Antibody data
- Antibody Data
- Antigen structure
- References [1]
- Comments [0]
- Validations
- Western blot [1]
- Immunocytochemistry [1]
- Other assay [1]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-99011 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- FAM83D Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Recombinant full-length protein
- Reactivity
- Human
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 0.9 mg/mL
- Storage
- -20°C or -80°C if preferred
Submitted references A weakened interface in the P182L variant of HSP27 associated with severe Charcot-Marie-Tooth neuropathy causes aberrant binding to interacting proteins.
Alderson TR, Adriaenssens E, Asselbergh B, Pritišanac I, Van Lent J, Gastall HY, Wälti MA, Louis JM, Timmerman V, Baldwin AJ, Lp Benesch J
The EMBO journal 2021 Apr 15;40(8):e103811
The EMBO journal 2021 Apr 15;40(8):e103811
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
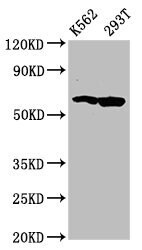
- Experimental details
- Western Blot analysis of FAM83D using a FAM83D Polyclonal antibody (Product # PA5-99011) at a concentration of 4.4 µg/mL. Positive WB detected in: K562 whole cell lysate, 293T whole cell lysate. A secondary Goat polyclonal antibody to rabbit IgG was applied at a 1:50,000 dilution. Observed band size: 65 kDa.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
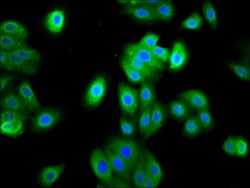
- Experimental details
- Immunofluorescent analysis of FAM83D in HepG2 cells using a FAM83D polyclonal antibody (Product # PA5-99011) at a dilution of 1:133. The cells were fixed in 4% formaldehyde, permeabilized using 0.2% Triton X-100 and blocked in 10% normal Goat Serum. The cells were then incubated with the antibody overnight at 4°C. The secondary antibody was Alexa Fluor 488-congugated Goat Anti-Rabbit IgG(H+L). Cells were counter-stained with DAPI.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
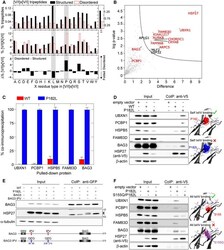
- Experimental details
- Figure 5 The P182L variant binds with enhanced affinity to IxI/V-containing proteins A Percentage of [V/I]x[V/I] tripeptides among ( top ) the total number of all tripeptides (% tripeptides) or ( middle ) the total number of [V/I]x[V/I] tripeptides in the structured (black) or disordered regions (red) ([V/I]x[V/I] %) shown as a function of the X residue type. ( Bottom ) The difference between [V/I]x[V/I] % for disordered and structured regions shows the enrichment (positive) or depletion (negative) of specific [V/I]x[V/I] motifs in disordered regions. The gray bars indicate the [V/I]L[V/I] and [V/I]P[V/I] motifs studied in this work, i.e ., ILV and IPV. B Volcano plot representing the interactors that are enriched for the P182L mutant versus GFP as a negative control, obtained with affinity-enrichment mass spectrometry. Proteins that co-immunoprecipitated significantly more ( P < 0.05) with the P182L variant are shown in the upper right quadrant. Interactors with one or more [I/V]x[I/V] motifs are displayed in red while other significantly enriched interactors are displayed in black. C, D Co-immunoprecipitation from HeLa cells stably overexpressing V5-epitope-tagged HSP27 (WT or P182L mutant) using anti-V5 beads. Co-immunoprecipitation was quantified and presented as a percentage relative to the P182L variant ( n = 3). The mean +- one standard deviation ( n = 3) is shown. Right : the location of Pro182 (red) in the crystal structure of peptide-bound cHSP27 (PDB: 4mjh) and in
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA