Antibody data
- Antibody Data
- Antigen structure
- References [42]
- Comments [0]
- Validations
- Immunocytochemistry [4]
- Immunohistochemistry [3]
- Flow cytometry [1]
- Other assay [24]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 700255 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Phospho-FAK (Tyr397) Recombinant Rabbit Monoclonal Antibody (31H5L17)
- Antibody type
- Monoclonal
- Antigen
- Synthetic peptide
- Description
- This antibody is predicted to react with mouse, rat, primate, bovine, equine, canine, chicken, opossum, zebra finch, zebrafish, Xenopus, and pufferfish based on sequence homology. This antibody was tested in IHC on breast, gastric, and thyroid carcinoma tissue and on HeLa and A549 cells in IF applications. This antibody has been used as a detector in a sandwich ELISA format. Recombinant rabbit monoclonal antibodies are produced using in vitro expression systems. The expression systems are developed by cloning in the specific antibody DNA sequences from immunoreactive rabbits. Then, individual clones are screened to select the best candidates for production. The advantages of using recombinant rabbit monoclonal antibodies include: better specificity and sensitivity, lot-to-lot consistency, animal origin-free formulations, and broader immunoreactivity to diverse targets due to larger rabbit immune repertoire.
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Antibody clone number
- 31H5L17
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Effects of Titanium Implant Surface Topology on Bone Cell Attachment and Proliferation in vitro.
KRAS Mutants Upregulate Integrin β4 to Promote Invasion and Metastasis in Colorectal Cancer.
A Novel CDK2/9 Inhibitor CYC065 Causes Anaphase Catastrophe and Represses Proliferation, Tumorigenesis, and Metastasis in Aneuploid Cancers.
Mechanosensitive expression of lamellipodin promotes intracellular stiffness, cyclin expression and cell proliferation.
Integrins Modulate Extracellular Matrix Organization to Control Cell Signaling during Hematopoiesis.
Oxygen Tension and the VHL-Hif1α Pathway Determine Onset of Neuronal Polarization and Cerebellar Germinal Zone Exit.
Surface Roughness Gradients Reveal Topography-Specific Mechanosensitive Responses in Human Mesenchymal Stem Cells.
Integrin and autocrine IGF2 pathways control fasting insulin secretion in β-cells.
Arginine methylation of SHANK2 by PRMT7 promotes human breast cancer metastasis through activating endosomal FAK signalling.
The histone deacetylase complex MiDAC regulates a neurodevelopmental gene expression program to control neurite outgrowth.
Electromagnetic fields alter the motility of metastatic breast cancer cells.
The effects of focal adhesion kinase and platelet-derived growth factor receptor beta inhibition in a patient-derived xenograft model of primary and metastatic Wilms tumor.
Differential nanoscale organisation of LFA-1 modulates T-cell migration.
Mechanical Durotactic Environment Enhances Specific Glioblastoma Cell Responses.
Matrix Rigidity-Dependent Regulation of Ca(2+) at Plasma Membrane Microdomains by FAK Visualized by Fluorescence Resonance Energy Transfer.
Matrix stiffness induces a tumorigenic phenotype in mammary epithelium through changes in chromatin accessibility.
The Adhesion G-Protein-Coupled Receptor, GPR56/ADGRG1, Inhibits Cell-Extracellular Matrix Signaling to Prevent Metastatic Melanoma Growth.
Downregulation of C-Terminal Tensin-Like Protein (CTEN) Suppresses Prostate Cell Proliferation and Contributes to Acinar Morphogenesis.
Cytoplasmic LIF reprograms invasive mode to enhance NPC dissemination through modulating YAP1-FAK/PXN signaling.
Hic-5 expression is a major indicator of cancer cell morphology, migration, and plasticity in three-dimensional matrices.
Overexpression of eIF4F components in meningiomas and suppression of meningioma cell growth by inhibiting translation initiation.
Nuclear FAK and its kinase activity regulate VEGFR2 transcription in angiogenesis of adult mice.
MARCKS-related protein regulates cytoskeletal organization at cell-cell and cell-substrate contacts in epithelial cells.
Acoustic Tweezing Cytometry Induces Rapid Initiation of Human Embryonic Stem Cell Differentiation.
Lysophosphatidic Acid Induces ECM Production via Activation of the Mechanosensitive YAP/TAZ Transcriptional Pathway in Trabecular Meshwork Cells.
Regulation of Focal Adhesion Kinase through a Direct Interaction with an Endogenous Inhibitor.
Hic-5 remodeling of the stromal matrix promotes breast tumor progression.
In vivo manipulation of the extracellular matrix induces vascular regression in a basal chordate.
Polypharmacology-based ceritinib repurposing using integrated functional proteomics.
Paxillin facilitates timely neurite initiation on soft-substrate environments by interacting with the endocytic machinery.
Epithelial contribution to the profibrotic stiff microenvironment and myofibroblast population in lung fibrosis.
Enhanced efficacy of AKT and FAK kinase combined inhibition in squamous cell lung carcinomas with stable reduction in PTEN.
LIMCH1 regulates nonmuscle myosin-II activity and suppresses cell migration.
αTAT1 downregulation induces mitotic catastrophe in HeLa and A549 cells.
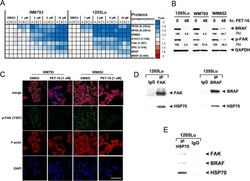
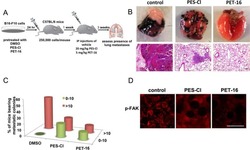
HSP70 Inhibition Limits FAK-Dependent Invasion and Enhances the Response to Melanoma Treatment with BRAF Inhibitors.
FMN2 Makes Perinuclear Actin to Protect Nuclei during Confined Migration and Promote Metastasis.
Mechanical Loading Stimulates Expression of Collagen Cross-Linking Associated Enzymes in Periodontal Ligament.
The FAK-Arp2/3 interaction promotes leading edge advance and haptosensing by coupling nascent adhesions to lamellipodia actin.
Focal adhesion kinase overexpression and its impact on human osteosarcoma.
Spatiotemporal dynamics of traction forces show three contraction centers in migratory neurons.
Matrix stiffening and β1 integrin drive subtype-specific fibroblast accumulation in lung cancer.
Loading-related regulation of transcription factor EGR2/Krox-20 in bone cells is ERK1/2 protein-mediated and prostaglandin, Wnt signaling pathway-, and insulin-like growth factor-I axis-dependent.
Levin M, Spiro RC, Jain H, Falk MM
Medical devices (Auckland, N.Z.) 2022;15:103-119
Medical devices (Auckland, N.Z.) 2022;15:103-119
KRAS Mutants Upregulate Integrin β4 to Promote Invasion and Metastasis in Colorectal Cancer.
Choi SH, Kim JK, Chen CT, Wu C, Marco MR, Barriga FM, O'Rourke K, Pelossof R, Qu X, Chang Q, de Stanchina E, Shia J, Smith JJ, Sanchez-Vega F, Garcia-Aguilar J
Molecular cancer research : MCR 2022 Aug 5;20(8):1305-1319
Molecular cancer research : MCR 2022 Aug 5;20(8):1305-1319
A Novel CDK2/9 Inhibitor CYC065 Causes Anaphase Catastrophe and Represses Proliferation, Tumorigenesis, and Metastasis in Aneuploid Cancers.
Kawakami M, Mustachio LM, Chen Y, Chen Z, Liu X, Wei CH, Roszik J, Kittai AS, Danilov AV, Zhang X, Fang B, Wang J, Heymach JV, Tyutyunyk-Massey L, Freemantle SJ, Kurie JM, Liu X, Dmitrovsky E
Molecular cancer therapeutics 2021 Mar;20(3):477-489
Molecular cancer therapeutics 2021 Mar;20(3):477-489
Mechanosensitive expression of lamellipodin promotes intracellular stiffness, cyclin expression and cell proliferation.
Brazzo JA, Biber JC, Nimmer E, Heo Y, Ying L, Zhao R, Lee K, Krause M, Bae Y
Journal of cell science 2021 Jun 15;134(12)
Journal of cell science 2021 Jun 15;134(12)
Integrins Modulate Extracellular Matrix Organization to Control Cell Signaling during Hematopoiesis.
Khadilkar RJ, Ho KYL, Venkatesh B, Tanentzapf G
Current biology : CB 2020 Sep 7;30(17):3316-3329.e5
Current biology : CB 2020 Sep 7;30(17):3316-3329.e5
Oxygen Tension and the VHL-Hif1α Pathway Determine Onset of Neuronal Polarization and Cerebellar Germinal Zone Exit.
Kullmann JA, Trivedi N, Howell D, Laumonnerie C, Nguyen V, Banerjee SS, Stabley DR, Shirinifard A, Rowitch DH, Solecki DJ
Neuron 2020 May 20;106(4):607-623.e5
Neuron 2020 May 20;106(4):607-623.e5
Surface Roughness Gradients Reveal Topography-Specific Mechanosensitive Responses in Human Mesenchymal Stem Cells.
Hou Y, Xie W, Yu L, Camacho LC, Nie C, Zhang M, Haag R, Wei Q
Small (Weinheim an der Bergstrasse, Germany) 2020 Mar;16(10):e1905422
Small (Weinheim an der Bergstrasse, Germany) 2020 Mar;16(10):e1905422
Integrin and autocrine IGF2 pathways control fasting insulin secretion in β-cells.
Arous C, Mizgier ML, Rickenbach K, Pinget M, Bouzakri K, Wehrle-Haller B
The Journal of biological chemistry 2020 Dec 4;295(49):16510-16528
The Journal of biological chemistry 2020 Dec 4;295(49):16510-16528
Arginine methylation of SHANK2 by PRMT7 promotes human breast cancer metastasis through activating endosomal FAK signalling.
Liu Y, Li L, Liu X, Wang Y, Liu L, Peng L, Liu J, Zhang L, Wang G, Li H, Liu DX, Huang B, Lu J, Zhang Y
eLife 2020 Aug 26;9
eLife 2020 Aug 26;9
The histone deacetylase complex MiDAC regulates a neurodevelopmental gene expression program to control neurite outgrowth.
Mondal B, Jin H, Kallappagoudar S, Sedkov Y, Martinez T, Sentmanat MF, Poet GJ, Li C, Fan Y, Pruett-Miller SM, Herz HM
eLife 2020 Apr 16;9
eLife 2020 Apr 16;9
Electromagnetic fields alter the motility of metastatic breast cancer cells.
Garg AA, Jones TH, Moss SM, Mishra S, Kaul K, Ahirwar DK, Ferree J, Kumar P, Subramaniam D, Ganju RK, Subramaniam VV, Song JW
Communications biology 2019;2:303
Communications biology 2019;2:303
The effects of focal adhesion kinase and platelet-derived growth factor receptor beta inhibition in a patient-derived xenograft model of primary and metastatic Wilms tumor.
Aye JM, Stafman LL, Williams AP, Garner EF, Stewart JE, Anderson JC, Mruthyunjayappa S, Waldrop MG, Goolsby CD, Markert HR, Quinn C, Marayati R, Mroczek-Musulman E, Willey CD, Yoon KJ, Whelan KF, Beierle EA
Oncotarget 2019 Sep 17;10(53):5534-5548
Oncotarget 2019 Sep 17;10(53):5534-5548
Differential nanoscale organisation of LFA-1 modulates T-cell migration.
Shannon MJ, Pineau J, Griffié J, Aaron J, Peel T, Williamson DJ, Zamoyska R, Cope AP, Cornish GH, Owen DM
Journal of cell science 2019 Oct 16;133(5)
Journal of cell science 2019 Oct 16;133(5)
Mechanical Durotactic Environment Enhances Specific Glioblastoma Cell Responses.
Palamà IE, D'Amone S, Ratano P, Donatelli A, Liscio A, Antonacci G, Testini M, Di Angelantonio S, Ragozzino D, Cortese B
Cancers 2019 May 9;11(5)
Cancers 2019 May 9;11(5)
Matrix Rigidity-Dependent Regulation of Ca(2+) at Plasma Membrane Microdomains by FAK Visualized by Fluorescence Resonance Energy Transfer.
Kim TJ, Lei L, Seong J, Suh JS, Jang YK, Jung SH, Sun J, Kim DH, Wang Y
Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2019 Feb 20;6(4):1801290
Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2019 Feb 20;6(4):1801290
Matrix stiffness induces a tumorigenic phenotype in mammary epithelium through changes in chromatin accessibility.
Stowers RS, Shcherbina A, Israeli J, Gruber JJ, Chang J, Nam S, Rabiee A, Teruel MN, Snyder MP, Kundaje A, Chaudhuri O
Nature biomedical engineering 2019 Dec;3(12):1009-1019
Nature biomedical engineering 2019 Dec;3(12):1009-1019
The Adhesion G-Protein-Coupled Receptor, GPR56/ADGRG1, Inhibits Cell-Extracellular Matrix Signaling to Prevent Metastatic Melanoma Growth.
Millar MW, Corson N, Xu L
Frontiers in oncology 2018;8:8
Frontiers in oncology 2018;8:8
Downregulation of C-Terminal Tensin-Like Protein (CTEN) Suppresses Prostate Cell Proliferation and Contributes to Acinar Morphogenesis.
Wu WM, Liao YC
International journal of molecular sciences 2018 Oct 16;19(10)
International journal of molecular sciences 2018 Oct 16;19(10)
Cytoplasmic LIF reprograms invasive mode to enhance NPC dissemination through modulating YAP1-FAK/PXN signaling.
Liu SC, Hsu T, Chang YS, Chung AK, Jiang SS, OuYang CN, Yuh CH, Hsueh C, Liu YP, Tsang NM
Nature communications 2018 Nov 30;9(1):5105
Nature communications 2018 Nov 30;9(1):5105
Hic-5 expression is a major indicator of cancer cell morphology, migration, and plasticity in three-dimensional matrices.
Gulvady AC, Dubois F, Deakin NO, Goreczny GJ, Turner CE
Molecular biology of the cell 2018 Jul 15;29(13):1704-1717
Molecular biology of the cell 2018 Jul 15;29(13):1704-1717
Overexpression of eIF4F components in meningiomas and suppression of meningioma cell growth by inhibiting translation initiation.
Oblinger JL, Burns SS, Huang J, Pan L, Ren Y, Shen R, Kinghorn AD, Welling DB, Chang LS
Experimental neurology 2018 Jan;299(Pt B):299-307
Experimental neurology 2018 Jan;299(Pt B):299-307
Nuclear FAK and its kinase activity regulate VEGFR2 transcription in angiogenesis of adult mice.
Sun S, Wu HJ, Guan JL
Scientific reports 2018 Feb 7;8(1):2550
Scientific reports 2018 Feb 7;8(1):2550
MARCKS-related protein regulates cytoskeletal organization at cell-cell and cell-substrate contacts in epithelial cells.
Van Itallie CM, Tietgens AJ, Aponte A, Gucek M, Cartagena-Rivera AX, Chadwick RS, Anderson JM
Journal of cell science 2018 Feb 2;131(3)
Journal of cell science 2018 Feb 2;131(3)
Acoustic Tweezing Cytometry Induces Rapid Initiation of Human Embryonic Stem Cell Differentiation.
Topal T, Hong X, Xue X, Fan Z, Kanetkar N, Nguyen JT, Fu J, Deng CX, Krebsbach PH
Scientific reports 2018 Aug 28;8(1):12977
Scientific reports 2018 Aug 28;8(1):12977
Lysophosphatidic Acid Induces ECM Production via Activation of the Mechanosensitive YAP/TAZ Transcriptional Pathway in Trabecular Meshwork Cells.
Ho LTY, Skiba N, Ullmer C, Rao PV
Investigative ophthalmology & visual science 2018 Apr 1;59(5):1969-1984
Investigative ophthalmology & visual science 2018 Apr 1;59(5):1969-1984
Regulation of Focal Adhesion Kinase through a Direct Interaction with an Endogenous Inhibitor.
Zak TJ, Koshman YE, Samarel AM, Robia SL
Biochemistry 2017 Sep 5;56(35):4722-4731
Biochemistry 2017 Sep 5;56(35):4722-4731
Hic-5 remodeling of the stromal matrix promotes breast tumor progression.
Goreczny GJ, Ouderkirk-Pecone JL, Olson EC, Krendel M, Turner CE
Oncogene 2017 May 11;36(19):2693-2703
Oncogene 2017 May 11;36(19):2693-2703
In vivo manipulation of the extracellular matrix induces vascular regression in a basal chordate.
Rodriguez D, Braden BP, Boyer SW, Taketa DA, Setar L, Calhoun C, Maio AD, Langenbacher A, Valentine MT, De Tomaso AW
Molecular biology of the cell 2017 Jul 7;28(14):1883-1893
Molecular biology of the cell 2017 Jul 7;28(14):1883-1893
Polypharmacology-based ceritinib repurposing using integrated functional proteomics.
Kuenzi BM, Remsing Rix LL, Stewart PA, Fang B, Kinose F, Bryant AT, Boyle TA, Koomen JM, Haura EB, Rix U
Nature chemical biology 2017 Dec;13(12):1222-1231
Nature chemical biology 2017 Dec;13(12):1222-1231
Paxillin facilitates timely neurite initiation on soft-substrate environments by interacting with the endocytic machinery.
Chang TY, Chen C, Lee M, Chang YC, Lu CH, Lu ST, Wang DY, Wang A, Guo CL, Cheng PL
eLife 2017 Dec 22;6
eLife 2017 Dec 22;6
Epithelial contribution to the profibrotic stiff microenvironment and myofibroblast population in lung fibrosis.
Gabasa M, Duch P, Jorba I, Giménez A, Lugo R, Pavelescu I, Rodríguez-Pascual F, Molina-Molina M, Xaubet A, Pereda J, Alcaraz J
Molecular biology of the cell 2017 Dec 15;28(26):3741-3755
Molecular biology of the cell 2017 Dec 15;28(26):3741-3755
Enhanced efficacy of AKT and FAK kinase combined inhibition in squamous cell lung carcinomas with stable reduction in PTEN.
Cavazzoni A, La Monica S, Alfieri R, Ravelli A, Van Der Steen N, Sciarrillo R, Madeddu D, Lagrasta CAM, Quaini F, Bonelli M, Fumarola C, Cretella D, Digiacomo G, Tiseo M, Peters GJ, Ardizzoni A, Petronini PG, Giovannetti E
Oncotarget 2017 Aug 8;8(32):53068-53083
Oncotarget 2017 Aug 8;8(32):53068-53083
LIMCH1 regulates nonmuscle myosin-II activity and suppresses cell migration.
Lin YH, Zhen YY, Chien KY, Lee IC, Lin WC, Chen MY, Pai LM
Molecular biology of the cell 2017 Apr 15;28(8):1054-1065
Molecular biology of the cell 2017 Apr 15;28(8):1054-1065
αTAT1 downregulation induces mitotic catastrophe in HeLa and A549 cells.
Chien JY, Tsen SD, Chien CC, Liu HW, Tung CY, Lin CH
Cell death discovery 2016;2:16006
Cell death discovery 2016;2:16006
HSP70 Inhibition Limits FAK-Dependent Invasion and Enhances the Response to Melanoma Treatment with BRAF Inhibitors.
Budina-Kolomets A, Webster MR, Leu JI, Jennis M, Krepler C, Guerrini A, Kossenkov AV, Xu W, Karakousis G, Schuchter L, Amaravadi RK, Wu H, Yin X, Liu Q, Lu Y, Mills GB, Xu X, George DL, Weeraratna AT, Murphy ME
Cancer research 2016 May 1;76(9):2720-30
Cancer research 2016 May 1;76(9):2720-30
FMN2 Makes Perinuclear Actin to Protect Nuclei during Confined Migration and Promote Metastasis.
Skau CT, Fischer RS, Gurel P, Thiam HR, Tubbs A, Baird MA, Davidson MW, Piel M, Alushin GM, Nussenzweig A, Steeg PS, Waterman CM
Cell 2016 Dec 1;167(6):1571-1585.e18
Cell 2016 Dec 1;167(6):1571-1585.e18
Mechanical Loading Stimulates Expression of Collagen Cross-Linking Associated Enzymes in Periodontal Ligament.
Kaku M, Rosales Rocabado JM, Kitami M, Ida T, Akiba Y, Yamauchi M, Uoshima K
Journal of cellular physiology 2016 Apr;231(4):926-33
Journal of cellular physiology 2016 Apr;231(4):926-33
The FAK-Arp2/3 interaction promotes leading edge advance and haptosensing by coupling nascent adhesions to lamellipodia actin.
Swaminathan V, Fischer RS, Waterman CM
Molecular biology of the cell 2016 Apr 1;27(7):1085-100
Molecular biology of the cell 2016 Apr 1;27(7):1085-100
Focal adhesion kinase overexpression and its impact on human osteosarcoma.
Ren K, Lu X, Yao N, Chen Y, Yang A, Chen H, Zhang J, Wu S, Shi X, Wang C, Sun X
Oncotarget 2015 Oct 13;6(31):31085-103
Oncotarget 2015 Oct 13;6(31):31085-103
Spatiotemporal dynamics of traction forces show three contraction centers in migratory neurons.
Jiang J, Zhang ZH, Yuan XB, Poo MM
The Journal of cell biology 2015 Jun 8;209(5):759-74
The Journal of cell biology 2015 Jun 8;209(5):759-74
Matrix stiffening and β1 integrin drive subtype-specific fibroblast accumulation in lung cancer.
Puig M, Lugo R, Gabasa M, Giménez A, Velásquez A, Galgoczy R, Ramírez J, Gómez-Caro A, Busnadiego Ó, Rodríguez-Pascual F, Gascón P, Reguart N, Alcaraz J
Molecular cancer research : MCR 2015 Jan;13(1):161-73
Molecular cancer research : MCR 2015 Jan;13(1):161-73
Loading-related regulation of transcription factor EGR2/Krox-20 in bone cells is ERK1/2 protein-mediated and prostaglandin, Wnt signaling pathway-, and insulin-like growth factor-I axis-dependent.
Zaman G, Sunters A, Galea GL, Javaheri B, Saxon LK, Moustafa A, Armstrong VJ, Price JS, Lanyon LE
The Journal of biological chemistry 2012 Feb 3;287(6):3946-62
The Journal of biological chemistry 2012 Feb 3;287(6):3946-62
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
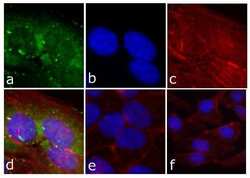
- Immunofluorescent analysis of FAK (pY397) was done on 80% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 15 minutes; permeabilized with 0.25% Triton X-100 for 10 minutes followed by blocking with 5% BSA for 1 hour at room temperature. The cells were incubated with FAK (pY397) Recombinant Rabbit Monoclonal Antibody (Product # 700255) at 1 µg\mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor® 488 Goat anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing cytoplasmic of FAK (pY397). Panel e shows no primary antibody. The images were captured at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
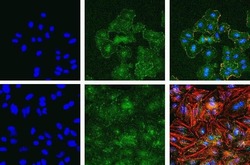
- Immunofluorescent analysis of Phospho-FAK pTyr397 in A549 (top) and HeLa cells (bottom) using a Phospho-FAK pTyr397 recombinant rabbit monoclonal antibody (Product # 700255) at a dilution of 1:2000 (A549) and 1:1000 (HeLa) followed by detection using an Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody at a dilution of 1:1000. Hoechst only (left), AF488 signal only (middle) and composite image (right) using an Alexa Fluor 568 Phalloidin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
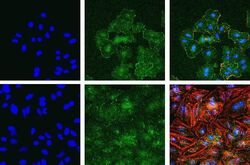
- Immunofluorescent analysis of Phospho-FAK pTyr397 in A549 (top) and HeLa cells (bottom) using a Phospho-FAK pTyr397 recombinant rabbit monoclonal antibody (Product # 700255) at a dilution of 1:2000 (A549) and 1:1000 (HeLa) followed by detection using an Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody at a dilution of 1:1000. Hoechst only (left), AF488 signal only (middle) and composite image (right) using an Alexa Fluor 568 Phalloidin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
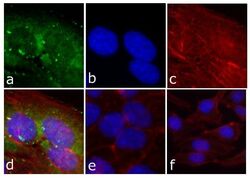
- Immunofluorescent analysis of FAK (pY397) was done on 80% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 15 minutes; permeabilized with 0.25% Triton X-100 for 10 minutes followed by blocking with 5% BSA for 1 hour at room temperature. The cells were incubated with FAK (pY397) Recombinant Rabbit Monoclonal Antibody (Product # 700255) at 1 µg\mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor® 488 Goat anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing cytoplasmic of FAK (pY397). Panel e shows no primary antibody. The images were captured at 20X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
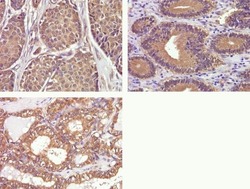
- Experimental details
- Immunohistochemistry analysis of Phospho-FAK pTyr397 in formalin-fixed, paraffin-embedded human breast (top left), gastric (top right), and thyroid carcimona (bottom) using a Phospho-FAK pTyr397 monoclonal antibody (Product # 700255) at a dilution of 1:50. Staining was visualized using DAB and images were taken at a magnification of 40x. Results show cytoplasmic staining in tumor cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
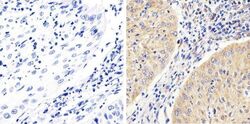
- Experimental details
- Immunohistochemistry analysis of FAK showing staining in the cytoplasm of paraffin-embedded human cervices carcinoma (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a FAK monoclonal antibody (Product # 700255) diluted in 3% BSA-PBS at a dilution of 1:100 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
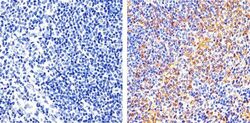
- Experimental details
- Immunohistochemistry analysis of FAK showing staining in the cytoplasm and membrane of paraffin-embedded mouse spleen tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a FAK monoclonal antibody (Product # 700255) diluted in 3% BSA-PBS at a dilution of 1:20 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
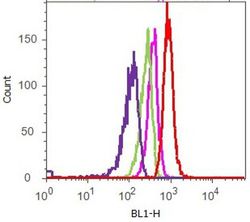
- Experimental details
- Flow cytometry analysis of FAK [pY397] was done on A549 cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Tritonª X-100 for 20 minutes, and blocked with 5% BSA for 1 hour at room temperature. Cells were labeled with ABfinityª FAK [pY397] Recombinant Rabbit Monoclonal Antibody (700255, red histogram) or with rabbit isotype control (pink histogram) at 1-3 µg/million cells in 2.5% BSA. After incubation at room temperature for 2-3 hours, the cells were labeled with Alexa Fluor¨ 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune¨ Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 4 Nuclear translocation of FAK in ECs. ( A , B ) MS1 cells were stimulated with 30 ng/ml VEGF for indicated times. They were lysed and fractionated to cytoplasmic and nuclear portions, and analyzed by immunoblotting using indicated antibodies. GAPDH was used as a cytoplasmic marker and RARP as a nuclear marker ( A ). The relative levels of FAK are quantified ( B ). n = 3, mean +- SEM. *p < 0.05. N.S., not significant. ( C , D ) MS1 cells were held in suspension for 30 min, and re-plated for 30 or 60 min on dishes coated with 10 mug/ml fibronectin. They were then analyzed as in A ( C ). The relative levels of FAK are quantified ( D ). n = 3, mean +- SEM. *p < 0.05. ( E , F ) MS1 cells were treated with FAK kinase inhibitor PF-271 or vehicle for 12 hours followed by VEGF stimulation for indicated times. They were then analyzed as in C. The relative levels of nuclear FAK are quantified ( F ). n = 3, mean +- SEM. *p < 0.05. Full-length western blots are presented in Supplementary Figures 7 - 9 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
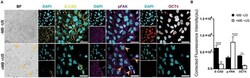
- Figure 3 ATC application activated FAK signaling and induced differentiation of hESCs. ( A ) Adherent colony of hESCs stained with DAPI (blue), E-cadherin (yellow) and pFAK (purple) with and without ATC stimulation (30 min). ( B ) Corrected fluorescence intensity of E-cadherin, pFAK activation and Oct4 after 30 min ATC stimulation compared with control group. Scale bars 50 um. All quantifications were from at least 3 independent experiments with two replicates per experiment. Unpaired t test p values *
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
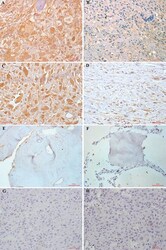
- Figure 1 Immunohistochemical staining of FAK (A, B, E, G) and pFAK (C, D, F, H) proteins in osteosarcoma and normal cancellous bone tissues A. FAK was overexpressed in 61.95% (70/113) of osteosarcoma cells. Tumor cells exhibited cytoplasmic and sometimes membranous immunoreactivity for FAK. B. Low FAK immunohistochemical staining was shown in 43 osteosarcoma patients. C. pFAK was mainly expressed in the cytoplasm of osteosarcoma cells in 37.17% (42/113) of cases. D. Low phospho-FAK immunohistochemical staining pFAK was shown in 71 osteosarcoma patients. E. No FAK immunohistochemical staining was observed in normal cancellous bone tissues. F. No pFAK immunohistochemical staining pFAK was observed in normal cancellous bone tissues. G. FAK immunohistochemical staining was not present in negative controls. H. pFAK immunohistochemical staining was not present in negative controls. Results shown here are representative images, x400 magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
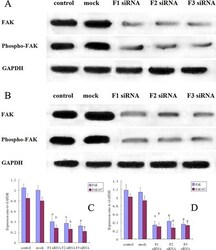
- Figure 3 FAK protein expression and FAK phosphorylation in siRNA treated osteosarcoma, control and mock group cells A. Western blot analysis of FAK and pFAK in MG-63 cells from control, mock, F1 siRNA, F2 siRNA and F3 siRNA groups. GAPDH was used as a control for protein load and integrity. B. Western blot analysis of FAK and pFAK in 143B cells from control, mock, F1 siRNA, F2 siRNA and F3 siRNA groups. GAPDH was used as a control for protein load and integrity. C. The bar chart demonstrates the ratio of FAK and pFAK protein to GAPDH by densitometry in MG-63 cells. The data are means +- SEM. D. The bar chart demonstrates the ratio of FAK and pFAK protein to GAPDH by densitometry in 143B cells. The data are means +- SEM.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
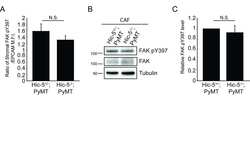
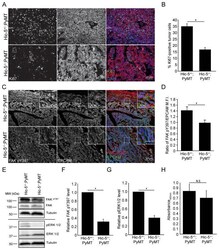
- Figure 3 Hic-5 expression in CAFs indirectly regulates tumor cell growth and signaling. A) Representative sections from end point tumors stained with Ki67. B) Quantification of the percentage of Ki67 and EPCAM positive cells. n=4 mice for each genotype. The slides were blinded prior to analysis. C) Representative sections of end point tumors stained for FAK pY397 and EPCAM. The inset shows reduced FAK pY397 fluorescence intensity in the Hic-5 -/- ;PyMT tumor cells, scale=25mum. D) Quantification of the ratio of mean fluorescence intensity (M.F.I.) of FAK pY397 and EPCAM, n=3 mice for each genotype. E) Representative western blots of whole tumor lysates and subsequent quantification of (F) FAK Y397 and (G) ERK 1/2 phosphorylation. n=3 mice of each genotype (H) Cell proliferation in vitro was assessed using an XTT assay. n=3 independent experiments. The data represent the mean +- S.E.M. * p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
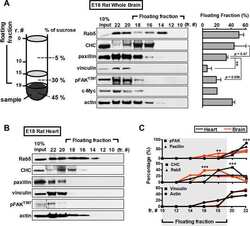
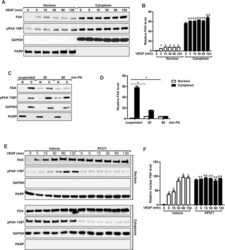
- FIGURE 8: Disruption of collagen matrix by BAPN results in loss of integrin signaling in vascular cells. (A) Flow cytometry gating strategy for BSA-488-labeled B. schlosseri vascular cells after formaldehyde fixation and methanol permeabilization. (B) Representative flow cytometry histogram for quantifying phosphorylated Y397 FAK (pY397FAK) in B. schlosseri vascular cells. FAK inhibitor (FAK14) was used a positive control for blocking FAK phosphorylation. (C) Percentage of vascular cells with high FAK phosphorylation for B. schlosseri colonies after indicated treatment for 4 h ( n = 3 per condition). * p < 0.05 as determined by an unpaired Student's t test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
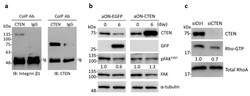
- Figure 5 CTEN acts as a binding partner of integrin beta1 and increases the activity of FAK and RhoA. ( a ) Whole cell lysate of RWPE-1 were coimmunoprecipitated (CoIP) with anti-CTEN antibody or normal rabbit IgG. The precipitates were examined by immunoblotting (IB) with anti-integrin beta1 and anti-CTEN antibodies. ( b ) Inducible EGFP-expressing (aON-EGFP) or CTEN-expressing (aON-CTEN) RWPE-1 cells were incubated in 3D culture system for 6 days in the presence of doxycycline (0.2 mug/mL). The cells were collected at the indicated time and the protein levels were examined by Western analyses using the indicated antibodies. alpha-tubulin was used as a loading control. The densities of the protein bands from the Western blot were quantified. The levels of the phosphorylated FAK Y397 (pFAK Y397 ) was normalized to those of the total FAK. The ratio of pFAKY397/FAK in each group was presented. ( c ) RWPE-1 cells were transfected with the indicated siRNA. The GTP-RhoA in RWPE-1 lysates was purified using GTP-RhoA pull-down assays as described in Materials and Methods and examined by Western blot analyses with an anti-RhoA antibody. The protein levels in whole cell lysates were also examined by Western analyses using the indicated antibodies. Total RhoA was used as loading control. The densities of the protein bands from the Western blot were quantified. The levels of Rho-GTP was normalized to those of total RhoA. The ratio of Rho-GTP/total RhoA was presented.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
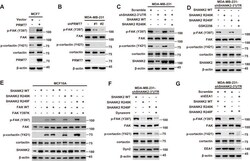
- Figure 6. SHANK2 R240 methylation activated endosomal FAK signals. ( A ) Phosphorylated/total FAK and cortactin were determined in MCF-7 cells expressing the indicated Flag-tagged PRMT7. Western blot was performed with the indicated antibodies. ( B ) Phosphorylated/total FAK and cortactin were determined in MDA-MB-231 cells with or without stable expression of the indicated SHANK2 shRNA or a control shRNA. Western blot was performed with the indicated antibodies. ( C ) Phosphorylated/total FAK and cortactin were determined in MDA-MB-231 cells with or without expressing SHANK2 shRNA and with or without reconstituted expression of WT SHANK2 or SHANK2 R240K mutant. Western blot was performed with the indicated antibodies. ( D ) Phosphorylated/total FAK and cortactin were determined in MDA-MB-231 cells expressing SHANK2 shRNA with or without reconstituted expression of WT SHANK2 or SHANK2 R240K or SHANK2 R240F mutant, SHANK2 R240F were treated with GSK2256098 (10 µM) or DMSO as control. Western blot was performed with the indicated antibodies. ( E ) Phosphorylated/total FAK and cortactin were determined in MCF10A cells co-transfected with SHANK2 and/or FAK plasmids. Western blot was performed with the indicated antibodies. ( F ) Phosphorylated/total FAK and cortactin were determined in MDA-MB-231 cells expressing SHANK2 shRNA with or without reconstituted expression of WT SHANK2, SHANK2 R240K or SHANK2 R240F mutant. SHANK2 R240F group was treated with dynasore (80 uM) or DM
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
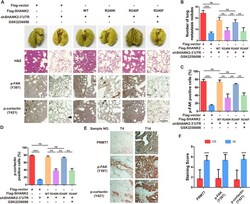
- Figure 8. Arginine methylation at SHANK2 R240 promoted metastasis through activates FAK signalling. ( A ) Equal numbers of MDA-MB-231 cells expressing SHANK2 shRNA with or without reconstituted expression of WT SHANK2, SHANK2 R240K or SHANK2 R240F mutant. SHANK2 R240F group was treated with GSK2256098 or DMSO. Cells were tail-vein injected into BALB/c nude mice, and lung metastasis was determined. The gross appearance of the lungs and the tumour nodules on the lungs were examined. Tumours were paraffin embedded and stained with H and E at Day12. n = 5 tumours in each group. IHC analyses of tumour tissues were performed with anti-p-FAK or anti-p-cortactin antibody. Scale bars,100 um. ( B ) The number of visible surface metastatic lesions in lungs was counted. (n = 5 mice for each group, **p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
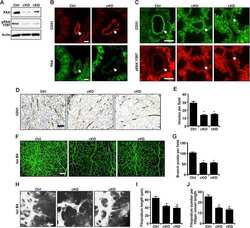
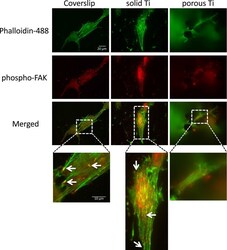
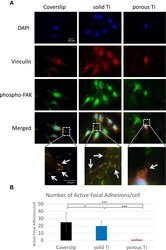
- Adhesion of MG-63 cells to different substrates, active focal adhesions and actin cytoskeleton. MG-63 cells were seeded on solid and porous titanium disks, and on poly-L-lysine-coated microscopic glass coverslips, and incubated for 4 days, then processed for indirect immunofluorescence analysis. Actively signaling focal adhesions were visualized using a phospho-specific (pTyr 397 ) anti-focal adhesion kinase (FAK) antibody (red), and the actin cytoskeleton was decorated with Alexa488-conjugated phalloidin (green). Merged images, and images zoomed in on selected areas (boxed) are shown below. Numerous active focal adhesions (red puncta, depicted by arrows) and actin stress fibers (green filaments) were clearly detectable on solid titanium and coverslips, but not on porous titanium consistent with data presented in Figure 3 . Scale bars representative for all images and zoomed in areas are given in mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Adhesion of MG-63 cells to different substrates, vinculin and phospho-FAK co-localization. MG-63 cells were seeded on solid and porous titanium disks, and on poly-L-lysine-coated microscopic glass coverslips, and incubated for 4 days, then processed for indirect immunofluorescence analysis. ( A ) Focal adhesions were visualized using anti-vinculin specific antibodies (red), active focal adhesions were visualized using phospho-specific (pTyr 397 ) anti-FAK antibodies (red), and cell nuclei were stained with DAPI (blue). Merged images, and images zoomed in on selected areas (boxed) are shown below. Numerous typical focal adhesions (yellow/orange puncta, depicted by arrows) double-labeled with both antibodies were clearly detectable on solid titanium and coverslips. Some may also be present in cells on porous titanium. ( B ) Total number of active focal adhesions per cell were determined by counting phospho-FAK positive adhesions (yellow/orange comma) in 18 cells on each substrate. Data are mean +- s.e.m. ; *p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
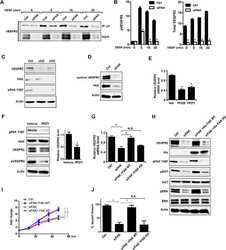
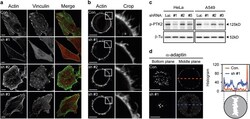
- Figure 2 alpha TAT1 downregulation decreased F-actin and altered alpha -adaptin distribution. ( a ) Confocal images of A549 cells visualized with Alexa Fluor 488 phalloidin (green) and immunostained with anti-vinculin antibody (red). ( b ) Confocal images of metaphase HeLa cells visualized with Alexa Fluor 488 phalloidin. Single optical section of the middle plane is shown. ( c ) pTyr397-PTK2 level determined by western blotting. The same protein extract was used in Figures 1a and 2c , therefore the same blotting of beta -Tu is presented in both figures. ( d ) Confocal images of metaphase HeLa cells immunostained with anti- alpha -adaptin antibody. Single optical section of the bottom or middle planes are shown. The signal intensity across the dashed lines is plotted in the right panel and the cartoon below indicates the relative position of the metaphase cell. Scale bar, 10 mu m.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
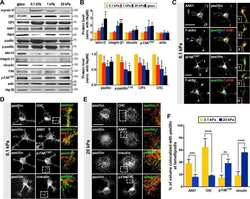
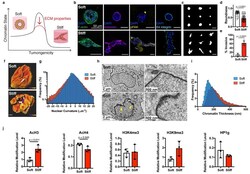
- Fig. 1 | Nuclear and chromatin alterations accompany phenotypic changes induced by ECM stiffness. a , The hypothesis underlying this study is that ECM properties can stabilize normal phenotypes or make phenotypic transitions more permissive through chromatin alterations. b , Immunofluorescence staining for F-actin, vimentin, phosphorylated FAK (Y397), and beta4 integrin in MCF10A acini after 14 days in soft (top) or stiff (bottom) matrices (representative images selected from 15, 3, 5, and 5 images per group respectively). c , Representative outlines of cell clusters from soft (top) and stiff (bottom) matrices. d , Quantification of roundness of cell clusters from at least three independent replicates (median +- 95% C.I.). Significance determined by Mann-Whitney test. e , Quantification of invasive clusters (n >= 5, mean +- S.D.) Significance determined by unpaired t-test. f , Color map of nuclear curvature for cells in soft (top) and stiff (bottom) matrices. Scale bar represents 2 mum and color bar ranges from -20 to 20 mum -1 . g , Distribution of curvature values, showing more regions of extreme curvature for cells in stiff matrices. Distributions were significantly different by Kolmogorov-Smirnov test (p < 0.0001, n >= 11). h , TEM micrographs of nuclei from cells in soft or stiff matrices at 2,000X (left) and 10,000X (right) magnification (representative images from at least 16 images per group). i , Distribution of measured chromatin thickness at each pixel around nucle
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
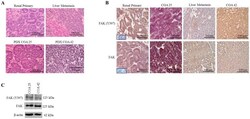
- Figure 1 FAK in human Wilms tumor (WT) samples and patient derived xenografts (PDX). ( A ) H&E staining demonstrating PDX COA 25 ( left lower panel ) recapitulated the predominant epithelial histology of the patient's primary renal WT ( left upper panel ) and PDX COA 42 ( right lower panel ) recapitulated the predominant blastemal histology of the patient's liver metastasis ( right upper panel ). ( B ) Immunohistochemistry staining with antibodies for FAK Y397 and total FAK was performed on the primary renal tumor, liver metastasis, and PDXs - COA 25 and COA 42. Negative controls were included for each sample ( inserts ). Staining for FAK Y397 and total FAK was positive in all samples and located in tumor cell cytoplasm and membrane. ( C ) Immunoblotting for FAK Y397 and total FAK was performed on COA 25 and COA 42 cell lysates. Y397 FAK and total FAK were detected in both PDXs.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
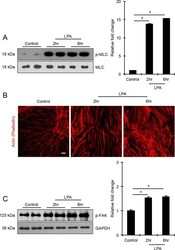
- Figure 2 LPA induces formation of actin stress fibers and phosphorylation of MLC and FAK in HTM cells. Serum-starved HTM cells (24 hours) treated with LPA (20 muM for 2 and 6 hours) showed significant increase in the levels of phosphorylated MLC (p-MLC; panel [A]) and FAK (p-FAK; panel [C]) compared with the control cells, based on quantitative immunoblot analyses. Values are mean +- SEM. n = 4, *P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
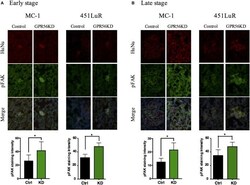
- Figure 5 Cell-extracellular matrix signaling is inhibited by GPR56 expression in vivo . (A) GPR56 depletion leads to increased p-FAK staining in 1-week metastases. Top: lung sections from mice injected with MC-1 or 451LuR cells expressing control-shRNA and GPR56-shRNA were sectioned and stained with the anti-p-FAK antibody (green). Red: human-specific anti-human nuclei (huNu) antibody to label human cells. Blue: DAPI, for nuclei. Bottom: quantitation of p-FAK staining intensity. The intensity was measured using the ImageJ software (see Materials and Methods ). (B) GPR56 depletion leads to increased p-FAK staining in 4-week (MC-1) and 3-week (451LuR) metastases. Top: lung sections from mice injected with MC-1 or 451LuR cells expressing control-shRNA and GPR56-shRNA were sectioned and stained with the anti-p-FAK antibody (green). Red: human-specific anti-huNu antibody to label human cells. Blue: DAPI, for nuclei. Bottom: quantitation of p-FAK staining intensity. The intensity was measured using the ImageJ software (see Materials and Methods ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
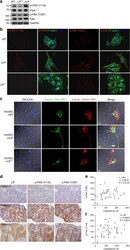
- Fig. 5 LIF regulates focal adhesion molecules. a Detection of endogenous focal adhesion kinases in cancer cells via western blot using GAPDH as a loading control. b LIF regulates the spatial distribution of activated focal adhesion kinases. Cells were labeled with antibodies against phospho-PXN (Y118) or phospho-FAK (Y397). Alexa Fluor 488 phalloidin (green) was used to stain F-actin. Blue, nuclear staining. Scale bars, 10 mum. c Live imaging of focal adhesion during transendothelial invasion. Cancer cells expressing LifeAct-RFP were pre-labeled with Talin-GFP and plated onto the HUVEC layer. Images were captured 24 h post-plating. Blue, nuclei labeled with Hoechst33342. Scale bars, 20 mum. The white arrow indicates the damaged area caused by Talin-rich elongated protrusion. d Representative images of LIF, p-PXN (Y118), and p-FAK (Y397) expression in paraffin-embedded consecutive NPC tissue sections. Scale bars, 50 mum. e , f Correlation analyses based on IHC scores (Spearman's correlation test). Correlations were evident between LIF and p-FAK (Y397) ( e ) and LIF and p-PXN (Y118) ( f )
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry