Antibody data
- Antibody Data
- Antigen structure
- References [1]
- Comments [0]
- Validations
- Immunocytochemistry [3]
- Immunohistochemistry [1]
- Flow cytometry [2]
- Other assay [1]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA5-15793 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- SMAD5 Monoclonal Antibody (3H9)
- Antibody type
- Monoclonal
- Antigen
- Purifed from natural sources
- Description
- MA5-15793 targets SMAD5 in indirect ELISA, FACS, IF, IHC, and WB applications and shows reactivity with Human and Rat samples. The MA5-15793 immunogen is purified recombinant fragment of human SMAD5 expressed in E. Coli. MA5-15793 detects SMAD5 which has a predicted molecular weight of approximately 52kDa.
- Reactivity
- Human, Rat
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 3H9
- Vial size
- 100 μL
- Concentration
- Conc. Not Determined
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Mitochondrial DNA alterations may influence the cisplatin responsiveness of oral squamous cell carcinoma.
Aminuddin A, Ng PY, Leong CO, Chua EW
Scientific reports 2020 May 12;10(1):7885
Scientific reports 2020 May 12;10(1):7885
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
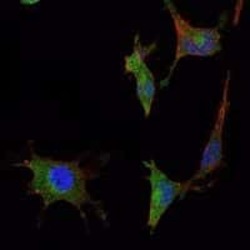
- Experimental details
- Immunofluorescence analysis of NTERA-2 cells using SMAD5 monoclonal antibody (Product # MA5-15793) (Green). Blue: DRAQ5 fluorescent DNA dye. Red: actin filaments have been labeled with phalloidin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
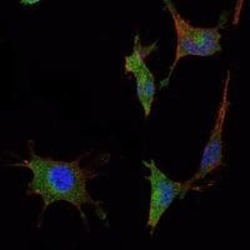
- Experimental details
- Immunofluorescence analysis of NTERA-2 cells using SMAD5 monoclonal antibody (Product # MA5-15793) (Green). Blue: DRAQ5 fluorescent DNA dye. Red: actin filaments have been labeled with phalloidin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
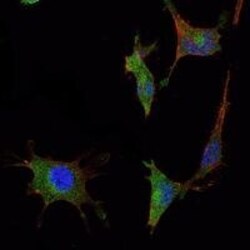
- Experimental details
- Immunofluorescence analysis of NTERA-2 cells using SMAD5 monoclonal antibody (Product # MA5-15793) (Green). Blue: DRAQ5 fluorescent DNA dye. Red: actin filaments have been labeled with phalloidin.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
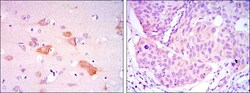
- Experimental details
- Immunohistochemical analysis of paraffin-embedded brain tissues (left) and lung cancer tissues (right) using SMAD5 monoclonal antibody (Product # MA5-15793) followed with DAB staining.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
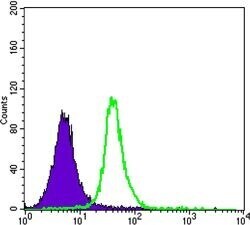
- Experimental details
- Flow cytometric analysis of Jurkat cells using SMAD5 monoclonal antibody (Product # MA5-15793) (green) and negative control (purple).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
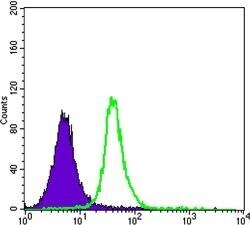
- Experimental details
- Flow cytometric analysis of Jurkat cells using SMAD5 monoclonal antibody (Product # MA5-15793) (green) and negative control (purple).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
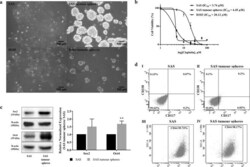
- Experimental details
- Figure 1 Derivation of cancer stem cells (CSCs) from OSCC cell lines via a sphere-forming assay and the characterization of their stem cell-like features. ( a ) The morphology of the parental SAS and H103 and their derived tumour spheres. SAS and H103 in normal culture media were observed as polygonal squamous epithelial cells with the adherent growth pattern. Within 7 d, tumour spheres, comprised of aggregated and suspended cells derived from SAS and H103, were formed in the specialized serum-free medium containing serum substitute, heparin, and growth factors and in a low attachment plate (100x magnification). The average diameters of the SAS and H103 tumour spheres were 133.4 +- 34.36 um and 68.1 +- 13.37 um, respectively. ( b ) Assessment of cell viability of SAS, SAS tumour spheres, and H103 after 72 h exposure to cisplatin. IC 50 was defined as the concentration of cisplatin required to reduce cell viability by half. Higher IC 50 values indicated lower sensitivity of the cells towards cisplatin and possibly cisplatin resistance. ( c ) Western blots of Sox2, Oct4 and beta-actin and the relative expression levels of the Sox2 and Oct4 transcription factors normalized to the beta-actin protein in SAS and SAS tumour spheres. The full-length blots are presented in Supplementary Figure S2 . ( d ) Expression of CD338, CD117 and CD44 surface markers in both SAS and SAS tumour spheres, as analyzed by flow cytometry. Multi-staining flow cytometry was used to analyse the surface ex
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry