Antibody data
- Antibody Data
- Antigen structure
- References [40]
- Comments [0]
- Validations
- Flow cytometry [2]
- Other assay [41]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-0909-82 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD90 (Thy-1) Monoclonal Antibody (eBio5E10 (5E10)), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The eBio5E10 monoclonal antibody reacts with human CD90, also known as Thy-1 (thymus cell antigen-1). CD90 is a 25-35 kD receptor expressed on thymocytes, CD34+ prothymocytes, hematopoietic stem cells, neurons, a small subset of human fetal liver cells, cord blood cells, and bone marrow cells. CD90 is expressed on a subset of immature, CD34+ cells and a distinct subset of mature CD34- cells that are CD3+CD4+. The CD90+CD34+ population is enriched for cells capable of long-term culture. CD90 is involved in regulation of adhesion and signal transduction by T cells. Applications Reported: This eBio5E10 (5E10) antibody has been reported for use in flow cytometric analysis, immunoprecipitation, immunoblotting (WB), and immunohistology staining of frozen tissue sections. Applications Tested: This eBio5E10 (5E10) antibody has been tested by flow cytometric analysis of human erythroleukemia (HEL) cells. This can be used at less than or equal to 0.5 µg per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- eBio5E10 (5E10)
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- 4°C
Submitted references Exosomes derived from stem cells of human deciduous exfoliated teeth inhibit angiogenesis in vivo and in vitro via the transfer of miR-100-5p and miR-1246.
Fused Cells between Human-Adipose-Derived Mesenchymal Stem Cells and Monocytes Keep Stemness Properties and Acquire High Mobility.
Aberrant Expression of COX-2 and FOXG1 in Infrapatellar Fat Pad-Derived ASCs from Pre-Diabetic Donors.
Exosomes Regulate Interclonal Communication on Osteogenic Differentiation Among Heterogeneous Osteogenic Single-Cell Clones Through PINK1/Parkin-Mediated Mitophagy.
Therapeutic potential of human umbilical cord mesenchymal stem cells on aortic atherosclerotic plaque in a high-fat diet rabbit model.
In Vitro Anti-cancer Activity of Adipose-Derived Mesenchymal Stem Cells Increased after Infection with Oncolytic Reovirus.
VAP-PLGA microspheres (VAP-PLGA) promote adipose-derived stem cells (ADSCs)-induced wound healing in chronic skin ulcers in mice via PI3K/Akt/HIF-1α pathway.
Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19.
TRPA1 triggers hyperalgesia and inflammation after tooth bleaching.
Down-Regulated Exosomal MicroRNA-221 - 3p Derived From Senescent Mesenchymal Stem Cells Impairs Heart Repair.
Differentiation Potential of Early- and Late-Passage Adipose-Derived Mesenchymal Stem Cells Cultured under Hypoxia and Normoxia.
The Effect of Angiotensin II, Retinoic Acid, EGCG, and Vitamin C on the Cardiomyogenic Differentiation Induction of Human Amniotic Fluid-Derived Mesenchymal Stem Cells.
PF-127 hydrogel plus sodium ascorbyl phosphate improves Wharton's jelly mesenchymal stem cell-mediated skin wound healing in mice.
TGF‑β induces periodontal ligament stem cell senescence through increase of ROS production.
LPS‑induced upregulation of the TLR4 signaling pathway inhibits osteogenic differentiation of human periodontal ligament stem cells under inflammatory conditions.
Visfatin Mediates Malignant Behaviors through Adipose-Derived Stem Cells Intermediary in Breast Cancer.
BNC1 regulates cell heterogeneity in human pluripotent stem cell-derived epicardium.
Thioredoxin 1 is associated with the proliferation and apoptosis of rheumatoid arthritis fibroblast-like synoviocytes.
BNIP3/Bcl-2-mediated apoptosis induced by cyclic tensile stretch in human cartilage endplate-derived stem cells.
CD90 promotes cell migration, viability and sphere‑forming ability of hepatocellular carcinoma cells.
Comprehensive characterization of chorionic villi-derived mesenchymal stromal cells from human placenta.
Human umbilical cord-derived mesenchymal stem cells ameliorate the enteropathy of food allergies in mice.
Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations.
A mobile endocytic network connects clathrin-independent receptor endocytosis to recycling and promotes T cell activation.
Nrf2 Inhibits Periodontal Ligament Stem Cell Apoptosis under Excessive Oxidative Stress.
Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism.
Endothelial and smooth muscle cells derived from human cardiac explants demonstrate angiogenic potential and suitable for design of cell-containing vascular grafts.
A Member of the Nuclear Receptor Superfamily, Designated as NR2F2, Supports the Self-Renewal Capacity and Pluripotency of Human Bone Marrow-Derived Mesenchymal Stem Cells.
CRISPR/Cas9-based genetic correction for recessive dystrophic epidermolysis bullosa.
Influence of aging on the quantity and quality of human cardiac stem cells.
MIF Plays a Key Role in Regulating Tissue-Specific Chondro-Osteogenic Differentiation Fate of Human Cartilage Endplate Stem Cells under Hypoxia.
Pericyte response to contraction mode-specific resistance exercise training in human skeletal muscle.
Role of intestinal myofibroblasts in HIV-associated intestinal collagen deposition and immune reconstitution following combination antiretroviral therapy.
Human esophageal myofibroblasts secrete proinflammatory cytokines in response to acid and Toll-like receptor 4 ligands.
Immunophenotypic comparison of heterogenous non-sorted versus sorted mononuclear cells from human umbilical cord blood: a novel cell enrichment approach.
Multi-kilobase homozygous targeted gene replacement in human induced pluripotent stem cells.
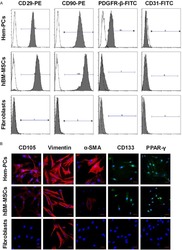
PDGFR-β (+) perivascular cells from infantile hemangioma display the features of mesenchymal stem cells and show stronger adipogenic potential in vitro and in vivo.
Epithelial cell differentiation of human mesenchymal stromal cells in decellularized lung scaffolds.
Hypoxia-cultured human adipose-derived mesenchymal stem cells are non-oncogenic and have enhanced viability, motility, and tropism to brain cancer.
Expression of Thy-1 on human hematopoietic progenitor cells.
Liu P, Zhang Q, Mi J, Wang S, Xu Q, Zhuang D, Chen W, Liu C, Zhang L, Guo J, Wu X
Stem cell research & therapy 2022 Mar 3;13(1):89
Stem cell research & therapy 2022 Mar 3;13(1):89
Fused Cells between Human-Adipose-Derived Mesenchymal Stem Cells and Monocytes Keep Stemness Properties and Acquire High Mobility.
Montalbán-Hernández K, Casado-Sánchez C, Avendaño-Ortiz J, Casalvilla-Dueñas JC, Bonel-Pérez GC, Prado-Montero J, Valentín-Quiroga J, Lozano-Rodríguez R, Terrón-Arcos V, de la Bastida FR, Córdoba L, Laso-García F, Diekhorst L, Del Fresno C, López-Collazo E
International journal of molecular sciences 2022 Aug 26;23(17)
International journal of molecular sciences 2022 Aug 26;23(17)
Aberrant Expression of COX-2 and FOXG1 in Infrapatellar Fat Pad-Derived ASCs from Pre-Diabetic Donors.
O'Donnell BT, Monjure TA, Al-Ghadban S, Ives CJ, L'Ecuyer MP, Rhee C, Romero-Lopez M, Li Z, Goodman SB, Lin H, Tuan RS, Bunnell BA
Cells 2022 Aug 1;11(15)
Cells 2022 Aug 1;11(15)
Exosomes Regulate Interclonal Communication on Osteogenic Differentiation Among Heterogeneous Osteogenic Single-Cell Clones Through PINK1/Parkin-Mediated Mitophagy.
Fei D, Xia Y, Zhai Q, Wang Y, Zhou F, Zhao W, He X, Wang Q, Jin Y, Li B
Frontiers in cell and developmental biology 2021;9:687258
Frontiers in cell and developmental biology 2021;9:687258
Therapeutic potential of human umbilical cord mesenchymal stem cells on aortic atherosclerotic plaque in a high-fat diet rabbit model.
Li Y, Shi G, Han Y, Shang H, Li H, Liang W, Zhao W, Bai L, Qin C
Stem cell research & therapy 2021 Jul 15;12(1):407
Stem cell research & therapy 2021 Jul 15;12(1):407
In Vitro Anti-cancer Activity of Adipose-Derived Mesenchymal Stem Cells Increased after Infection with Oncolytic Reovirus.
Babaei A, Bannazadeh Baghi H, Nezhadi A, Jamalpoor Z
Advanced pharmaceutical bulletin 2021 Feb;11(2):361-370
Advanced pharmaceutical bulletin 2021 Feb;11(2):361-370
VAP-PLGA microspheres (VAP-PLGA) promote adipose-derived stem cells (ADSCs)-induced wound healing in chronic skin ulcers in mice via PI3K/Akt/HIF-1α pathway.
Jiang W, Zhang J, Zhang X, Fan C, Huang J
Bioengineered 2021 Dec;12(2):10264-10284
Bioengineered 2021 Dec;12(2):10264-10284
Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19.
Li Z, Wang Z, Dinh PC, Zhu D, Popowski KD, Lutz H, Hu S, Lewis MG, Cook A, Andersen H, Greenhouse J, Pessaint L, Lobo LJ, Cheng K
Nature nanotechnology 2021 Aug;16(8):942-951
Nature nanotechnology 2021 Aug;16(8):942-951
TRPA1 triggers hyperalgesia and inflammation after tooth bleaching.
Chen C, Huang X, Zhu W, Ding C, Huang P, Li R
Scientific reports 2021 Aug 31;11(1):17418
Scientific reports 2021 Aug 31;11(1):17418
Down-Regulated Exosomal MicroRNA-221 - 3p Derived From Senescent Mesenchymal Stem Cells Impairs Heart Repair.
Sun L, Zhu W, Zhao P, Zhang J, Lu Y, Zhu Y, Zhao W, Liu Y, Chen Q, Zhang F
Frontiers in cell and developmental biology 2020;8:263
Frontiers in cell and developmental biology 2020;8:263
Differentiation Potential of Early- and Late-Passage Adipose-Derived Mesenchymal Stem Cells Cultured under Hypoxia and Normoxia.
Zhao AG, Shah K, Freitag J, Cromer B, Sumer H
Stem cells international 2020;2020:8898221
Stem cells international 2020;2020:8898221
The Effect of Angiotensin II, Retinoic Acid, EGCG, and Vitamin C on the Cardiomyogenic Differentiation Induction of Human Amniotic Fluid-Derived Mesenchymal Stem Cells.
Gasiūnienė M, Valatkaitė E, Navakauskaitė A, Navakauskienė R
International journal of molecular sciences 2020 Nov 19;21(22)
International journal of molecular sciences 2020 Nov 19;21(22)
PF-127 hydrogel plus sodium ascorbyl phosphate improves Wharton's jelly mesenchymal stem cell-mediated skin wound healing in mice.
Deng Q, Huang S, Wen J, Jiao Y, Su X, Shi G, Huang J
Stem cell research & therapy 2020 Apr 3;11(1):143
Stem cell research & therapy 2020 Apr 3;11(1):143
TGF‑β induces periodontal ligament stem cell senescence through increase of ROS production.
Fan C, Ji Q, Zhang C, Xu S, Sun H, Li Z
Molecular medicine reports 2019 Oct;20(4):3123-3130
Molecular medicine reports 2019 Oct;20(4):3123-3130
LPS‑induced upregulation of the TLR4 signaling pathway inhibits osteogenic differentiation of human periodontal ligament stem cells under inflammatory conditions.
Yu B, Li Q, Zhou M
International journal of molecular medicine 2019 Jun;43(6):2341-2351
International journal of molecular medicine 2019 Jun;43(6):2341-2351
Visfatin Mediates Malignant Behaviors through Adipose-Derived Stem Cells Intermediary in Breast Cancer.
Huang JY, Wang YY, Lo S, Tseng LM, Chen DR, Wu YC, Hou MF, Yuan SF
Cancers 2019 Dec 20;12(1)
Cancers 2019 Dec 20;12(1)
BNC1 regulates cell heterogeneity in human pluripotent stem cell-derived epicardium.
Gambardella L, McManus SA, Moignard V, Sebukhan D, Delaune A, Andrews S, Bernard WG, Morrison MA, Riley PR, Göttgens B, Gambardella Le Novère N, Sinha S
Development (Cambridge, England) 2019 Dec 13;146(24)
Development (Cambridge, England) 2019 Dec 13;146(24)
Thioredoxin 1 is associated with the proliferation and apoptosis of rheumatoid arthritis fibroblast-like synoviocytes.
Lu T, Zong M, Fan S, Lu Y, Yu S, Fan L
Clinical rheumatology 2018 Jan;37(1):117-125
Clinical rheumatology 2018 Jan;37(1):117-125
BNIP3/Bcl-2-mediated apoptosis induced by cyclic tensile stretch in human cartilage endplate-derived stem cells.
Yuan C, Pu L, He Z, Wang J
Experimental and therapeutic medicine 2018 Jan;15(1):235-241
Experimental and therapeutic medicine 2018 Jan;15(1):235-241
CD90 promotes cell migration, viability and sphere‑forming ability of hepatocellular carcinoma cells.
Zhang K, Che S, Su Z, Zheng S, Zhang H, Yang S, Li W, Liu J
International journal of molecular medicine 2018 Feb;41(2):946-954
International journal of molecular medicine 2018 Feb;41(2):946-954
Comprehensive characterization of chorionic villi-derived mesenchymal stromal cells from human placenta.
Ventura Ferreira MS, Bienert M, Müller K, Rath B, Goecke T, Opländer C, Braunschweig T, Mela P, Brümmendorf TH, Beier F, Neuss S
Stem cell research & therapy 2018 Feb 5;9(1):28
Stem cell research & therapy 2018 Feb 5;9(1):28
Human umbilical cord-derived mesenchymal stem cells ameliorate the enteropathy of food allergies in mice.
Yan N, Xu J, Zhao C, Wu Y, Gao F, Li C, Zhou W, Xiao T, Zhou X, Shao Q, Xia S
Experimental and therapeutic medicine 2018 Dec;16(6):4445-4456
Experimental and therapeutic medicine 2018 Dec;16(6):4445-4456
Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations.
Philippeos C, Telerman SB, Oulès B, Pisco AO, Shaw TJ, Elgueta R, Lombardi G, Driskell RR, Soldin M, Lynch MD, Watt FM
The Journal of investigative dermatology 2018 Apr;138(4):811-825
The Journal of investigative dermatology 2018 Apr;138(4):811-825
A mobile endocytic network connects clathrin-independent receptor endocytosis to recycling and promotes T cell activation.
Compeer EB, Kraus F, Ecker M, Redpath G, Amiezer M, Rother N, Nicovich PR, Kapoor-Kaushik N, Deng Q, Samson GPB, Yang Z, Lou J, Carnell M, Vartoukian H, Gaus K, Rossy J
Nature communications 2018 Apr 23;9(1):1597
Nature communications 2018 Apr 23;9(1):1597
Nrf2 Inhibits Periodontal Ligament Stem Cell Apoptosis under Excessive Oxidative Stress.
Liu Y, Yang H, Wen Y, Li B, Zhao Y, Xing J, Zhang M, Chen Y
International journal of molecular sciences 2017 May 17;18(5)
International journal of molecular sciences 2017 May 17;18(5)
Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism.
Li J, Mao Q, He J, She H, Zhang Z, Yin C
Stem cell research & therapy 2017 Mar 9;8(1):55
Stem cell research & therapy 2017 Mar 9;8(1):55
Endothelial and smooth muscle cells derived from human cardiac explants demonstrate angiogenic potential and suitable for design of cell-containing vascular grafts.
Zakharova IS, Zhiven' MK, Saaya SB, Shevchenko AI, Smirnova AM, Strunov A, Karpenko AA, Pokushalov EA, Ivanova LN, Makarevich PI, Parfyonova YV, Aboian E, Zakian SM
Journal of translational medicine 2017 Mar 3;15(1):54
Journal of translational medicine 2017 Mar 3;15(1):54
A Member of the Nuclear Receptor Superfamily, Designated as NR2F2, Supports the Self-Renewal Capacity and Pluripotency of Human Bone Marrow-Derived Mesenchymal Stem Cells.
Zhu N, Wang H, Wang B, Wei J, Shan W, Feng J, Huang H
Stem cells international 2016;2016:5687589
Stem cells international 2016;2016:5687589
CRISPR/Cas9-based genetic correction for recessive dystrophic epidermolysis bullosa.
Webber BR, Osborn MJ, McElroy AN, Twaroski K, Lonetree CL, DeFeo AP, Xia L, Eide C, Lees CJ, McElmurry RT, Riddle MJ, Kim CJ, Patel DD, Blazar BR, Tolar J
NPJ Regenerative medicine 2016;1:16014-
NPJ Regenerative medicine 2016;1:16014-
Influence of aging on the quantity and quality of human cardiac stem cells.
Nakamura T, Hosoyama T, Kawamura D, Takeuchi Y, Tanaka Y, Samura M, Ueno K, Nishimoto A, Kurazumi H, Suzuki R, Ito H, Sakata K, Mikamo A, Li TS, Hamano K
Scientific reports 2016 Mar 7;6:22781
Scientific reports 2016 Mar 7;6:22781
MIF Plays a Key Role in Regulating Tissue-Specific Chondro-Osteogenic Differentiation Fate of Human Cartilage Endplate Stem Cells under Hypoxia.
Yao Y, Deng Q, Song W, Zhang H, Li Y, Yang Y, Fan X, Liu M, Shang J, Sun C, Tang Y, Jin X, Liu H, Huang B, Zhou Y
Stem cell reports 2016 Aug 9;7(2):249-62
Stem cell reports 2016 Aug 9;7(2):249-62
Pericyte response to contraction mode-specific resistance exercise training in human skeletal muscle.
Farup J, De Lisio M, Rahbek SK, Bjerre J, Vendelbo MH, Boppart MD, Vissing K
Journal of applied physiology (Bethesda, Md. : 1985) 2015 Nov 15;119(10):1053-63
Journal of applied physiology (Bethesda, Md. : 1985) 2015 Nov 15;119(10):1053-63
Role of intestinal myofibroblasts in HIV-associated intestinal collagen deposition and immune reconstitution following combination antiretroviral therapy.
Asmuth DM, Pinchuk IV, Wu J, Vargas G, Chen X, Mann S, Albanese A, Ma ZM, Saroufeem R, Melcher GP, Troia-Cancio P, Torok NJ, Miller CJ, Powell DW
AIDS (London, England) 2015 May 15;29(8):877-88
AIDS (London, England) 2015 May 15;29(8):877-88
Human esophageal myofibroblasts secrete proinflammatory cytokines in response to acid and Toll-like receptor 4 ligands.
Gargus M, Niu C, Vallone JG, Binkley J, Rubin DC, Shaker A
American journal of physiology. Gastrointestinal and liver physiology 2015 Jun 1;308(11):G904-23
American journal of physiology. Gastrointestinal and liver physiology 2015 Jun 1;308(11):G904-23
Immunophenotypic comparison of heterogenous non-sorted versus sorted mononuclear cells from human umbilical cord blood: a novel cell enrichment approach.
Indumathi S, Harikrishnan R, Rajkumar JS, Dhanasekaran M
Cytotechnology 2015 Jan;67(1):107-14
Cytotechnology 2015 Jan;67(1):107-14
Multi-kilobase homozygous targeted gene replacement in human induced pluripotent stem cells.
Byrne SM, Ortiz L, Mali P, Aach J, Church GM
Nucleic acids research 2015 Feb 18;43(3):e21
Nucleic acids research 2015 Feb 18;43(3):e21
PDGFR-β (+) perivascular cells from infantile hemangioma display the features of mesenchymal stem cells and show stronger adipogenic potential in vitro and in vivo.
Yuan SM, Guo Y, Zhou XJ, Shen WM, Chen HN
International journal of clinical and experimental pathology 2014;7(6):2861-70
International journal of clinical and experimental pathology 2014;7(6):2861-70
Epithelial cell differentiation of human mesenchymal stromal cells in decellularized lung scaffolds.
Mendez JJ, Ghaedi M, Steinbacher D, Niklason LE
Tissue engineering. Part A 2014 Jun;20(11-12):1735-46
Tissue engineering. Part A 2014 Jun;20(11-12):1735-46
Hypoxia-cultured human adipose-derived mesenchymal stem cells are non-oncogenic and have enhanced viability, motility, and tropism to brain cancer.
Feng Y, Zhu M, Dangelmajer S, Lee YM, Wijesekera O, Castellanos CX, Denduluri A, Chaichana KL, Li Q, Zhang H, Levchenko A, Guerrero-Cazares H, Quiñones-Hinojosa A
Cell death & disease 2014 Dec 11;5(12):e1567
Cell death & disease 2014 Dec 11;5(12):e1567
Expression of Thy-1 on human hematopoietic progenitor cells.
Craig W, Kay R, Cutler RL, Lansdorp PM
The Journal of experimental medicine 1993 May 1;177(5):1331-42
The Journal of experimental medicine 1993 May 1;177(5):1331-42
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
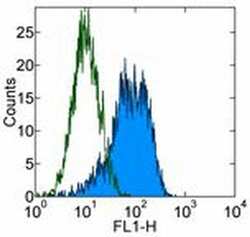
- Experimental details
- Staining of human erythroleukemia (HEL) cell line with 0.25 µg of Mouse IgG1 kappa Isotype Control Purified (Product # 14-4714-82) (open histogram) or 0.25 µg of Anti-Human CD90 (Thy-1) Purified (filled histogram) followed by Anti-Mouse IgG FITC (Product # 11-4011-85). Total viable cells were used for analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
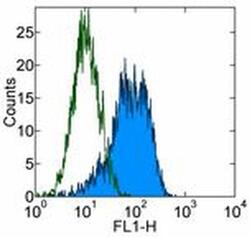
- Experimental details
- Staining of human erythroleukemia (HEL) cell line with 0.25 µg of Mouse IgG1 kappa Isotype Control Purified (Product # 14-4714-82) (open histogram) or 0.25 µg of Anti-Human CD90 (Thy-1) Purified (filled histogram) followed by Anti-Mouse IgG FITC (Product # 11-4011-85). Total viable cells were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
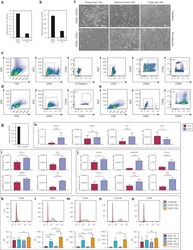
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
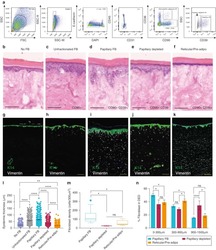
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
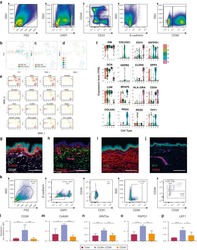
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 1 Flow cytometry analysis of phenotype characterization of hUCMSCs. Phenotype of CD73, CD90, CD105, CD14, CD34, CD45, CD79a and HLA-DR of hUCMSCs was detected by flow cytometry. Intensity >= 95% represented strong expression while
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 1. Immunophenotypic profile of cultured cartilage endplate-derived stem cells. The blue lines represent the fluorescence intensity of cells stained with the indicated antibodies and the red lines represent the negative controls cells, which were stained with a non-immunoreactive isotype control antibody. FITC, fluorescein isothiocyanate; PerCP, peridinin chlorophyll; PE, phycoerythrin; HLA-DR, human leukocyte antigen-antigen D related.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
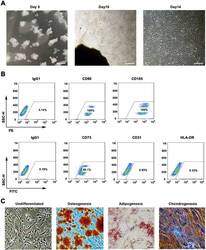
- Experimental details
- Fig. 1 WJMSCs isolation and characterization. a Primary cell isolation procedure from Wharton''s jelly tissue. The migrated cells exhibited typical fibroblast-like morphology. Scale bar, 500 mum. b Flow cytometry analysis of P4 cells using mesenchymal stem cell markers (CD90, CD105, CD73), endothelial cell marker (CD31), and MHC class II protein HLA-DR. Isotypic antibodies (IgG1-PE and IgG1-FITC) were used as negative controls. c Representative stained images show that the fourth passage WJMSCs could differentiate into osteocytes (Alizarin Red S), adipocytes (Oil Red O), and chondrocytes (Alcian blue). Scale bar, 100 mum
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
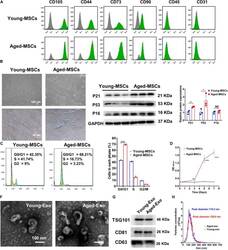
- FIGURE 1 Characterization of young and aged MSCs and exosomes. (A) Surface marker profiling of young-MSCs and aged-MSCs. (B) SA-beta-Gal staining showed that senescence increased significantly in aged MSCs. (C) Representative immunoblot images and quantitative analysis of p21, p53, and p16 protein level in young and aged-MSCs. ( n = 3). (D) Quantitation of cell cycle phases by propidium iodide staining. ( n = 3). (E) The CCK-8 assay showed that aged MSCs grew more slowly than young MSCs. ( n = 6). (F) Young and aged exosomes were observed using TEM. (G) The exosome surface markers were analyzed by Western blot. (H) Nanoparticle tracking analysis was used to analyze the particle size and concentration of Young-Exo and Aged-Exo. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; NS, not significant.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
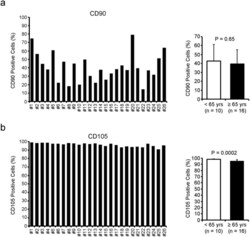
- Figure 2 The phenotype of human CDCs varies according to the individual patient. The percentage of CD90- ( a ) and CD105-positive ( b ) cells was measured by flow cytometry. The average of CD90 and CD105 positivity was compared between CDCs from younger (=65 yrs) patients.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
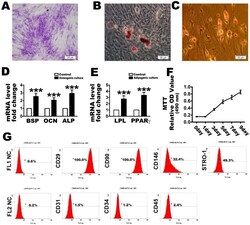
- Figure 1 Identification of human periodontal ligament stem cells (PDLSCs) used in this research. Representative image of colony-forming units and ( A ) a random single-cell clone on day 14. Bars = 25 um; ( B ) Representative images of mineralized cell nodules following a 21-day osteogenic induction period and ( C ) lipid droplets following a 14-day adipogenic induction period. Bars = 50 um; ( D ) Osteogenesis and ( E ) adipogenesis mRNA expression after the culture conditions compared with control; ( F ) Proliferation of locally isolated PDLSCs, as assessed by MTT assays after cultured at days 1, 3, 5, 7 and 9; ( G ) Cell surface markers identified by flow cytometric analysis. FL1 NC and FL2 NC means the negative control to the FL1 and FL2, respectively. The data are expressed as the mean +- SD. n = 5. *** p < 0.001 represent significant differences between the indicated columns.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
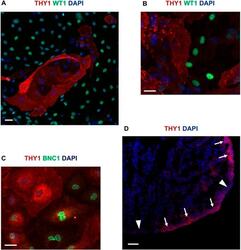
- Fig. 5. THY1 expression in epicardium. (A) Immunofluorescence double labelling for THY1 and WT1 in hPSC-epi. (B,C) Immunofluorescence double labelling in an epicardial explant from an 8 week pc human embryo for THY1 and WT1 (B) or THY1 and BNC1 (C). (D) THY1 immunofluorescence staining on a cryosection of a human heart at 8 weeks pc. The regions of high or low expression of THY1 are indicated by arrows or arrowheads, respectively. Scale bars: 40 mum (A); 20 mum (B,C); 50 mum (D).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
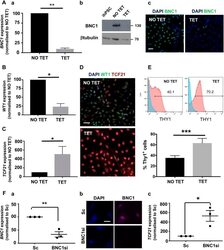
- Fig. 8. BNC1 function in developing epicardial cells. (A) hPSC-epi developed from TET-inducible knockdown hPSC showed more than 90% reduction in BNC1 RNA under the TET condition (Aa) and 98% reduction at the protein level by western blot (Ab) as also visualised by immunofluorescence (Ac) ( n =5). (B,C) These cells showed more than 75% reduction of WT1 RNA (B) and a 5-fold increase in TCF21 RNA (C). (D,E) When BNC1 is silenced during its development, the hPSC-epi is enriched in the TCF21 high population as revealed by double immunofluorescence WT1/TCF21 (D) and THY1 flow cytometry analysis [E; histograms of a representative experiment (top) and recapitulative graph (bottom) of n =5; brackets indicate the percentage of positive cells]. (F) BNC1 silencing can be achieved in human foetal primary epicardium using siRNA as shown by RT-PCR (Fa) and immunofluorescence (Fb). The knockdown of BNC1 in human foetal primary epicardium leads to a greater than 5-fold increase in TCF21 RNA (Fc) ( n =3). The RT-PCR data shown in Aa, B, C, Fa and Fc were obtained by the quantitative relative standard curve protocol as described in Materials and Methods. RNA measurements were normalised to housekeeper genes porphobilinogen deaminase ( PBGD ) or GAPDH . Statistics were performed with Prism 7 from GraphPad with a ratio paired t -test. Error bars represent s.e.m. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
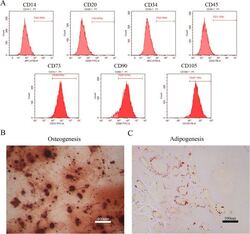
- Figure 1 Identification of dental pulp stem cells (DPSCs). Human DPSCs were positive for the cell surface antigens CD73, CD90, and CD105, as well as negative for CD14, CD20, CD34, and CD45 demonstrated by flow cytometry ( A ). DPSCs were cultured under osteogenic ( B , 14 days) or adipogenic ( C , 21 days) conditions, and showed mineralized nodules and lipid clusters as revealed by alizarin red and oil red staining, respectively. Scale bar = 400 ( B ) or 100 ( C ) mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2 Identification and purification of hPDLSCs by flow cytometry. hPDLSCs, human periodontal ligament stem cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
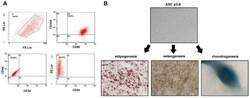
- Figure 1 Identification of ADSCs. ( A ) ADSCs were isolated from the adipose tissue of breast tumors. After two to three passages, the expressions of ADSCs markers (CD90FITC, CD105PE, and CD44FITC) and the lack of CD34PE and CD45FITC were confirmed by flow cytometry. ( B ) The differentiation ability of ADSCs was tested by adipogenesis, osteogenesis, and chondrogenesis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
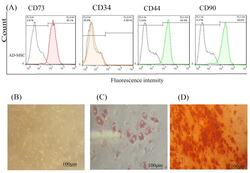
- Figure 1 AD-MSCs characterization; (A) Flow cytometry assay to assess the CD markers in the surface of extracted AD-MSCs. Target cells were positive for CD73 (95.1%), and CD90 (90.6%) surface markers and were negative for CD44 and CD34 markers. (B) Morphology of AD-MSCs at passage 3. (C) Oil red staining to prove the adipogenic potential differentiation of AD-MSCs. (D) Alizarin-red staining to confirm the osteogenic potential differentiation of AD-MSCs. Scale bar = 100 mum. Abbreviations: AD-MSCs: adipose-derived mesenchymal stem cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 1 Characterization of UCSCs. A UCSCs display a spindle shaped and fibroblast-like morphology. B High UCSCs expression of CD90, CD29, CD73, and CD105, and low expression of HLA using flow cytometry
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
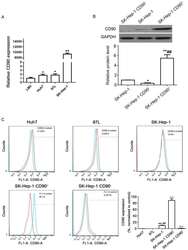
- Figure 1 Distribution of CD90 cells in human HCC cell lines. (A) Expression levels of CD90 in 4 different HCC cell lines (LM3, Huh7, MHCC 97L and SK-Hep-1) were determined by quantitative polymerase chain reaction. P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
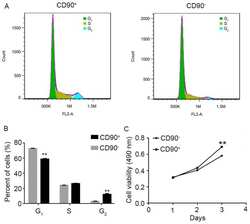
- Figure 2 Effect of SK-Hep-1 CD90 + cells on cell cycle and viability. (A) Representative image and (B) quantitative analysis of flow cytometry assay to analyze the cell cycle of CD90 + and CD90 - cells. Data are presented as the mean +- standard deviation of three independent experiments. (C) MTT assay used to determine the viability of CD90 + and CD90 - cells. The results demonstrated that the viability of CD90 + cells was significantly increased compared with CD90 - cells. ** P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
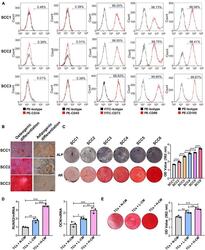
- FIGURE 1 Single-cell colonies with high osteogenic ability had a greater mineralization promotion ability than L-SCCs. (A) The presence of cell surface markers of three SCCs was detected by flow cytometry. (B) The osteogenic and adipogenic differentiation potential of the three SCCs were assessed by Alizarin red and Oil Red O staining. (C) The osteogenic differentiation abilities of SCCs were tested by alkaline phosphatase (ALP) and Alizarin red staining. Alizarin red staining was quantified after the addition of 10% cetylpyridinium chloride. (D) Conditioned medium collected from H-SCCs or L-SCCs was mixed with equal volumes of twofold concentrated osteogenic induction medium, and was used to stimulate the TCs toward osteogenic differentiation for 7 days, following which, the levels of the osteogenic differentiation-related genes in the TCs were tested by RT-qPCR ( n = 3). (E) Conditioned medium collected from H-SCCs or L-SCCs was mixed with equal volumes of twofold concentrated osteogenic induction medium, and was used to stimulate the TCs toward osteogenic differentiation for 28 days, following which, the TCs were subjected to Alizarin red staining ( n = 3). CM, conditioned medium; TCs, target cells; AR, Alizarin red; N-CM, normal culture medium; L-CM, conditioned medium from L-SCCs; H-CM, conditioned medium from H-SCCs. Scale bar, 50 mum. * P < 0.05; ** P < 0.01; *** P < 0.001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
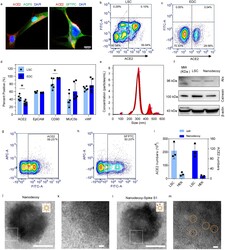
- Figure 1. Characterizations of lung spheroid cell-derived nanodecoys. (a) Representative confocal images of LSCs labeled with ACE2, AQP5, and SFTPC antibodies. Three images were taken. Scale bars, 20 mum. (b) Representative flow cytometry analysis of LSCs (b) and EDCs (c) for ACE2 expression and (d) quantitative results of flow cytometry analysis of EDCs and LSCs for ACE2, EpCAM, CD90, MUC5b, and vWF. Data are shown as mean +- SD, n =4 or 6 independent experiments. Statistical analysis was performed by two-way ANOVA with a Tukey post hoc test. See Supplementary Figure S17 for gating strategies. (e) Size measurement of nanodecoys using NTA Nanosight. (f) Western blot of Alix and Calnexin in LSC-nanodecoys and LSCs. Flow cytometry analysis showing the expressions of ACE2 (g) and type II pneumocytes maker SFPTC (h) on LSC-nanodecoys. See Supplementary Figure S17 for gating strategies. (i) Measurement of ACE2 numbers on both cells and nanodecoys. HEK indicates HEK293. Data are shown as mean +- SD, n =3 independent experiments. Transmission electron microscopy (TEM) images showing naked nanodecoys (j) and enlarged figure (k). TEM images showing spike S1-bound nanodecoys (l) and enlarged figure (m). Spike S1 was detected using gold nanoparticle-labeled secondary antibodies with diameters of 10 nm. Cartoon pictures (insets in Figure j and l) were created with BioRender.com .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
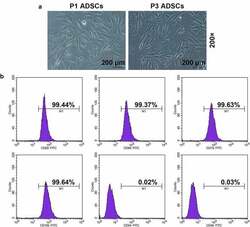
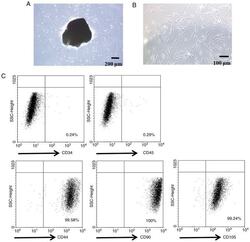
- Figure 1. Morphology and immune phenotype of adipose-derived stem cells (ADSCs) were identified by morphological observation and flow cytometry. (a) Morphology of the primary (P1) and third passage (P3) of ADSCs. Images were acquired at 200x magnification. (b) Immune phenotype of ADSCs. The average data from three independent experiments were shown as mean +- standard deviation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
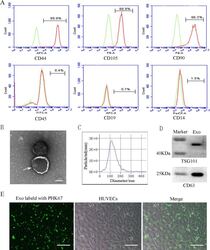
- Characterization and uptake of SHED-Exos. A Surface markers of SHED cells were analyzed by flow cytometry (FACS) and were positive for mesenchymal markers (CD44, CD105 and CD90) and negative for endothelial markers (CD45, CD19 and CD14). B The morphology of exosomes (indicated by arrows) was observed using a transmission electron microscope (TEM). Scale bar = 100 nm. C Particle size distribution of SHED-Exos assessed by nanoparticle tracking analysis (NTA). D Expression of exosome-specific CD63 and TSG101 validated using western blotting. E Efficient uptake of PKH67-labeled exosomes by HUVECs was detected at 24 h. Scale bars = 100 mum
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
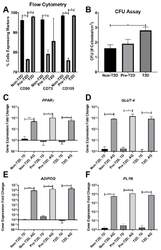
- Decreased expression of CD90, CD73, and CD105 on Pre-T2D IPFP-ASCs. ( A ) Flow cytometry for CD90 and CD105 demonstrated decreased expression in Pre-T2D IPFP-ASCs compared to those from Non-T2D and T2D groups ( n = 3, * p < 0.05). ( B ) CFU assay illustrated increased self-renewal properties in T2D IPFP-ASCs compared to Non-T2D ( n = 3, * p < 0.05). ( C - F ) RT-qPCR for common adipokines in ASCs and adipocyte differentiated ASCs demonstrated no significant difference in adipogenic potential between Non-T2D, Pre-T2D, and T2D IPFP-ASCs ( n = 3, * p < 0.05, *** p < 0.001, **** p < 0.0001). Non-T2D IPFP ASCs 7-Day is the control Group set as 1. Non-T2D: IPFP-ASCs from donors without Type II diabetes mellitus, Pre-T2D: IPFP-ASCs from donors with pre-Type II diabetes mellitus, T2D: IPFP-ASCs from donors with type II diabetes mellitus, 7D: Confluent ASCs, AQ: Adipocyte Differentiated ASCs.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
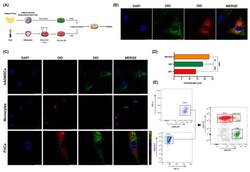
- Human-adipose-derived mesenchymal stem cells fuse with human monocytes ex vivo. ( A ), Schematic representation of experimental co-culture conditions. ( B ), Representative confocal images at 63x magnification of co-cultured DIO stained hADMSCs (green) with DID stained monocytes (red) and DAPI for nuclei (blue) is shown. Merges show co-cultured cells DID + DIO + (tangerine). ( C ), Representative confocal images at 63x magnification of DIO stained hADMSCs (green), DID stained monocytes (red) and hybrid cells (FHCs) (tangerine) are shown. DAPI was used for nuclei labelling (blue). Diameter sizes are shown in merge panels. ( D ) , Diameter comparisons between hADMSCs (green), monocytes (red), and FHCs (tangerine) are shown (n = 3). ( E ), Left panels, representative FACS analysis gating strategy illustrating CD90 + and CD14 + single staining's. Right panel, dot plot after 5-day co-culture illustrates CD90 + hADMSCs (green), CD14 + human monocytes (red), and CD90 + CD14 + (FHCs, tangerine). **, p < 0.01; ***, p < 0.001; ****, p < 0.0001 in one-way ANOVA test with Tukey's multiple comparison post-hoc test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 1 Primary human adipose-derived cells cultured in hypoxia (hAMSCs-H) and normoxia (hAMSCs-N) are both MSCs but normoxia-cultured cells show increased signs of senescence, such as increased area and elongated morphology, compared with hypoxia-cultured cells. ( a ) hAMSCs were isolated from human fat tissue and cultured in hypoxic (1.5% oxygen) or normoxic (21% oxygen) conditions in vitro . The viability, mobility, tumor tropism, safety, and tumorigenic potential were subsequently compared in vitro and in vivo . ( b ) Differentiation assay. hAMSCs were cultured in control media and differentiation media for 3 weeks, 10 days after the second passage. Three different stains were performed to assess differentiation capabilities (scale bar, 100 mu m). ( c ) Flow cytometric analysis was performed to confirm the absence of CD31-, CD34-, and CD45-positive cells in both cell cultures. In addition, primary hAMSC cultures expressed high levels of CD73, CD90, and CD105, both in hypoxic and normoxic culture conditions at day 10 after passage 2. ( d ) Representative images of cell morphologies of hAMSCs on 2D surface (scale bar, 200 mu m). ( e ) Schematic of 3D-nanopatterned surface used to assess morphology and motility. ( f ) Images of cell morphologies of hAMSCs on 3D-nanopatterned surface (scale bar, 200 mu m). ( g - j ) The length, width, area, and length-to-width ratio were measured and compared after cell aligned on the nanopattern surface. Error bars represent S.E.M. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
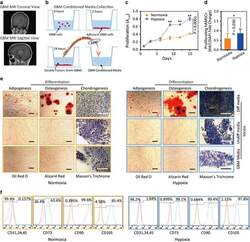
- Figure 3 Hypoxia-cultured primary human adipose-derived mesenchymal stem cells (hAMSCs-H) retain a greater proliferation capacity compared with normoxia-cultured primary hAMSCs (hAMSCs-N) when exposed to GBM media. hAMSCs-H maintain stem cell characteristics when exposed to GBM media. ( a ) Representative MRI of GBM from a patient. ( b ) Schema showing the collection of GBM CM and culture of hAMSCs in filtered GBM CM for proliferation and migration assays. ( c ) MTT assay was used to determine the effects of hypoxic conditions on the proliferative capacity of primary hAMSCs in GBM CM. In GBM CM, hAMSCs-H showed greater proliferation at day 10 and 15 compared with hAMSCs-N. ( d ) Ki-67 immunostaining was performed to quantify the number of proliferating cells in GBM CM. Proliferative capacities of hAMSCs-H and hAMSCs-N are shown in GBM CM (normalized to hAMSC-N proliferative capacity in control media). In GBM CM, hAMSCs-H had a greater proportion of proliferating cells than hAMSCs-N. ( e) Differentiation assay. hAMSCs were cultured in control media, differentiation media, and GBM CM for 3 weeks, 10 days after the second passage. Three stainings were performed to assess the differentiation capabilities (scale bar, 100 mu m). Both hAMSCs-N and hAMSCs-H maintained tri-lineage differentiation capability in GBM CM. ( f ) Flow cytometric analysis for CD31, CD34, CD45, CD73, CD90, and CD105 in hAMSC-N and hAMSC-H cultures after exposure to GBM CM for 20 days. hAMSCs-H maintained MSC
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
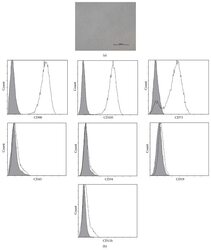
- Figure 1 Characteristics of BM-MSCs. (a) Representative morphology of BM-MSCs. Scale bar = 500 mu m. (b) Representative flow cytometric characterization of cell surface markers expressed on BM-MSCs. Isotypic controls were represented by the gray filled histograms.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2 Characteristics of transfected BM-MSCs. (a) Representative morphology of transfected BM-MSCs in negative control group. (b) More than 90% of BM-MSCs expressed GFP in negative control group. (c) Representative morphology of transfected BM-MSCs in knock-down group. (d) More than 90% of BM-MSCs expressed GFP in knock-down group. (e) Representative flow cytometric characterization of cell surface markers expressed on transfected BM-MSCs. Isotypic controls were represented by black line. The red line represented the negative control group and the blue line represented the knock-down group. (f) The knock-down of NR2F2 was confirmed by western blot analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
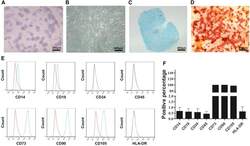
- Figure 2 CESCs Shared Features with BM-MSCs Regarding Morphology, Stem Cell Surface Markers, and Differentiation Ability (A) H&E staining of the tissue section. (B) Morphology of CESCs in agarose after seeding 6 weeks later. (C) Histologic section stained with Alcian blue of chondrified pellets in which CESCs formed in chondrogenic induction medium after 3 weeks. (D) Alizarin red staining of CESCs that underwent osteogenic induction for 3 weeks. (E) Immunophenotypic profile of stem cells in CESCs by flow cytometric analysis. The green lines indicate the fluorescence intensity of cells stained with the corresponding antibodies, and the red lines represent isotype-matched negative control cells. (F) Percentages of CESCs expressing different stem cell markers (n = 6 independent experiments). Data represent the mean +- SD.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
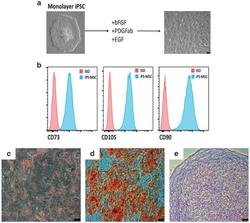
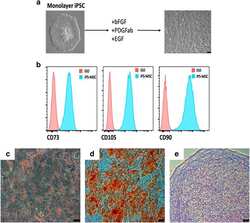
- Figure 3 Mesenchymal stem cell derivation. ( a ) MSC differentiation. Mono-layer iPSCs were subjected to bFGF, PDGFab and EGF resulting in differentiation to a cell population with spindle-shaped morphology (right). ( b ) FACS analysis. Passage 3 MSCs were analysed for cell surface expression of CD73, CD105, and CD90 ( n =3 experiments), and histogram analysis is shown in blue. Isotype antibody control FACS histograms are shown in pink. ( c , d ) Tri-lineage differentiation. ( c ) Oil red-O staining demonstrating the ability of iPSC-derived MSCs to form adipose cells. ( d ) Alizarin red staining of osteogenic progeny. ( e ) Toluidine blue staining of chondrogenic cells from MSCs. ( c - e ) Representative images of at least two different MSC pools and n =3-4 replicates. FACS, fluorescence-activated cell sorting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 3 Mesenchymal stem cell derivation. ( a ) MSC differentiation. Mono-layer iPSCs were subjected to bFGF, PDGFab and EGF resulting in differentiation to a cell population with spindle-shaped morphology (right). ( b ) FACS analysis. Passage 3 MSCs were analysed for cell surface expression of CD73, CD105, and CD90 ( n =3 experiments), and histogram analysis is shown in blue. Isotype antibody control FACS histograms are shown in pink. ( c , d ) Tri-lineage differentiation. ( c ) Oil red-O staining demonstrating the ability of iPSC-derived MSCs to form adipose cells. ( d ) Alizarin red staining of osteogenic progeny. ( e ) Toluidine blue staining of chondrogenic cells from MSCs. ( c - e ) Representative images of at least two different MSC pools and n =3-4 replicates. FACS, fluorescence-activated cell sorting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
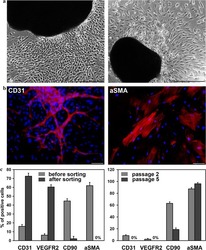
- Fig. 1 Cells isolated from cardiac explants in culture. a Morphology of primary cells migrated from cardiac explants. Phase contrast images of cells cultivated in an endothelial growth medium ( left panel ) and cells derived in a smooth muscle growth medium ( right panel ). Scale bar 100 mum. b CD31-positive endothelial ( left panel ) and alphaSMA-positive smooth muscle ( right panel ) cells were detected by immunofluorescent staining of primary cardiac explant cultures growing in endothelial or smooth muscle medium, respectively. Scale bar 100 mum. c Flow cytometric analysis of surface markers. Comparison of cells cultivated in endothelial growth medium before and after MACS separation ( left panel ). Comparison of cells cultivated in smooth muscle cell growth medium at the second and fifth passages ( right panel )
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
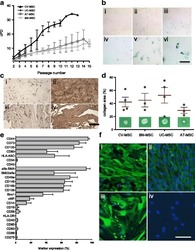
- Fig. 2 a Cumulative population-doubling (cPD) levels versus passage number for the four different sources of MSC. Black represents CV-MSC ( n = 7), dark gray UC-MSC ( n = 4), medium gray AT-MSC ( n = 5), and light gray BM-MSC ( n = 6). b IHC-based senescence-associated beta-galactosidase (SA-beta-gal) staining of CV-MSC in early (i, passage 4) and late (ii, passage 9) passages, AT-MSC in passage 6 (iii), BM-MSC in passage 6 (iv), and UC-MSC in passage 2 (v) and passage 4 (vi). Scale = 200 mum. c IHC of CV-MSC (i, ii) and BM-MSC (iii, iv) stained for osteopontin (i, iii) and fibronectin (ii, iv). Scale = 1 mm. d Collagen area (%) after collagen contraction assay for CV-MSC ( n = 4), BM-MSC ( n = 3), UC-MSC ( n = 4), and AT-MSC ( n = 3). Cells in passage 3 were used. Results expressed as mean +- SD, percentage of the total collagen area of the collagen gels without cells. e Surface marker expression of CV-MSC in early passages ( n = 5). Results expressed as mean +- SD (%). f Representative immunofluorescence of early passaged CV-MSC (i, iii) and BM-MSC (iii, iv) stained for SM22alpha (i, iii) and alpha-SMA (ii, iv). Scale = 50 mum. AT adipose tissue, BM bone marrow, CV chorionic villi, MSC mesenchymal stromal cells, UC umbilical cord
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 1. Characterization of PDLSCs. (A) PDL cell clusters exhibited radiating or whirlpool-like morphology. The central structure in this image is a fragment of PDL tissue. Scale bar, 200 mum (B) CD146 + PDLSCs were small, round, fusiform and triangular. Scale bar, 100 mum. (C) PDLSCs were positive for the stem cell markers CD44, CD90 and CD105, but negative for CD34 and CD45, as detected by flow cytometry. PDL, periodontal ligament; PDLSCs, PDL stem cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
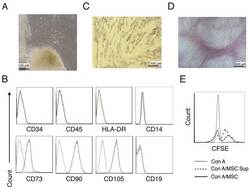
- Figure 1. Characterization of hUC-MSCs. (A) Morphological observations of hUC-MSCs. Umbilical cord tissues were cultured for >15 days and long spindle-shaded fibroblastic cells were observed around the tissue using Zeiss light microscopy (scale bar, 100 um). (B) Phenotyping of hUC-MSCs. hUC-MSCs were stained with a fluorescein-labeled antibodies (CD34, CD45, CD73, CD90, CD105, CD14, CD19 and HLA-DR) and analyzed with a flow cytometer. (C) Adipogenic and (D) osteogenic differentiation of hUC-MSCs. hUC-MSCs were cultured in adipogenic and osteogenic medium, respectively. Lipid droplets in the adipocytes are presented with Oil Red O staining (scale bar, 100 um). hUC-MSCs-derived osteoblasts were detected with Alizarin Red staining (scale bar, 200 um). (E) hUC-MSCs inhibit the proliferation of CFSE-labeled CD4 + T cells, which were activated by Con A stimulation. Experiments were repeated three times and representative graphs and images are presented. hUC-MSC, human umbilical cord-derived mesenchymal stem cell; MSC Sup, culture supernatant of hUC-MSCs; Con A, concanavalin A; CFSE, carboxyfluorescein succinimidyl ester.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2 Flow cytometry of CD cell surface markers for cells cultured under hypoxia and normoxia. The positive CD markers for MSCs as detected by the fluorescent antibodies anti-CD73 FITC, anti-CD105 PE, and anti-CD90 PE Cy7. The negative markers of MSCs were detected using anti-CD14 FITC, anti-CD45 PerCP, anti-CD34-R-PE, and anti-CD19 PE-Cy7 antibodies. Unstained cell for each condition was used as negative controls.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 1 Human amniotic fluid-derived mesenchymal stem cells (AF-MSCs) characterization. ( A ) The typical morphology of human amniotic fluid-derived mesenchymal stem cells, grown in cell culture. Scale bar = 400 um. ( B ) The expression of the main cell surface markers CD44, CD90, CD105, and CD34 as detected by flow cytometry. Unlabeled ctrl: unlabeled, undifferentiated control cells. Results are presented as the mean +- SD ( n = 3). ( C ) The relative expression of pluripotency gene markers, namely, OCT4, SOX2, NANOG, and REX1, as determined by RT-qPCR. Data, relative to GAPDH, are presented as the mean +- SD ( n = 3).
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Flow cytometry
Flow cytometry