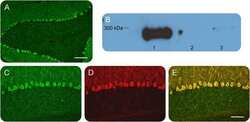
- Figure 1 Identification and validation of the anti-inositol 1,4,5-triphosphate receptor 1 (IP3R1) antibody Patient sera with a working diagnosis of cerebellar ataxia were screened using immunohistochemistry in mouse brain slices. A specific pattern was identified, where the antibodies bound primarily the Purkinje cells (A). Following antibody identification, the precipitate used for the mass spectrometry was blotted and probed with a commercial antibody against IP3R1. The antibody recognized the band, with an apparent molecular weight of 270-280 kDa (lane 1). The same antibody did not recognize precipitates from healthy control subjects (lanes 2 and 3) (B). We double-labeled mouse brain with patient serum (C) and the commercial antibody against IP3R1 (D). The colocalization was near perfect, confirming the identity of the autoantibody (E). (A) Scale bar 200 mum; (C-E) scale bar 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
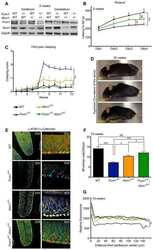
- Figure 5 Experimental induction ER-mitochondria interactions increases mitochondrial calcium flux and impairs glucose homeostasis (A) Schematic illustrating the mitochondrial-ER synthetic linker. The construct encodes a monomeric red fluorescent protein (RFP) fused to the OMM targeting sequence of mAKAP1 at the N terminus and the ER targeting sequence of yUBC6 at the C terminus with a length of 25 nm 25 . Representative TEM of Hepa 1-6 cells expressing control (upper panel) and linker plasmids (bottom panel) at 9300x, scale bar: 500nm. (B) FACS analysis of Annexin V positivity in control and linker-expressing cells, n=3. (C) Representative trace of mitochondrial Ca 2+ dynamics in single Hepa 1-6 cells expressing the control or linker construct, measured by FRET of 4mtD3cpv following treatment with ATP (100muM). (C inset) Quantification of the peak in Ca 2+ after ATP stimulation, 3 independent experiments. (D) Measurement of oxygen consumption rate (OCR) in control and linker-expressing Hepa 1-6 cells in the presence of 2muM Oligomycin, 0.4 muM FCCP and 2muM Rotenone (Rot) and 2muM antimycin (AA). n=15 from 4 independent experiments. (E) Representative TEMs of liver sections from lean mice expressing the control or linker construct, scale bar: 500nm upper panel and 100nm bottom panel (F) Liver sections from HFD-fed mice expressing control or linker constructs, stained with hematoxylin and eosin, scale bar 50um. (G) OCR of primary hepatocytes from HFD-fed mice expressing contro
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 1 Identification and validation of the anti-inositol 1,4,5-triphosphate receptor 1 (IP3R1) antibody Patient sera with a working diagnosis of cerebellar ataxia were screened using immunohistochemistry in mouse brain slices. A specific pattern was identified, where the antibodies bound primarily the Purkinje cells (A). Following antibody identification, the precipitate used for the mass spectrometry was blotted and probed with a commercial antibody against IP3R1. The antibody recognized the band, with an apparent molecular weight of 270-280 kDa (lane 1). The same antibody did not recognize precipitates from healthy control subjects (lanes 2 and 3) (B). We double-labeled mouse brain with patient serum (C) and the commercial antibody against IP3R1 (D). The colocalization was near perfect, confirming the identity of the autoantibody (E). (A) Scale bar 200 mum; (C-E) scale bar 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
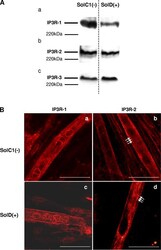
- Figure 5. Determination and immunolocalization of the expression pattern of IP3R proteins. (A) Western blot analysis of IP3R-1 (a), IP3R-2 (b), and IP3R-3 (c), respectively, in SolC1(-) and SolD(+) myotubes. 30 mug protein from each type of myotube was separated on 5% SDS-PAGE, transferred onto a pore nitrocellulose membrane, and processed for Western blot analysis with specific antibodies. The 220-kD band corresponded to the molecular weight of the myosin protein. (B) IP3R isoform immunolabeling in SolC1(-) and SolD(+) myotubes was observed with laser scanning confocal microscopy. IP3R-1 and IP3R-2 localizations were detected using polyclonal antibodies, PA1-901 and PA1-904, respectively. IP3R-1 staining is localized in the nuclear envelope region and in the cytoplasm in SolC1(-) (a) and in SolD(+) (c) myotubes. IP3R-2 showed a peripheral and fully cytoplasmic localization and, as indicated with white arrows, striated staining pattern appeared at this differentiation stage in SolC1(-) (b) and SolD(+) (d) myotubes. Bars, 25 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
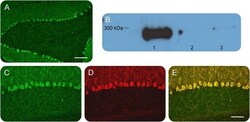
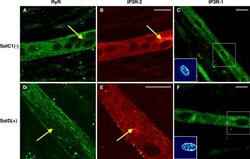
- Figure 7. Immunolocalization of RyRs and IP3Rs in SolC1(-) and SolD(+) myotubes. Immunolabelings of SR calcium release channels in SolC1(-) (A-C) and in SolD(+) myotubes (D-F) were observed with laser scanning confocal microscopy (LSCM). The same random distribution of RyRs (anti-RyR; Calbiochem) was observed in SolC1(-) myotubes (A) and in SolD(+) myotubes (D). In the same myotubes, immunostaining with antibodies against IP3R-2 was performed in SolC1(-) (B) and in SolD(+) (E). Yellow arrows, locations corresponding to elevated staining for IP3R-2 and low staining for RyR. IP3R-1 staining is localized in the nuclear envelope region and in the cytoplasm in SolC1(-) (C) and in SolD(+) (F) myotubes. Insets, staining of selected nuclei (dotted squares) loaded with the fluorescent TO-PRO-3 probe. IP3R-1 and IP3R-2 localizations were detected using polyclonal antibodies, PA1-901 and PA1-904, respectively.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
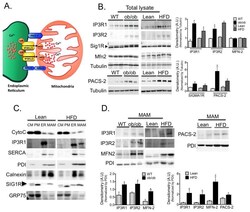
- Figure 3 Regulation of MAM enriched proteins in obesity (A) Schematic illustration of functional and structural MAM proteins. (B) Western blot and image J based quantification analysis of indicated proteins in liver total lysates from wt/ ob/ob and lean/HFD-fed (16 weeks) mice. Arrow indicates the specific Sig1R band, n=3 representative of 2-3 independent experiments. (C) Western blot analysis of indicated proteins of subcellular fractions from mouse livers. CM: crude mitochondria. PM: Pure mitochondria, ER: endoplasmic reticulum and MAM: mitochondria associated ER membranes. Cytochrome C (Cyto C) was used as a mitochondrial marker; IP3R and SERCA were found mostly in the bulk ER. PDI and Calnexin were equally distributed between ER and MAM, and Sigma 1 receptor (Sig1R) enrichment served as a known MAM marker. This figure is representative of at least 5 different preparations (D) Western blot and quantification analysis of indicated proteins in MAMs from wt/ ob/ob and lean/HFD mice, n=3. PDI served as a loading control. All the graphs represent mean +- SEM, *P < 0.05, Student's t-test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
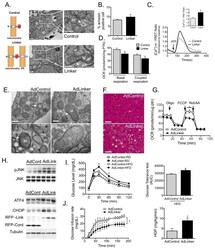
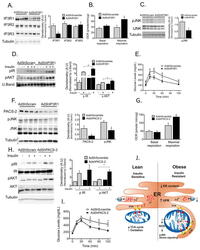
- Figure 6 Experimental suppression of MAM function or formation alters mitochondrial calcium flux and improves glucose homeostasis (A) Western blot and quantification analysis demonstrating specific knockdown of IP3R1 in liver lysates following adenoviral shRNA transduction, n= 3, representative of 2 independent experiments. (B) Oxygen consumption rate of primary hepatocytes from mice expressing control or shIP3R1, n=6. (C) Western blot and quantification analysis showing reduced JNK phosphorylation in liver lysates following IP3R1 knockdown. (D) Western blot and quantification analysis of the insulin-action in the liver samples following expression of shScramble or shIP3R1. n=3. (E) Glucose tolerance test in mice expressing shScramble or shIP3R1, n=7. (F) Western blot and quantification analysis demonstrating specific knockdown of PACS-2 in liver lysates following adenoviral shRNA transduction, n=4 representative of 2 independent experiments. (G) Oxygen consumption rate of primary hepatocytes from mice expressing control or shPACS-2, n=5 for shscramble and n=8 for shPACS-2. (H) Western blot and quantification analysis of the insulin-action in the liver samples following expression of shScramble or shPACS-2, n=3. (I) Glucose tolerance test in mice expressing shScramble or shPACS-2, n=7 for shScramble and 9 for shPACS-2. (J) We propose here that an early development in the course of obesity-induced metabolic disease is increased MAM formation in the liver. Increased MAM formati
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
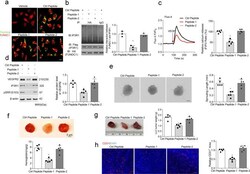
- Experimental details
- Fig. 6 A specific therapeutic peptide blocks the FUNDC1-IP3R1 interaction and angiogenesis. a Transduction efficiency of peptides 1, 2, and a control peptide in Human umbilical vein endothelial cells (HUVECs) ( n = 5 independent experiments). b HUVECs were treated with different peptides (20 muM) for 24 h, then subjected to immunoprecipitation with antibody against FUNDC1(HA) to quantify the interaction of FUNDC1 and IP3R1 ( n = 3 independent experiments). c HUVECs were treated with different peptides (20 muM) for 24 h, then cytosolic Ca 2+ was detected using the fluorescent probe Fluo-4, AM ( n = 5 independent experiments). d HUVECs were treated with different peptides. After 24 h, the cell lysates were collected and subjected to western blot assays to detect the expression of VEGFR2, IP3R1, SRF, and phosphorylated SRF at Ser103 (pSRF) ( n = 5 independent experiments). e Three-dimensional spheroids and representative images of spheroid-sprouting were analyzed ( n = 5 independent experiments). Scale bar, 100 um. f Matrigel containing vascular endothelial growth factor (VEGF) was injected subcutaneously into 6-week-old wild-type mice, then the mice received an intravenous injection of the indicated peptides. After 10 days, matrigel plugs were removed for analysis of new vessel formation by histological and Hb assay ( n = 5 mice/group). Quantification of Hb extracted from matrigel plugs of different groups. g C57BL/6 mice with LLC tumors that were approximately 90 mm 3 in size
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4 Increased expression of endoplasmic reticulum (ER) stress sensor proteins and ER-mitochondrial axis proteins due to stigmasterol treatment in ovarian cancer cells. ( A , B ) Immunoblots representing the expression of unfolded protein response (UPR) proteins following stigmasterol treatments (0, 5, 10, and 20 mug/mL). TUBA was used as a control and is shown at the bottom of the figure. ( C , D ) Immunoblots representing the expression of ER-mitochondrial axis proteins following stigmasterol treatments (0, 5, 10, and 20 mug/mL). TUBA was used as a control and is shown at the bottom of the figure. ( E , F ) Immunoblots representing the expression of autophagy proteins following stigmasterol treatment. TUBA was used as a control and is shown at the bottom of the figure.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
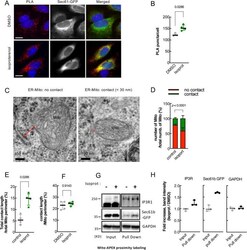
- Experimental details
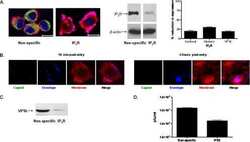
- Figure 2 beta2-AR agonists enhance physical contacts between ER and Mito. ( A ) Representative images of PLA in HeLa cells treated with DMSO or isoproterenol (1 muM). PLA signal (red) indicates a close contact between ER and Mito. Sec61-GFP (green): to label ER; DAPI (blue): nucleus. ( B ) Quantifications of PLA signal as shown in A ( n = 4 experiments with 77-97 cells each; Mann-Whitney test). ( C ) Representative TEM images of Mito (HEK293T cells) showing no contact (left) or a close contact (right) with ER membrane. Red lines: the distance between ER and Mito membrane. ( D ) Quantitative analysis of the percentage of the number of Mito showing contact or no contact with ER ( n = 3 experiments with 346, 405 Mito for control, isoprot; Chi-square test). ( E , F ) The percent of total contact length divided by the total Mito perimeter measured among all mitochondria ( E ) ( n = 4 experiments with 122, 171 mitochondria for control, isoprot; Mann Whitney test), and the percent of contact length divided by Mito perimeter using only Mito showing a close contact with ER ( F ) ( n = 4 experiments with 27, 95 mitochondria for control, isoprot; Mann Whitney test) in TEM images. ( G ) Representative images of Western blots of biotinylated proteins proximity-labeled by Mito-APEX (for 3 min) followed by streptavidin affinity pull down, in control- vs isoprot-treated HEK293T cells. GAPDH used for control. Full-length blots are presented in Supplementary Fig. S6 . ( H ) Quantification of I
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
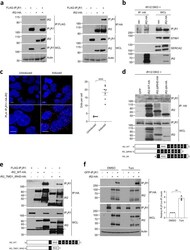
- Experimental details
- Fig. 4 iRhoms bind to IP 3 Rs. a Levels of iRhom2 and IP 3 R1 were analysed by immunoblotting in whole cell lysate (WCL) and immunoprecipitation (IP: HA or IP: FLAG) from HEK293T cells transiently transfected for 36 h with FLAG-IP 3 R1 and iRhom2-HA. b Levels of iRhom2 and endogenous IP 3 R1, STIM1 and SERCA2 were determined by immunoblotting in whole cell lysate (WCL) and after immunoprecipitation (IP: HA) from iRhom1/2 DKO MEFs stably expressing iRhom2-HA. c Proximity ligation assay (PLA) in iRhom1/2 DKO HEK293T cells reconstituted with HA-iRhom2 under a tetracycline-inducible promoter were stained using antibodies to detect endogenous IP 3 R1 and N-terminally HA-tagged iRhom2 after 24 h of 250 ng/ml of doxycycline to induce iRhom2. Dots per cell were quantified using ImageJ and shown as mean +- SEM, uninduced ( n = 4 images/166 cells), induced ( n = 7 images/259 cells) from two biologically independent experiments. Two-tailed unpaired Student's t -test **** p < 0.0001. Bottom panels are magnified sections of above corresponding panels. Scale bars = 10 mum. d Levels of WT and deletion mutants of iRhom2 and endogenous IP 3 R1 were determined by immunoblotting in whole cell lysate (WCL) and immunoprecipitation (IP: HA) from iRhom1/2 DKO MEFs stably expressing iRhom2-WT-HA or iRhom2-DeltaIRHD-HA or iRhom2-DeltaN-HA. Schematics show iRhom2 mutants with domains deleted (numbering refers to TMDs and IRHD refers to iRhom homology domain). e Levels of WT and deletion mutant of iRho
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
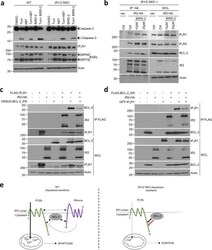
- Experimental details
- Fig. 6 iRhoms control BCL-2 antiapoptotic effect on IP 3 Rs. a Cell lysates from WT and iRhom1/2 DKO MEFs treated with tunicamycin (0.5 ug/ml) alone or in the presence of BIRD-2 (10 uM) or ABT-199/Venetoclax (ABT, 1 uM) were analysed by immunoblotting with indicated antibodies. b Levels of iRhom2 and endogenous IP 3 R1, IP 3 R3 and BCL-2 were analysed by immunoblotting in whole cell lysate (WCL) and immunoprecipitation (IP: HA) from iRhom1/2 DKO MEFs stably expressing iRhom2-HA after treatment for 18 h with control or BIRD-2 peptides (10 uM or 20 uM). c , d Levels of iRhom2, IP 3 R1 and BCL-2 were analysed by immunoblotting in whole cell lysate (WCL) and immunoprecipitation (IP: FLAG) from HEK293T cells transiently transfected for 36 h with FLAG-IP 3 R1 (1.25 ug), iRhom2-HA (0.25 ug) and VENUS-BCL-2_ER (0.25 ug; BCL-2 targeted specifically to ER) in ( c ) or GFP-IP 3 R1 (1.25 ug), iRhom2-HA (0.25 ug) and FLAG-BCL-2_ER (0.25 ug; Bcl-2 targeted specifically to ER) in ( d ). Immunoblotting data are representative of 2-3 independent experiments. e Proposed model for the role of iRhoms in regulating IP 3 R function via BCL-2. In WT cells, IP 3 R, iRhoms and BCL-2 interact at the ER within a regulatory complex, where iRhom2 attenuates the inhibitory effect of BCL-2 on IP 3 R activity. This enhances the flow of Ca 2+ from the ER into mitochondria under basal conditions and during acute release of Ca 2+ (e.g. ER stress) to regulate apoptosis. In the absence of iRhoms, the inhibitory
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
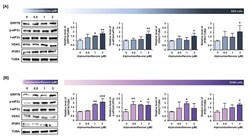
- Experimental details
- Alpinumisoflavone activates the ER stress and ER-mitochondria contact proteins in ES2 and OV90 cells. ( A , B ) In both ES2 ( A ) and OV90 ( B ) cells treated with alpinumisoflavone for 24 h, immunoblots of GRP78, phosphor-eIF2alpha, total-eIF2alpha, VDAC, IP3R1 and TUBA were captured using the ChemiDoc system. Relative intensities of each target protein are presented as bar graphs next to sorted immunoblots. All experiments were performed in triplicate. The significance between the vehicle and treatment groups is depicted by asterisks (* p < 0.05, ** p < 0.01 and *** p < 0.001).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
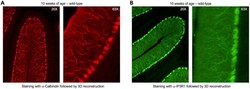
- Experimental details
- Typical outcome of Purkinje cell imaging in 3D reconstruction with a single antibody (A and B) 3D image reconstruction of Purkinje neurons acquired at 20x ( left ) and 63x ( right ) in wild-type mice at 10 weeks of age with staining against Calbindin (A, red) or IP3R1 (B, green).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
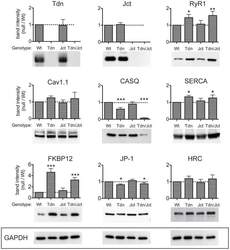
- Experimental details
- Figure 1 Relative levels of CRU proteins in crude homogenates from skeletal muscle. Identical amounts (25 ug/lane) of microsomal fraction of skeletal muscles from WT, Tdn-, Jct- and Tdn-/Jct mice were loaded and immunoblotted with several antibodies. Membranes were tested for expression of triadin (Tdn), junctin (Jct), ryanodine receptor (RyR1), Dihydropyridine receptors (Cav1.1), Calsequestrin (CASQ), SERCA-1 pump (SERCA), FK506 binding protein (FKBP12), Junctophilin-1 (JP-1) and Histidin-rich Ca 2+ binding protein (HRC) as described in Material and Methods. Band intensity for each protein was normalized to GAPDH expression to correct for loading and plotted as fraction of its WT counterpart (dotted line). Data presented as mean +- SD of 3-7 independent blots. * p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- 8 Figure Osthole influenced intracellular mechanisms related to endoplasmic reticulum (ER)-mitochondrial functions. Expression of ER-mitochondrial function targets such as (a) ULK1, (b) GRP75, (c) MFN2, (d) P62, (e) FAM82A2, (f) VAPB, (g) VDAC, (h) IP3R1, and (i) IP3R2 was analyzed by western blot analysis in ES2 and OV90 cells treated with various doses of osthole (0, 5, 10, and 20 muM). Intensity of the target protein was detected and analyzed relative to total protein or alpha-tubulin protein. *** p < .001, ** p < .01, and * p < .05 indicate significant differences compared to vehicle-treated cells. IP3R1, inositol 1,4,5-trisphosphate (IP3) receptor type 1; IP3, inositol trisphosphate; MFN2, mitofusin-2; VAPB, vesicle-associated membrane protein-associated protein B/C; VDAC, voltage-dependent anion channel
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 6 Stimulation of endoplasmic reticulum (ER) stress sensor, ER-mitochondria tethering proteins, and autophagy regulators by fucoidan in ES-2 and OV-90 cells. ( A - F ) The stimulation of ER stress sensor proteins, ( A ) IRE1alpha, ( B ) ATF6alpha, ( C ) p-PERK, ( D ) GADD153, ( E ) p-eIF2alpha, and ( F ) GRP78 was observed through western blot analysis in ES-2 and OV-90 cell lines incubated with fucoidan. ( G - L ) The activation of ER-mitochondria axis-associated proteins, ( G ) VDAC, ( H ) IP3R1, ( I ) IP3R2, ( J ) GRP75, ( K ) MFN2, and ( L ) VAPB was analyzed by western blotting in fucoidan treated ES-2 and OV-90 cells. ( M - O ) The activation of autophagy-associated proteins, ( M ) LC3B, ( N ) BECN1, and ( O ) ATG5 was observed through western blot in ES-2 and OV-90 cells incubated with fucoidan. The graph of the signals was calculated compared with total signal or alpha tubulin (TUBA). *** p < 0.001, ** p < 0.01, and * p < 0.05 show significances.
 Explore
Explore Validate
Validate Learn
Learn