Antibody data
- Antibody Data
- Antigen structure
- References [23]
- Comments [0]
- Validations
- Flow cytometry [2]
- Other assay [6]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-1239-37 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD123 Monoclonal Antibody (6H6), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The 6H6 monoclonal antibody reacts with human CD123, the alpha chain of the IL-3 receptor. This 60-70 kDa transmembrane protein binds to IL-3 with low affinity by itself, and when associated with CD131 (common beta chain) binds IL-3 with high affinity. CD123 is expressed by myeloid precursors, macrophages, dendritic cells, mast cells, basophils, and megakaryocytes. Applications Reported: This 6H6 antibody has been reported for use in flow cytometric analysis, immunohistology staining of frozen tissue sections, and immunohistology staining of paraffin embedded tissue sections. Applications Tested: The 6H6 antibody has been tested by flow cytometric analysis of normal human peripheral blood cells. This can be used at less than or equal to 1 µg per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 6H6
- Vial size
- 2 mg
- Concentration
- 0.5 mg/mL
- Storage
- 4°C
Submitted references Unsupervised Analysis of Flow Cytometry Data in a Clinical Setting Captures Cell Diversity and Allows Population Discovery.
Astrocytic interleukin-3 programs microglia and limits Alzheimer's disease.
Human pDCs Are Superior to cDC2s in Attracting Cytolytic Lymphocytes in Melanoma Patients Receiving DC Vaccination.
Quantifying in situ adaptive immune cell cognate interactions in humans.
Neutrophils Inhibit Synthesis of Mineralized Extracellular Matrix by Human Bone Marrow-Derived Stromal Cells In Vitro.
High Expression of Galectin-3 in Patients with IgG4-Related Disease: A Proteomic Approach.
Self-reactive IgE exacerbates interferon responses associated with autoimmunity.
Humanized Mice Reveal Differential Immunogenicity of Cells Derived from Autologous Induced Pluripotent Stem Cells.
Brief Report: IFIH1 Mutation Causes Systemic Lupus Erythematosus With Selective IgA Deficiency.
Thymic HIV-2 infection uncovers posttranscriptional control of viral replication in human thymocytes.
Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice.
Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection.
Frailty in old age is associated with decreased interleukin-12/23 production in response to toll-like receptor ligation.
R-phycoerythrin-conjugated antibodies are inappropriate for intracellular staining of murine plasma cells.
Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma.
Possible implication of local immune response in Darier's disease: an immunohistochemical characterization of lesional inflammatory infiltrate.
Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease.
Human spleen contains different subsets of dendritic cells and regulatory T lymphocytes.
CD56-positive haematological neoplasms of the skin: a multicentre study of the Cutaneous Lymphoma Project Group of the European Organisation for Research and Treatment of Cancer.
Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI.
Local activation of the innate immune system in Buruli ulcer lesions.
Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation.
Expression of the plasmacytoid dendritic cell marker BDCA-2 supports a spectrum of maturation among CD4+ CD56+ hematodermic neoplasms.
Baumgaertner P, Sankar M, Herrera F, Benedetti F, Barras D, Thierry AC, Dangaj D, Kandalaft LE, Coukos G, Xenarios I, Guex N, Harari A
Frontiers in immunology 2021;12:633910
Frontiers in immunology 2021;12:633910
Astrocytic interleukin-3 programs microglia and limits Alzheimer's disease.
McAlpine CS, Park J, Griciuc A, Kim E, Choi SH, Iwamoto Y, Kiss MG, Christie KA, Vinegoni C, Poller WC, Mindur JE, Chan CT, He S, Janssen H, Wong LP, Downey J, Singh S, Anzai A, Kahles F, Jorfi M, Feruglio PF, Sadreyev RI, Weissleder R, Kleinstiver BP, Nahrendorf M, Tanzi RE, Swirski FK
Nature 2021 Jul;595(7869):701-706
Nature 2021 Jul;595(7869):701-706
Human pDCs Are Superior to cDC2s in Attracting Cytolytic Lymphocytes in Melanoma Patients Receiving DC Vaccination.
van Beek JJP, Flórez-Grau G, Gorris MAJ, Mathan TSM, Schreibelt G, Bol KF, Textor J, de Vries IJM
Cell reports 2020 Jan 28;30(4):1027-1038.e4
Cell reports 2020 Jan 28;30(4):1027-1038.e4
Quantifying in situ adaptive immune cell cognate interactions in humans.
Liarski VM, Sibley A, van Panhuys N, Ai J, Chang A, Kennedy D, Merolle M, Germain RN, Giger ML, Clark MR
Nature immunology 2019 Apr;20(4):503-513
Nature immunology 2019 Apr;20(4):503-513
Neutrophils Inhibit Synthesis of Mineralized Extracellular Matrix by Human Bone Marrow-Derived Stromal Cells In Vitro.
Bastian OW, Croes M, Alblas J, Koenderman L, Leenen LPH, Blokhuis TJ
Frontiers in immunology 2018;9:945
Frontiers in immunology 2018;9:945
High Expression of Galectin-3 in Patients with IgG4-Related Disease: A Proteomic Approach.
Salah A, Yoshifuji H, Ito S, Kitagori K, Kiso K, Yamada N, Nakajima T, Haga H, Tsuruyama T, Miyagawa-Hayashino A
Pathology research international 2017;2017:9312142
Pathology research international 2017;2017:9312142
Self-reactive IgE exacerbates interferon responses associated with autoimmunity.
Henault J, Riggs JM, Karnell JL, Liarski VM, Li J, Shirinian L, Xu L, Casey KA, Smith MA, Khatry DB, Izhak L, Clarke L, Herbst R, Ettinger R, Petri M, Clark MR, Mustelin T, Kolbeck R, Sanjuan MA
Nature immunology 2016 Feb;17(2):196-203
Nature immunology 2016 Feb;17(2):196-203
Humanized Mice Reveal Differential Immunogenicity of Cells Derived from Autologous Induced Pluripotent Stem Cells.
Zhao T, Zhang ZN, Westenskow PD, Todorova D, Hu Z, Lin T, Rong Z, Kim J, He J, Wang M, Clegg DO, Yang YG, Zhang K, Friedlander M, Xu Y
Cell stem cell 2015 Sep 3;17(3):353-9
Cell stem cell 2015 Sep 3;17(3):353-9
Brief Report: IFIH1 Mutation Causes Systemic Lupus Erythematosus With Selective IgA Deficiency.
Van Eyck L, De Somer L, Pombal D, Bornschein S, Frans G, Humblet-Baron S, Moens L, de Zegher F, Bossuyt X, Wouters C, Liston A
Arthritis & rheumatology (Hoboken, N.J.) 2015 Jun;67(6):1592-7
Arthritis & rheumatology (Hoboken, N.J.) 2015 Jun;67(6):1592-7
Thymic HIV-2 infection uncovers posttranscriptional control of viral replication in human thymocytes.
Nunes-Cabaço H, Matoso P, Foxall RB, Tendeiro R, Pires AR, Carvalho T, Pinheiro AI, Soares RS, Sousa AE
Journal of virology 2015 Feb;89(4):2201-8
Journal of virology 2015 Feb;89(4):2201-8
Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice.
Watchmaker PB, Lahl K, Lee M, Baumjohann D, Morton J, Kim SJ, Zeng R, Dent A, Ansel KM, Diamond B, Hadeiba H, Butcher EC
Nature immunology 2014 Jan;15(1):98-108
Nature immunology 2014 Jan;15(1):98-108
Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection.
Ataide MA, Andrade WA, Zamboni DS, Wang D, Souza Mdo C, Franklin BS, Elian S, Martins FS, Pereira D, Reed G, Fitzgerald KA, Golenbock DT, Gazzinelli RT
PLoS pathogens 2014 Jan;10(1):e1003885
PLoS pathogens 2014 Jan;10(1):e1003885
Frailty in old age is associated with decreased interleukin-12/23 production in response to toll-like receptor ligation.
Compté N, Zouaoui Boudjeltia K, Vanhaeverbeek M, De Breucker S, Tassignon J, Trelcat A, Pepersack T, Goriely S
PloS one 2013;8(6):e65325
PloS one 2013;8(6):e65325
R-phycoerythrin-conjugated antibodies are inappropriate for intracellular staining of murine plasma cells.
Kim MS, Kim TS
Cytometry. Part A : the journal of the International Society for Analytical Cytology 2013 May;83(5):452-60
Cytometry. Part A : the journal of the International Society for Analytical Cytology 2013 May;83(5):452-60
Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma.
Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD
Science translational medicine 2013 Feb 27;5(174):174ra26
Science translational medicine 2013 Feb 27;5(174):174ra26
Possible implication of local immune response in Darier's disease: an immunohistochemical characterization of lesional inflammatory infiltrate.
Miracco C, Pietronudo F, Mourmouras V, Pellegrino M, Onorati M, Mastrogiulio MG, Cantarini L, Luzi P
Mediators of inflammation 2010;2010:350304
Mediators of inflammation 2010;2010:350304
Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease.
Freeman CM, Martinez FJ, Han MK, Ames TM, Chensue SW, Todt JC, Arenberg DA, Meldrum CA, Getty C, McCloskey L, Curtis JL
American journal of respiratory and critical care medicine 2009 Dec 15;180(12):1179-88
American journal of respiratory and critical care medicine 2009 Dec 15;180(12):1179-88
Human spleen contains different subsets of dendritic cells and regulatory T lymphocytes.
Velásquez-Lopera MM, Correa LA, García LF
Clinical and experimental immunology 2008 Oct;154(1):107-14
Clinical and experimental immunology 2008 Oct;154(1):107-14
CD56-positive haematological neoplasms of the skin: a multicentre study of the Cutaneous Lymphoma Project Group of the European Organisation for Research and Treatment of Cancer.
Assaf C, Gellrich S, Whittaker S, Robson A, Cerroni L, Massone C, Kerl H, Rose C, Chott A, Chimenti S, Hallermann C, Petrella T, Wechsler J, Bagot M, Hummel M, Bullani-Kerl K, Bekkenk MW, Kempf W, Meijer CJ, Willemze R, Sterry W
Journal of clinical pathology 2007 Sep;60(9):981-9
Journal of clinical pathology 2007 Sep;60(9):981-9
Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI.
Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, Ding A, Knowles DM, Santini PA, Cerutti A
Nature immunology 2007 Mar;8(3):294-303
Nature immunology 2007 Mar;8(3):294-303
Local activation of the innate immune system in Buruli ulcer lesions.
Peduzzi E, Groeper C, Schütte D, Zajac P, Rondini S, Mensah-Quainoo E, Spagnoli GC, Pluschke G, Daubenberger CA
The Journal of investigative dermatology 2007 Mar;127(3):638-45
The Journal of investigative dermatology 2007 Mar;127(3):638-45
Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation.
Lan P, Tonomura N, Shimizu A, Wang S, Yang YG
Blood 2006 Jul 15;108(2):487-92
Blood 2006 Jul 15;108(2):487-92
Expression of the plasmacytoid dendritic cell marker BDCA-2 supports a spectrum of maturation among CD4+ CD56+ hematodermic neoplasms.
Jaye DL, Geigerman CM, Herling M, Eastburn K, Waller EK, Jones D
Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2006 Dec;19(12):1555-62
Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2006 Dec;19(12):1555-62
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
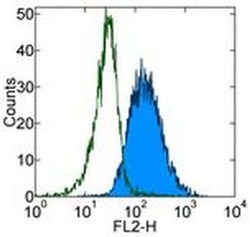
- Experimental details
- Staining of TF-1 cell line with Mouse IgG1 kappa Isotype Control Purified (Product # 14-4714-82) (open histogram) or Anti-Human CD123 Purified (filled histogram) followed by F (ab')2 Anti-Mouse IgG PE (Product # 12-4012). Total viable cells were used for analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
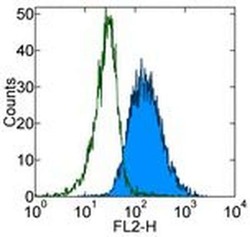
- Experimental details
- Staining of TF-1 cell line with Mouse IgG1 kappa Isotype Control Purified (Product # 14-4714-82) (open histogram) or Anti-Human CD123 Purified (filled histogram) followed by F (ab')2 Anti-Mouse IgG PE (Product # 12-4012). Total viable cells were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
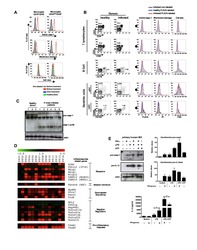
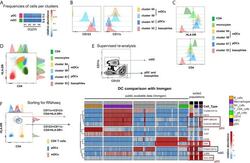
- Figure 5 Identification of multiple mDC and pDC subsets by MegaClust. (A) Comparison of the different clusters identified by MegaClust as mDCs and pDCs (example for one patient for four time points). (B) Expression level of CD123 and CD11c of the two mDC clusters and the two pDC clusters identified by MegaClust. (C) Expression levels of HLA-DR and CD4 for the two pDC clusters and the two mDC clusters identified by MegaClust. Cluster 53 could be assigned as basophils due to the lack of expression of CD4 and HLA-DR (). The expression of CD4 is shown for CD4 T cells and CD14 + monocyte clusters for comparison. (D) Overlay of CD4 and HLA-DR of MegaClust identified clusters for mDC, pDC, basophils, CD4 and monocytes. (E) mDCs and pDCs were discriminated according to CD11c and CD123 expression in the supervised flow cytometry re-analysis according to the new gating strategy described in Supplementary Figure 5 (one representative patient 0QZW). (F) Representative illustration of HLA-DR and CD4 co-expression on mDCs and pDCs prior to FACS sorting CD11c+CD123 - CD4 + HLA-DR+ mDCs and CD123 + CD11c - CD4 + HLA-DR + were FACS sorted (dotted lines). (G) Gene expression profiles of flow cytometry sorted CD11c + CD123 - CD4 + HLA-DR + mDCs, CD123 + CD11c - CD4 + HLA-DR + CD4 + HLA-DR + pDC and CD14 + CD16 - monocytes from 3 healthy donors are shown in comparison to the RNA expression profile of public available RNAseq datasets (Immgen) indicating the gene expression profile of B cells, mac
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
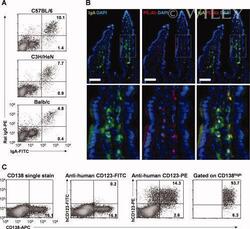
- 2 ""Pan-specific"" staining of LP-PCs by PE-antibodies. ( A ) Selective staining of IgA + LP-PCs by PE-conjugated antibody was analyzed in LPCs from C57BL/6, C3H/HeN, and Balb/c mice. ( B ) Fluorescent immunohistochemistry of C57BL/6 small intestine sections that were stained with anti-IgA (green), PE-conjugated antibody (red), and DAPI (blue). Images were overlaid with the indicated colors on top of each image. The white rectangles are enlarged into the lower panels. Scale bars, 50 mum. Pearson''s Correlation Coefficient, r > 0.75. ( C ) LP-PC-specific staining by PE-antibody was analyzed with mouse anti-human CD123 antibodies. LPCs from the murine small intestine were stained with FITC- or PE-conjugated anti-human CD123 antibodies, and fluorescent intensity in CD138 + plasma cells was compared by flow cytometry. Data are representative of three independent experiments. [Color figure can be viewed in the online issue which is available at wileyonlinelibrary.com.]
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
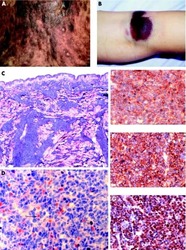
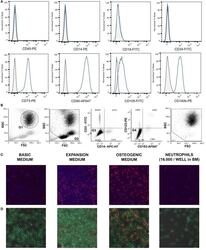
- Figure 1 (A) Surface antigen expression of bone marrow stromal cells (BMSCs) isolated from the talus bone marrow using flow cytometry. >95% of cells were negative for CD45 and CD14, and >99% of cells were negative for CD19 and CD34. In addition, >95% were positive for CD73, CD90, CD105, and CD140b. Since plastic adherence is a well-established and validated technique to isolate multipotent stromal cells (MSCs), we have only characterized one BMSC donor using flowcytometry instead of all donors. The blue lines are stained cells and the gray lines are negative (unstained) controls. Adapted from Croes et al. ( 25 ). (B) Fluorescence-activated cell sorting (FACS) gating strategy used to isolate granulocytes/neutrophils from peripheral blood leukocytes. Granulocytes were either isolated from unlabeled leukocytes using gate 1 (G1) within the forward/sideward scatter (FSC/SSC). Alternatively, leukocytes were stained using CD3, CD14, CD193, and CD123. Within the FSC/SSC of these labeled cells, debris was first excluded [gate 2 (G2)]. Subsequently, CD3+ cells (lymphocytes) and CD14+ (monocytes) were excluded [gate 3 (G3)]. In addition, CD193+ cells (eosinophils) and CD123+ cells (basophils) were excluded [gate 4 (G4)]. The remaining CD3- CD14- CD193- CD123- cells were defined FACS-sorted neutrophils (G2+, G3+, G4+ sorted neutrophils). Re-analysis of FACS-sorted neutrophils shows adequate exclusion of lymphocytes and monocytes based on their FSC/SSC. (C) Images of BMSCs obtained by arr
 Explore
Explore Validate
Validate Learn
Learn Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry