Antibody data
- Antibody Data
- Antigen structure
- References [21]
- Comments [0]
- Validations
- Immunocytochemistry [4]
- Other assay [15]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 53-6503-82 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Myosin 4 Monoclonal Antibody (MF20), Alexa Fluor™ 488, eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: This MF20 monoclonal antibody recognizes the heavy chain of myosin II, specificially the light meromyosin portion, in cardiac and skeletal muscle of vertebrates. Myosin II is composed of two heavy chains and four light chains. The 220-kDa myosin heavy chain exists as four different isoforms due to alternative splicing. Myosins interact with actin and hydrolyze ATP to function in muscle contraction, cytokinesis, and phagocytosis. The MF20 has been shown to react to myosin from a variety of mammalian, avian and amphibian species, including rat, mouse, human, chicken, zebrafish, and dog. Applications Reported: This MF20 antibody has been reported for use in immunohistochemical staining, immunocytochemistry, and immunohistochemical staining of frozen tissue sections. Applications Tested: This MF20 antibody has been tested by immunocytochemistry on fixed and permeabilized C2C12 cells that were differentiated for 2 days in low serum medium prior to staining. Staining can be performed using less than or equal to 5 µg/mL. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Excitation: 488 nm; Emission: 519 nm; Laser: Blue Laser. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human, Mouse, Rat, Bovine, Canine, Chicken/Avian, Feline, Guinea Pig, Rabbit
- Host
- Mouse
- Conjugate
- Green dye
- Isotype
- IgG
- Antibody clone number
- MF20
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- 4°C, store in dark, DO NOT FREEZE!
Submitted references Impaired Skeletal Muscle Development and Regeneration in Transglutaminase 2 Knockout Mice.
TAM kinase signaling is indispensable for proper skeletal muscle regeneration in mice.
hiPSC-Derived Neurons Provide a Robust and Physiologically Relevant In Vitro Platform to Test Botulinum Neurotoxins.
TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice.
Comprehensive histological investigation of age-related changes in dermal extracellular matrix and muscle fibers in the upper lip vermilion.
Data on the proliferation and differentiation of C2C12 myoblast treated with branched-chain ketoacid dehydrogenase kinase inhibitor.
In vivo detection of programmed cell death during mouse heart development.
Micropatterned substrates with physiological stiffness promote cell maturation and Pompe disease phenotype in human induced pluripotent stem cell-derived skeletal myocytes.
Assessment of different strategies for scalable production and proliferation of human myoblasts.
Ternary Aligned Nanofibers of RGD Peptide-Displaying M13 Bacteriophage/PLGA/Graphene Oxide for Facilitated Myogenesis.
Classification of C2C12 cells at differentiation by convolutional neural network of deep learning using phase contrast images.
Peptidyl-prolyl cis-trans isomerase NIMA interacting 1 regulates skeletal muscle fusion through structural modification of Smad3 in the linker region.
An Engineered Optogenetic Switch for Spatiotemporal Control of Gene Expression, Cell Differentiation, and Tissue Morphogenesis.
NOTCH1-Dependent Nitric Oxide Signaling Deficiency in Hypoplastic Left Heart Syndrome Revealed Through Patient-Specific Phenotypes Detected in Bioengineered Cardiogenesis.
Natural underlying mtDNA heteroplasmy as a potential source of intra-person hiPSC variability.
Targeted Ablation of Periostin-Expressing Activated Fibroblasts Prevents Adverse Cardiac Remodeling in Mice.
Ultrastructure of medial rectus muscles in patients with intermittent exotropia.
Single-molecule analysis of myocyte differentiation reveals bimodal lineage commitment.
Inhibition of TGFβ signaling increases direct conversion of fibroblasts to induced cardiomyocytes.
iPS cell-derived cardiogenicity is hindered by sustained integration of reprogramming transgenes.
Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success.
Budai Z, Al-Zaeed N, Szentesi P, Halász H, Csernoch L, Szondy Z, Sarang Z
Cells 2021 Nov 9;10(11)
Cells 2021 Nov 9;10(11)
TAM kinase signaling is indispensable for proper skeletal muscle regeneration in mice.
Al-Zaeed N, Budai Z, Szondy Z, Sarang Z
Cell death & disease 2021 Jun 12;12(6):611
Cell death & disease 2021 Jun 12;12(6):611
hiPSC-Derived Neurons Provide a Robust and Physiologically Relevant In Vitro Platform to Test Botulinum Neurotoxins.
Lamotte JD, Roqueviere S, Gautier H, Raban E, Bouré C, Fonfria E, Krupp J, Nicoleau C
Frontiers in pharmacology 2020;11:617867
Frontiers in pharmacology 2020;11:617867
TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice.
Ono Y, Maejima Y, Saito M, Sakamoto K, Horita S, Shimomura K, Inoue S, Kotani J
Scientific reports 2020 Jan 20;10(1):694
Scientific reports 2020 Jan 20;10(1):694
Comprehensive histological investigation of age-related changes in dermal extracellular matrix and muscle fibers in the upper lip vermilion.
Gomi T, Imamura T
International journal of cosmetic science 2020 Aug;42(4):359-368
International journal of cosmetic science 2020 Aug;42(4):359-368
Data on the proliferation and differentiation of C2C12 myoblast treated with branched-chain ketoacid dehydrogenase kinase inhibitor.
Sato Y, Tate H, Yoshizawa F, Sato Y
Data in brief 2020 Aug;31:105766
Data in brief 2020 Aug;31:105766
In vivo detection of programmed cell death during mouse heart development.
Martínez-Lagunas K, Yamaguchi Y, Becker C, Geisen C, DeRuiter MC, Miura M, Fleischmann BK, Hesse M
Cell death and differentiation 2020 Apr;27(4):1398-1414
Cell death and differentiation 2020 Apr;27(4):1398-1414
Micropatterned substrates with physiological stiffness promote cell maturation and Pompe disease phenotype in human induced pluripotent stem cell-derived skeletal myocytes.
Jiwlawat N, Lynch EM, Napiwocki BN, Stempien A, Ashton RS, Kamp TJ, Crone WC, Suzuki M
Biotechnology and bioengineering 2019 Sep;116(9):2377-2392
Biotechnology and bioengineering 2019 Sep;116(9):2377-2392
Assessment of different strategies for scalable production and proliferation of human myoblasts.
Chua MJ, Yildirim ED, Tan JE, Chua YB, Low SC, Ding SLS, Li CW, Jiang Z, Teh BT, Yu K, Shyh-Chang N
Cell proliferation 2019 May;52(3):e12602
Cell proliferation 2019 May;52(3):e12602
Ternary Aligned Nanofibers of RGD Peptide-Displaying M13 Bacteriophage/PLGA/Graphene Oxide for Facilitated Myogenesis.
Shin YC, Kim C, Song SJ, Jun S, Kim CS, Hong SW, Hyon SH, Han DW, Oh JW
Nanotheranostics 2018;2(2):144-156
Nanotheranostics 2018;2(2):144-156
Classification of C2C12 cells at differentiation by convolutional neural network of deep learning using phase contrast images.
Niioka H, Asatani S, Yoshimura A, Ohigashi H, Tagawa S, Miyake J
Human cell 2018 Jan;31(1):87-93
Human cell 2018 Jan;31(1):87-93
Peptidyl-prolyl cis-trans isomerase NIMA interacting 1 regulates skeletal muscle fusion through structural modification of Smad3 in the linker region.
Islam R, Yoon H, Shin HR, Bae HS, Kim BS, Yoon WJ, Woo KM, Baek JH, Lee YS, Ryoo HM
Journal of cellular physiology 2018 Dec;233(12):9390-9403
Journal of cellular physiology 2018 Dec;233(12):9390-9403
An Engineered Optogenetic Switch for Spatiotemporal Control of Gene Expression, Cell Differentiation, and Tissue Morphogenesis.
Polstein LR, Juhas M, Hanna G, Bursac N, Gersbach CA
ACS synthetic biology 2017 Nov 17;6(11):2003-2013
ACS synthetic biology 2017 Nov 17;6(11):2003-2013
NOTCH1-Dependent Nitric Oxide Signaling Deficiency in Hypoplastic Left Heart Syndrome Revealed Through Patient-Specific Phenotypes Detected in Bioengineered Cardiogenesis.
Hrstka SC, Li X, Nelson TJ, Wanek Program Genetics Pipeline Group
Stem cells (Dayton, Ohio) 2017 Apr;35(4):1106-1119
Stem cells (Dayton, Ohio) 2017 Apr;35(4):1106-1119
Natural underlying mtDNA heteroplasmy as a potential source of intra-person hiPSC variability.
Perales-Clemente E, Cook AN, Evans JM, Roellinger S, Secreto F, Emmanuele V, Oglesbee D, Mootha VK, Hirano M, Schon EA, Terzic A, Nelson TJ
The EMBO journal 2016 Sep 15;35(18):1979-90
The EMBO journal 2016 Sep 15;35(18):1979-90
Targeted Ablation of Periostin-Expressing Activated Fibroblasts Prevents Adverse Cardiac Remodeling in Mice.
Kaur H, Takefuji M, Ngai CY, Carvalho J, Bayer J, Wietelmann A, Poetsch A, Hoelper S, Conway SJ, Möllmann H, Looso M, Troidl C, Offermanns S, Wettschureck N
Circulation research 2016 Jun 10;118(12):1906-17
Circulation research 2016 Jun 10;118(12):1906-17
Ultrastructure of medial rectus muscles in patients with intermittent exotropia.
Yao J, Wang X, Ren H, Liu G, Lu P
Eye (London, England) 2016 Jan;30(1):146-51
Eye (London, England) 2016 Jan;30(1):146-51
Single-molecule analysis of myocyte differentiation reveals bimodal lineage commitment.
Gibson TM, Gersbach CA
Integrative biology : quantitative biosciences from nano to macro 2015 Jun;7(6):663-71
Integrative biology : quantitative biosciences from nano to macro 2015 Jun;7(6):663-71
Inhibition of TGFβ signaling increases direct conversion of fibroblasts to induced cardiomyocytes.
Ifkovits JL, Addis RC, Epstein JA, Gearhart JD
PloS one 2014;9(2):e89678
PloS one 2014;9(2):e89678
iPS cell-derived cardiogenicity is hindered by sustained integration of reprogramming transgenes.
Martinez-Fernandez A, Nelson TJ, Reyes S, Alekseev AE, Secreto F, Perez-Terzic C, Beraldi R, Sung HK, Nagy A, Terzic A
Circulation. Cardiovascular genetics 2014 Oct;7(5):667-76
Circulation. Cardiovascular genetics 2014 Oct;7(5):667-76
Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success.
Addis RC, Ifkovits JL, Pinto F, Kellam LD, Esteso P, Rentschler S, Christoforou N, Epstein JA, Gearhart JD
Journal of molecular and cellular cardiology 2013 Jul;60:97-106
Journal of molecular and cellular cardiology 2013 Jul;60:97-106
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
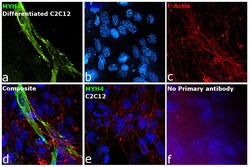
- Experimental details
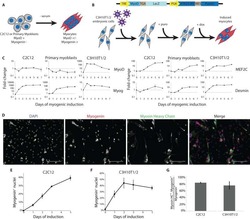
- Immunofluorescence analysis of MYH4 was performed using 70% confluent log phase C2C12 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with Myosin 4 Monoclonal Antibody (MF20), Alexa Fluor 488, eBioscience™ (Product # 53-6503-82) at 1:100 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor™ Plus 647 (Product # A32787, 1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Blue) was stained withAlexa Fluor™ 488 Phalloidin (Product # A12379, 1:300). Panel d represents the merged image showing Cytoskeleton localization. Panel e represents C2c12 undifferentiated. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
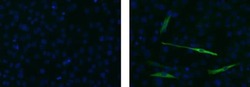
- Experimental details
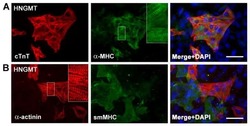
- Immunocytochemistry of differentiated C2C12 cells using 5 µg/mL of Mouse IgG2b Isotype Control Alexa Fluor® 488 (Product # 53-4732-80) (left) or 5 µg/mL Anti-Myosin Heavy Chain Alexa Fluor® 488 (right). Myosin is positive in a subpopulation of differentiated cells. Nuclei are counterstained with DAPI.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
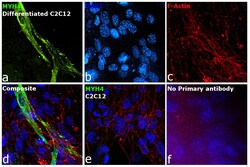
- Experimental details
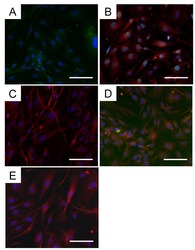
- Immunofluorescence analysis of MYH4 was performed using 70% confluent log phase C2C12 and C2C12 differentiated cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with Myosin 4 Monoclonal Antibody (MF20), Alexa Fluor 488, eBioscience™ (Product # 53-6503-82) at 1:100 in 0.1% BSA, incubated at 4 degree Celsius overnight (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing Cytoskeleton localization. Panel e represents C2C12 undifferentiated cells with no expression of MYH4. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
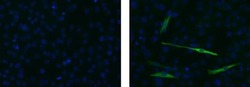
- Experimental details
- Immunocytochemistry of differentiated C2C12 cells using 5 µg/mL of Mouse IgG2b Isotype Control Alexa Fluor® 488 (Product # 53-4732-80) (left) or 5 µg/mL Anti-Myosin Heavy Chain Alexa Fluor® 488 (right). Myosin is positive in a subpopulation of differentiated cells. Nuclei are counterstained with DAPI.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
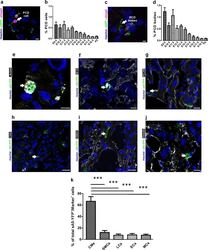
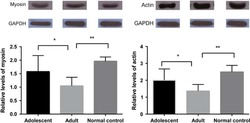
- Experimental details
- Fig. 8 Identification and quantitation of the different cell types undergoing PCD during heart development. a Sagittal section of a transgenic heart at E12.5 depicting PCD cells. PCD cells were defined by condensed nuclei, a clear ring-like membrane sA5-YFP accumulation, and/or cCasp3 + signal. b Quantitation of PCD cells (white arrow in a). c Sagittal section of a transgenic heart at E12.5 depicting bodies of PCD cells. PCD Bodies were defined as smaller particles (2-4 um) without nuclear signal and positive for either sA5-YFP or cCasp3 or both. d Quantitation of PCD bodies (white arrow in c ). e - j Co-stainings in sections of transgenic embryonic hearts (E13.5) stained for cardiomyocyte (CMs, alpha-actinin, and MF20), endothelial cell (ECs, PECAM), leukocyte (LCs, CD45), smooth muscle cell (SMCs, ASMAC), and mesenchymal cell (MCs, vimentin) markers. k Quantitation of the different cell types that undergo PCD during heart development. Hoechst nuclear staining and sA5-YFP secreted human Annexin V-yellow fluorescent protein. Bars are 5 um ( a , c , e , f ), 10 um ( g , j ), and 20 um ( h , i ). Data are shown as mean +- s.e.m.,*** P < 0.001 ANOVA with Bonferroni post hoc test; n = 7-13
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
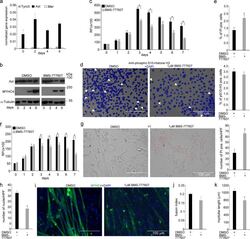
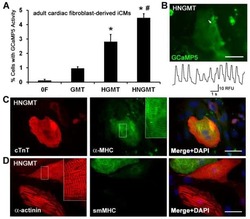
- Experimental details
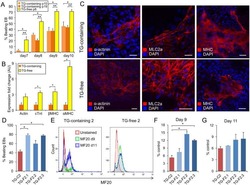
- Fig. 6 In vitro administration of the pan-TAM tyrosine kinase inhibitor BMS-777607 impairs myogenesis of C2C12 myoblast cells. a mRNA expression levels of Tyro3, Axl, and Mer during the differentiation of C2C12 myoblast cells determined by qRT-PCR. b Protein expression levels of Axl and myosin heavy chain 4 (MYHC4) in differentiating C2C12 myoblasts in the presence or absence of 1 muM BMS-777607 determined by Western blot analysis. alpha-Tubulin was used as a loading control. One representative blot of three is shown. c Alterations in the number of viable C2C12 cells grown in growth medium in the presence or absence of 1 muM BMS-777607 determined by PrestoBlue staining. d Percent of cells in G2/M phase grown in growth medium in the presence or absence of 1 muM BMS-777607 as indicated by anti-phospho-histone H3 (Ser10) and DAPI co-staining (at least 20 HPF were analyzed). Arrows point to the anti-phospho-histone H3 positive nuclei. e Percent of PI-positive cells grown in growth medium in the presence or absence of 1 muM BMS-777607. f Alterations in the number of viable C2C12 cells grown in differentiation medium in the presence or absence of 1 muM BMS-777607 determined by PrestoBlue staining. BMS-777607 was added when the medium was changed to differentiation medium. g Representative light microscopic images of C2C12 myoblasts differentiated for 6 days in the absence or presence of 1 muM BMS-777607 after staining dead cells with propidium iodide and quantification of propidium
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
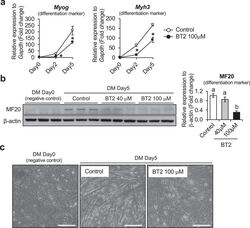
- Experimental details
- Fig. 2 The effect of BT2 on C2C12 myoblast differentiation. a) Time-course qRT-PCR analysis of the effect of BT2 treatment (100 muM) on the expression of myogenic differentiation markers ( Myh3 and Myog ) of C2C12 myoblasts. mRNA expression levels were calculated relative to Gapdh and the data are expressed as a fold-increase. Significance was determined with the two-tailed Student's t-test (vs. control, *p < 0.05) (n = 6). Values are expressed as means +- SEM. b) The effect of BT2 treatment (40 muM and 100 muM) on total MyHC expression (anti-MF20) of C2C12 myoblasts for 5 days after induction of differentiation. Myoblasts at DM 0day (cultured in growth media) is used as negative control. Graph shows the relative intensity of each band after normalization to beta-actin. Different superscripts indicate a significant difference between 2 groups. All assessments of significance were performed with 1-way ANOVA with Tukey post hoc test (p < 0.05) (n = 3). Values are expressed as means +- SEM. c) Representative images of Control and BT2-treated (100 muM) C2C12 myoblasts for 5 days after induction of differentiation. Myoblasts at DM day0 was shown as negative control. Bar = 100 mum. Fig 2
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
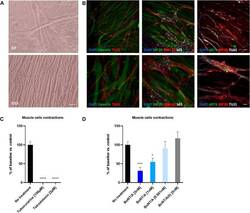
- Experimental details
- FIGURE 7 Coculture of human myotubes and human motor neurons. (A) Phase images of co-cultures maintained in P96 during 7 or 13 days. Scale bar: 25 um. (B) Immunocytochemical characterization of NMJ formation. Muscular fibers are identified with Myosin/MF20 or Desmin, Motor Neurons neurites are identified with TUJ1 or SMI-32 and MN nuclei are identified with Islet 1 and Acetylcholine receptor with alpha-bungarotoxin. Nuclei are represented in blue (DAPI). Top: 20X images; scale bar: 50 um. Bottom: 40X images; scale bar: 25 um. (C) Effect of TTX and Tubocurarine on muscle cells contractions after 6 h of exposure. (D) Dose-response effects of BoNT/A on contraction of myotubes after 6 h of exposure. * p < 0.05; ** p > 0.01; *** p < 0.001; **** p < 0.0001.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
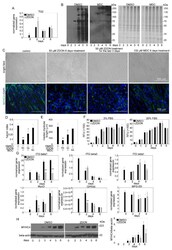
- Experimental details
- Figure 7 TG2 promotes fusion of C2C12 myoblasts without using its crosslinking activity. ( A ) mRNA expression levels of Table 2 . in differentiating C2C12 myoblasts in the presence or absence of 50 uM ZDON, a conformation inhibitor of TG2 crosslinking activity, added at day 0 of differentiation ( B ) Biotin-cadaverine incorporation into proteins of differentiating C2C12 myoblasts in the presence and absence of 100 uM monodansylcadaverine, a competitive inhibitor of TG2 crosslinking activity, detected by Western blot analysis. Protein loadings on the Coomassie dye-stained gel are also shown. ( C ) Representative light- and fluorescent microscopic images of C2C12 myoblasts differentiated for 6 days in the absence or presence of 50 uM ZDON added at day 0 or day 4 of differentiation, or in the presence of 100 uM monodansylcadaverine added at day 0 of differentiation. MYHC4 was visualized by using anti-MYHC4 antibody (green) and nuclei by DAPI (blue). Scale bars, 100 um. ( D ) Fusion index of C2C12 cells differentiated for 6 days in the absence and presence of TG2 inhibitors. ( E ) The length of myotubes generated from C2C12 myoblasts differentiated for 6 days in the absence or presence of TG2 inhibitors ( F ) Alterations in the number of viable C2C12 cells grown in growth and differentiation medium in the presence or absence of 50 uM ZDON added at day 0 determined by PrestoBlue staining. ( G ) mRNA expression levels of myogenic genes in differentiating C2C12 myoblasts in the pre
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
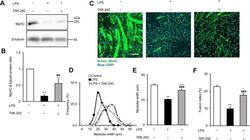
- Experimental details
- Figure 1 TAK-242 reduces LPS-induced atrophy of C2C12 myotubes. ( A,B ) Western blot analysis ( A ) and quantification ( B ) of MyHC expression in C2C12 myotubes treated for 48 h with vehicle, LPS (1 mug/mL), or LPS and TAK-242 (1 muM). Data in ( B ) were normalised to beta-tubulin protein levels, and the ratio in vehicle-treated control cells was set to 1. N = 5/group. Full-length blots are presented in Supplementary Figure S4. ( C ) Representative immunofluorescence staining of MyHC in C2C12 myotubes treated as described for ( A,B ). Scale bar = 100 mum. ( D-F ) Distribution of myotube widths ( D ), mean myotube width ( E ), and fusion index ( F ) of C2C12 cells treated as described in ( A,B ). Myotube width was measured as described in the Methods. N = 97-114. The fusion index was calculated from five randomly selected fields as described in the Methods. For all panels, data are presented as the mean +- s.e.m. ***p < 0.001, **p < 0.01, *p < 0.05 vs vehicle control; ### p < 0.001, ## p < 0.01 vs LPS-treated group. P-values were derived from one-way ANOVA followed by Tukey's honest significant difference test or Kruskal-Wallis test followed by Dunn's post hoc tests with Bonferroni correction.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
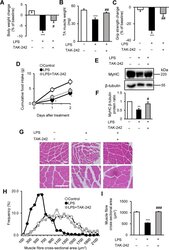
- Figure 3 TAK-242 reduces LPS-induced muscle atrophy and weakness in mice. ( A - D ) Wild-type C57BL/6 mice (8-12-week-old males) were injected with vehicle (PBS containing 0.9% DMSO) or TAK-242 (3 mg/kg) and then with PBS or LPS (1 mg/kg) 1 h later. After 2 days, mice were assessed for body weight ( A ), TA muscle weight ( B ), and grip strength ( C ). Food intake ( D ) was measured every 24 h up to 48 h. N = 4-5/group. (E , F ) Western blot analysis ( E ) and quantification ( F ) of MyHC expression in TA muscles at 2 days after administration of LPS (1 mg/kg) and TAK-242 (3 mg/kg) as described for ( A-D ). Data were normalised to beta-tubulin protein levels, and the ratio in vehicle control-treated mice was set at 1.0. N = 7-8/group. Full-length blots are presented in Supplementary Figure S6 . ( G-I ) Representative images of H&E-stained TA muscle sections ( G ) and quantification of the distribution ( H ) and mean ( I ) cross-sectional areas of TA muscle fibres at 2 days after administration of LPS (1 mg/kg) and TAK-242 (3 mg/kg) as described for ( A-D ). The cross-sectional area of TA muscle fibres was measured as described in the Methods. Scale bar, 100 muM. N = 506-523/group. For all panels, data are presented as the mean +- s.e.m. ***p < 0.001, **p < 0.01, *p < 0.05 vs vehicle control, ### p < 0.001, ## p < 0.01, # p < 0.05 vs LPS-treated group by one-way ANOVA followed by Tukey's honest significant difference test.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
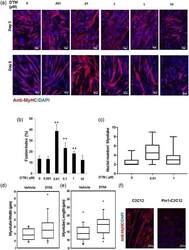
- Figure 2 Loss of peptidyl-prolyl cis-trans isomerase NIMA interacting 1 (Pin1) enhances muscle fusion in vitro. (a) Low dose of the Pin1 inhibitor dipentamethylene thiuram monosulfide (DTM; 0.01 muM) treatment caused more enhanced fusion than higher dosage (1 muM) in C2C12 cells. C2C12 cells were differentiated for 5 days with different dosage of Pin1 inhibitor DTM ranging from 0.001 to 10 muM. Cells were then immunostained for myosin heavy chain (MyHC) with anti-MyHC antibody and Cy3-conjugated secondary antimouse antibody. Scale bar= 50 muM in Day 3 images and scale bar= 100 muM in Day 5 images. (b) DTM treatment at 0.01 muM concentration shows highest fusion index. Graph showing the fusion index calculated by dividing the total number of nuclei in multinucleated cells (which have more than two nuclei) in each field by the total number of nuclei and making percentage from cells treated with DTM or without DTM ( **p < 0.001). DTM treatment at 0.01 muM concentration shows the highest fusion index. (c) Nuclei number in each myotube ( x -axis in the graph) of cells treated with different dosages of DTM. Statistics was calculated with GraphPad Prism software, and p < 0.01. (d) Myotube width and (e) length shown graphically in a box plot made with data derived from DTM (0.01 muM)-treated C2C12 cells after 3 days of differentiation. Pin1 inhibition increases both the width and length of myotubes. (f) Overexpression of Pin1 inhibiting myotube formation. Pin1-overexpressing st
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
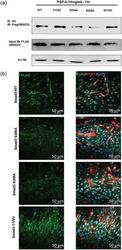
- Figure 6 Peptidyl-prolyl cis-trans isomerase NIMA interacting 1 (Pin1)-binding serine 204 residue in the smad3 linker region is crucial for fusion. (a) Pin1 binds to serine 204 and serine 208 of the Smad3 linker region in postmitotic C2C12 cells. Smad3 wild type and SP/TP site mutants in the linker region of Smad3 (CS2 Smad3-WT, CS2 Smad3 T179V, CS2 Smad3 S204A, CS2 Smad3 S208A, and CS2 Smad3 S213A) were coexpressed with PCNDA3.1 HA-Pin1. 24 hr posttransfection and postculture in a differentiation medium, cells were harvested and Immunoprecipitatioin (IP) was done with the anti-HA antibody. Later, western blot analysis was done for IP samples and input samples followed by immunoblotting for the Flag tag and actin. (b) Cells overexpressing CS2 Smad3 S204A only showed rescue of myotube fusion, and cells overexpressing CS2 Smad3 T179V showed many cells with two nuclei. C2C12 cells were transfected with constructs for Smad3 wild type and SP-TP site mutants in the linker region of Smad3 (CS2 Smad3-WT, CS2 Smad3 S204A, CS2 Smad3 S208A, and CS2 Smad3 T179V). The cells were allowed to differentiate for 4 days before fixation and immunostained with the Anti-MyHC antibody and Anti-Flag antibody. Scale bar= 50 muM [Color figure can be viewed at wileyonlinelibrary.com]
- Conjugate
- Green dye
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunohistochemistry
Immunohistochemistry