PA1-018
antibody from Invitrogen Antibodies
Targeting: HSPB1
CMT2F, Hs.76067, Hsp25, HSP27, HSP28
 Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [6]
- Comments [0]
- Validations
- Immunocytochemistry [4]
- Immunoprecipitation [1]
- Flow cytometry [1]
- Other assay [9]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-018 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Phospho-HSP27 (Ser15) Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA1-018 detects phosphorylated heat shock protein 27(hsp27) from rat and human tissue tissues. PA1-018 has been successfully used in Western blot, immunoprecipitation, immunofluorscence, immunocytochemistry and immunohistochemistry procedures. Immunocytochemical staining of phospho-hsp27 (Ser15) in HeLa cells with this antibody results in cytoplasmic staining. The PA1-018 immunogen is a synthetic phosphopeptide corresponding to the residues L(10) L R G P (pS) W D P F R C(21) of human HSP27. The immunizing peptide is 81% homologous in mice, rats, and hamsters. This peptide (Cat. # PEP-187) is available for use in neutralization and control experiments.
- Reactivity
- Human, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Neuroprotective Effects of Asparagus officinalis Stem Extract in Transgenic Mice Overexpressing Amyloid Precursor Protein.
Cell stress promotes the association of phosphorylated HspB1 with F-actin.
Distension of the uterus induces HspB1 expression in rat uterine smooth muscle.
The hsp27kD heat shock protein and p38-MAPK signaling are required for regular epidermal differentiation.
Small heat shock protein 27 (Hsp27) expression is highly induced in rat myometrium during late pregnancy and labour.
Hsp27 and axonal growth in adult sensory neurons in vitro.
Peng Z, Bedi S, Mann V, Sundaresan A, Homma K, Gaskey G, Kowada M, Umar S, Kulkarni AD, Eltzschig HK, Doursout MF
Journal of immunology research 2021;2021:8121407
Journal of immunology research 2021;2021:8121407
Cell stress promotes the association of phosphorylated HspB1 with F-actin.
Clarke JP, Mearow KM
PloS one 2013;8(7):e68978
PloS one 2013;8(7):e68978
Distension of the uterus induces HspB1 expression in rat uterine smooth muscle.
White BG, MacPhee DJ
American journal of physiology. Regulatory, integrative and comparative physiology 2011 Nov;301(5):R1418-26
American journal of physiology. Regulatory, integrative and comparative physiology 2011 Nov;301(5):R1418-26
The hsp27kD heat shock protein and p38-MAPK signaling are required for regular epidermal differentiation.
Jonak C, Mildner M, Klosner G, Paulitschke V, Kunstfeld R, Pehamberger H, Tschachler E, Trautinger F
Journal of dermatological science 2011 Jan;61(1):32-7
Journal of dermatological science 2011 Jan;61(1):32-7
Small heat shock protein 27 (Hsp27) expression is highly induced in rat myometrium during late pregnancy and labour.
White BG, Williams SJ, Highmore K, Macphee DJ
Reproduction (Cambridge, England) 2005 Jan;129(1):115-26
Reproduction (Cambridge, England) 2005 Jan;129(1):115-26
Hsp27 and axonal growth in adult sensory neurons in vitro.
Williams KL, Rahimtula M, Mearow KM
BMC neuroscience 2005 Apr 8;6:24
BMC neuroscience 2005 Apr 8;6:24
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
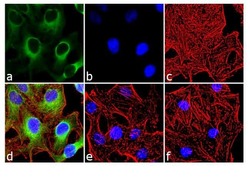
- Experimental details
- Immunofluorescence analysis of Phospho-HSP27 pSer15 was done on 70% confluent log phase HeLa cells. The cells were treated with 25 µg of Anisomycin for 30 minutes and cell were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with of Phospho-HSP27 pSer15 Rabbit Polyclonal Antibody (Product # PA1-018) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic localization. Panel e is untreated cell with no signal. Panel f is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
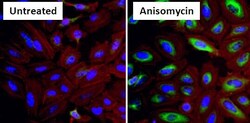
- Experimental details
- Immunofluorescent analysis of Phospho Heat Shock Protein 27 (p-HSP27) pSer15 (green) in HeLa cells either left untreated (left panel) or treated with 10uM Anisomysin (right panel) for 30 minutes. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature and blocked with 1% Blocker BSA (Product # 37525) for 15 minutes at room temperature. Cells were probed with a p-HSP27 pSer15 polyclonal antibody (Product # PA1-018) at a dilution of 1:50 for at least 1 hour at room temperature, washed with PBS, and incubated with DyLight 488 goat anti-rabbit IgG secondary antibody (Product # 35552) at a dilution of 1:400 for 30 minutes at room temperature. F-Actin (red) was stained with DyLight 554 Phalloidin (Product # 21834) and nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ArrayScan at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
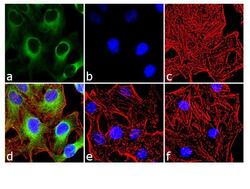
- Experimental details
- Immunofluorescence analysis of Phospho-HSP27 pSer15 was done on 70% confluent log phase HeLa cells. The cells were treated with 25 µg of Anisomycin for 30 minutes and cell were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with of Phospho-HSP27 pSer15 Rabbit Polyclonal Antibody (Product # PA1-018) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic localization. Panel e is untreated cell with no signal. Panel f is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
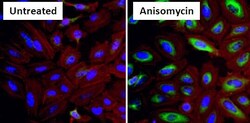
- Experimental details
- Immunofluorescent analysis of Phospho Heat Shock Protein 27 (p-HSP27) pSer15 (green) in HeLa cells either left untreated (left panel) or treated with 10uM Anisomysin (right panel) for 30 minutes. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature and blocked with 1% Blocker BSA (Product # 37525) for 15 minutes at room temperature. Cells were probed with a p-HSP27 pSer15 polyclonal antibody (Product # PA1-018) at a dilution of 1:50 for at least 1 hour at room temperature, washed with PBS, and incubated with DyLight 488 goat anti-rabbit IgG secondary antibody (Product # 35552) at a dilution of 1:400 for 30 minutes at room temperature. F-Actin (red) was stained with DyLight 554 Phalloidin (Product # 21834) and nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ArrayScan at 20X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
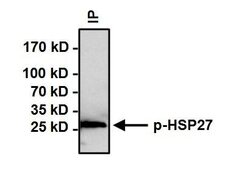
- Experimental details
- Immunoprecipitation of Phospho Heat Shock Protein 27 (p-HSP27) pSer15 was performed on HeLa cells treated with 10uM Anisomysin for 30 minutes. Antigen-antibody complexes were formed by incubating 500 µg of whole cell lysate with 3 µg of p-HSP27 pSer15 polyclonal antibody (Product # PA1-018) overnight on a rocking platform at 4°C. The immune complexes were captured on 50 µL Protein A/G Agarose (Product # 20421), washed extensively, and eluted with Lane Marker Reducing Sample Buffer (Product # 39000). Samples were then resolved on a 4-20% Tris-HCl polyacrylamide gel, transferred to a PVDF membrane, and blocked with 5% BSA/TBST for at least 1 hour. The membrane was probed with a p-HSP27 pSer15 polyclonal antibody (Product # PA1-018) at a dilution of 1:500 overnight rotating at 4°C, washed with TBST, and probed with Clean-Blot IP Detection Reagent (Product # 21230) at a dilution of 1:1000 for at least 1 hour. Chemiluminescent detection was performed using SuperSignal West Dura (Product # 34075).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
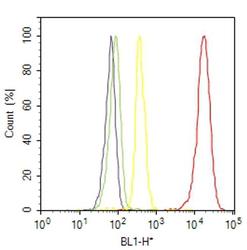
- Experimental details
- Flow cytometry analysis of Phospho-HSP27 [pSer15] was done on HeLa cells treated with anisomycin (25ug/mL, 30 minutes). Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with Phospho-HSP27 [pSer15] Rabbit Polyclonal Antibody (PA1018, red histogram) or with rabbit isotype control (yellow histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
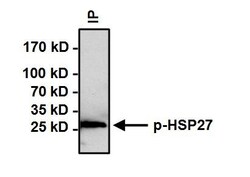
- Experimental details
- Immunoprecipitation of Phospho Heat Shock Protein 27 (p-HSP27) pSer15 was performed on HeLa cells treated with 10uM Anisomysin for 30 minutes. Antigen-antibody complexes were formed by incubating 500 µg of whole cell lysate with 3 µg of p-HSP27 pSer15 polyclonal antibody (Product # PA1-018) overnight on a rocking platform at 4°C. The immune complexes were captured on 50 µL Protein A/G Agarose (Product # 20421), washed extensively, and eluted with Lane Marker Reducing Sample Buffer (Product # 39000). Samples were then resolved on a 4-20% Tris-HCl polyacrylamide gel, transferred to a PVDF membrane, and blocked with 5% BSA/TBST for at least 1 hour. The membrane was probed with a p-HSP27 pSer15 polyclonal antibody (Product # PA1-018) at a dilution of 1:500 overnight rotating at 4°C, washed with TBST, and probed with Clean-Blot IP Detection Reagent (Product # 21230) at a dilution of 1:1000 for at least 1 hour. Chemiluminescent detection was performed using SuperSignal West Dura (Product # 34075).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
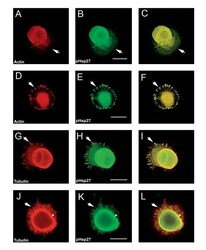
- Experimental details
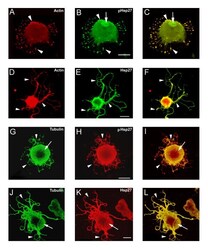
- Figure 3 pHsp27 also co-localizes with actin and tubulin at early stages of neurite growth. Neurons plated on polylysine and stimulated with laminin for 1-6 hrs were also immunostained with antibodies directed against phosphorylated Hsp27 (pHsp27 Ser15 ) to examine colocalization with actin or tubulin. A, D - rhodamine-phalloidin (red); B, E, H, K - pHsp27 (green); G, J - tubulin (red). The respective merged images are presented in panels C, F, I, L. Actin and pHsp27 appear to be colocalized in the body of the lamellopodium in A-C, but actin seems to be excluded from the leading edge (arrows). There is also localization of pHsp27 and actin in focal contacts (D-F, arrows). pHsp27 also colocalizes with tubulin in focal contacts (G-I, arrow), in a cortical ring (arrowhead) and in processes emerging from the cell body (J-L, arrow). Scale bar - 20 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
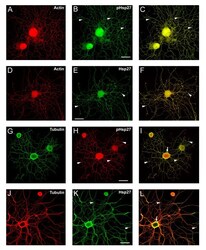
- Figure 4 Hsp27 continues to be expressed and localized with cytoskeletal elements in neurons and neuritic networks. In these experiments, neurons were plated on laminin (no added neurotrophins) and fixed 24 hrs after plating. As shown in the images, many neurons exhibit extensive neuritic growth under these conditions. A-F: Neurons were labelled with rhodamine-phalloidin (A, D, red), and immunostained for pHsp27 (B, green) and Hsp27 (E, green); C, F - merged images. G-L: Neurons were immunostained for tubulin (G, green; J, red), pHsp27 (H, red), Hsp27 (K, green); I, L - merged images. Hsp27 and pHsp27 are expressed throughout the neuritic network, and there is colocalization of these with actin (C, F) and less so with tubulin (I, L). Note the accumulation of pHsp27 and Hsp27 at point of branching of neurites (arrowheads- B, C, E, F, H, I, K, L). The cortical colocalization of tubulin with pHsp27 and Hsp27 is still evident at this stage of neurite growth (arrows - I, L). Scale bar - 50 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
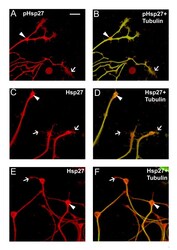
- Figure 5 Co-localization of Hsp27 and actin in growth cones of growing neurites. Growth cones from neurons plated on laminin as outlined for Figure 4 were observed to express both pHsp27 and Hsp27. pHsp27 (A) and Hsp27 (C, E, red), shown together with tubulin (green-yellow) in the merged images (B, D, F), are present in growth cones and filopodia extending from the growth cones (arrows). There is also an accumulation in the core of growth cones and at points of neurite branching (arrowheads- A-F). Note that the tubulin staining does not completely overlap with pHsp27 or Hsp27, particularly in some of the extending filopodia (B, arrow) and the core of the growth cones in D, F (arrowheads). Scale bar - 10 mm.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 6 Disruption of the actin cytoskeleton results in aberrant neurite growth. Neurons plated on LN were treated with cytochalasin D (2 mM, added 3 hrs after plating), fixed 24 hrs later and stained for pHsp27 (B, H), Hsp27 (E, K), actin (A, D) or tubulin (G, J). The respective merged images are presented in panels C, F, I and L. The cytochalasin D treatment resulted in various atypical patterns of growth. One phenotype was the elaboration of numerous processes or microspikes as seen in panels A-C, with obvious accumulation of actin and pHsp27 especially at the tips of the microspikes (arrowheads); pHsp27, but not actin, accumulates in the nucleus (B, C, arrow). Abnormal process extension was also observed. In the neuron shown in D-F, some extension was observed although there was now less colocalization of actin with the Hsp27 (arrowheads). Panels G-L show tubulin staining along with either pHsp27 or Hsp27; the nature of the cytoskeletal network is clearer in these examples (arrows). Arrowheads point to atypical neurite growth, eg., lacking the usual radial branching pattern as seen in Fig 4. Scale bar - 20 mm.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
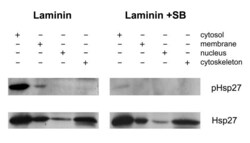
- Figure 7 p38 MAPK inhibition blocks phosphorylation of Hsp27. Neurons plated on laminin were exposed to p38 MAPK inhibitors, SB203580 and SB202190 (10 mM each). Cells were sampled at 24 hrs post SB addition, using cellular subfractionation (as described in the Methods). The resulting protein from cytosol, membrane, nucleus and cytoskeleton fractions was electrophoresed and the blot subsequently probed for pHsp27 and Hsp27. Inhibition of p38 MAPK activity (laminin+SB) results in attenuation of the Hsp27 phosphorylation.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
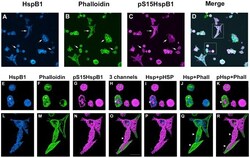
- Experimental details
- Figure 2 Cellular localization of HspB1 and F-actin in PC12 cells subsequent to a heat shock. Low passage PC12 cells were cultured on collagen-coated coverslips and were subjected to a 30 min heat shock (HS) followed by immediate fixation, and processing for immunocytochemistry using antibodies directed against total HspB1 or phospho-S15-HspB1 followed by fluorescently tagged secondary antibodies; F-actin was labelled using Alexa-488-phalloidin. Panel A-D: PC12 cells visualized with confocal microscopy; images represent the z-stack projections. A - HspB1 (blue); B - phalloidin (green); C-pS15-HspB1 (magenta); D- merged image. Panels E-K, L-R: three-dimensional rendering of immunostained surfaces as compiled by Imaris software of cells (boxes in D) selected from the low power view. E, L -HspB1 (blue); F, M - Phalloidin (green); G, N - pHspB1(magenta); H, O - all 3 together; I, P - HspB1+pHspB1; J, Q - HspB1+Phalloidin; K, R - pHspB1+Phalloidin. Scale bar -10 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
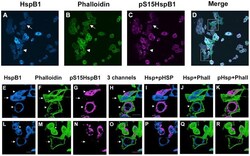
- Experimental details
- Figure 3 Cellular localization of HspB1 and F-actin in PC12 cells subsequent to a heat shock in the presence of a p38MAPK inhibitor. Low passage PC12 cells were cultured on collagen-coated coverslips, incubated with 10 uM SB203580 for 1 hr prior to and during a 30 min heat shock (HS), followed by immediate fixation and processing for immunocytochemistry using antibodies directed against total HspB1 (Stressgen SPA801) or phospho-S15-HspB1 followed by fluorescently tagged secondary antibodies; F-actin was labelled using Alexa-488-phalloidin. Panel A-D: PC12 cells visualized with confocal microscopy; images represent the z-stack projections. A - HspB1 (blue); B- phalloidin (green); C-pS15-HspB1 (magenta); D- merged image. Panels E-K, L-R: three-dimensional rendering of immunostained surfaces as compiled by Imaris software of cells (boxes in D) selected from the low power view. E, L -HspB1 (blue); F, M - Phalloidin (green); G, N - pHspB1(magenta); H, O - all 3 together; I, P - HspB1+pHspB1; J, Q - HspB1+Phalloidin; K, R - pHspB1+Phalloidin. Scale bar -10 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
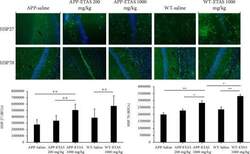
- Experimental details
- Figure 3 Quantification of heat shock protein (HSP27) and HSP70 in the hippocampus of APP-overexpressed mice treated with ETAS (200 mg/kg and 1000 mg/kg) and WT mice treated with ETAS (1000 mg/kg) for 1 month, respectively; RFUs: relative fluorescence units; green represents HSP27/70 expression, and blue represents DAPI. Arrows represent fluorescent green staining, indicating positive expression. Magnification is represented x200. HSP27: ** p < 0.01 APP-ETAS (1000 mg/kg) vs. APP-ETAS (200 mg/kg) and APP saline-treated mice; ** p < 0.01 WT-ETAS (1000 mg/kg) vs. WT saline-treated mice; RFUs: relative fluorescence units. Four mice per group and four sections per mouse were used. HSP70: * p < 0.05 APP-ETAS (1000 mg/kg) vs. APP-ETAS (200 mg/kg); ** p < 0.01 APP-ETAS (1000 mg/kg) vs. APP saline-treated mice; ** p < 0.01 WT-ETAS (1000 mg/kg) vs. WT saline-treated mice. RFUs: relative fluorescence units. Four mice per group and four sections per mouse were used.
 Explore
Explore Validate
Validate Learn
Learn