Antibody data
- Antibody Data
- Antigen structure
- References [17]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Flow cytometry [1]
- Other assay [18]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-749 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Ataxin 7 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA1-749 detects ataxin 7 protein from transfected cell lysate. PA1-749 has successfully been used in immunohistochemistry and Western blot procedures. By Western blot, this antibody detects an ~96 kDa protein representing human ataxin 7 in transfected COS-1 cells. The PA1-749 immunizing peptide corresponds to amino acid residues 1-17 from human ataxin 7. This sequence is completely conserved in mouse ataxin 7. This peptide (Cat. # PEP-217) is available for use in neutralization and control experiments.
- Reactivity
- Human, Drosophila
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references SCA7 Mouse Cerebellar Pathology Reveals Preferential Downregulation of Key Purkinje Cell-Identity Genes and Shared Disease Signature with SCA1 and SCA2.
DNAzyme Cleavage of CAG Repeat RNA in Polyglutamine Diseases.
Respiratory dysfunction in a mouse model of spinocerebellar ataxia type 7.
Metabolic and Organelle Morphology Defects in Mice and Human Patients Define Spinocerebellar Ataxia Type 7 as a Mitochondrial Disease.
Lentiviral vector-mediated overexpression of mutant ataxin-7 recapitulates SCA7 pathology and promotes accumulation of the FUS/TLS and MBNL1 RNA-binding proteins.
Mutant CAG Repeats Effectively Targeted by RNA Interference in SCA7 Cells.
Proteolytic cleavage of ataxin-7 promotes SCA7 retinal degeneration and neurological dysfunction.
RNA interference-based therapy for spinocerebellar ataxia type 7 retinal degeneration.
Nonallele specific silencing of ataxin-7 improves disease phenotypes in a mouse model of SCA7.
Histone deacetylase-3 interacts with ataxin-7 and is altered in a spinocerebellar ataxia type 7 mouse model.
Reduction of mutant ataxin-7 expression restores motor function and prevents cerebellar synaptic reorganization in a conditional mouse model of SCA7.
Interferon β induces clearance of mutant ataxin 7 and improves locomotion in SCA7 knock-in mice.
Spinocerebellar ataxia type 7 cerebellar disease requires the coordinated action of mutant ataxin-7 in neurons and glia, and displays non-cell-autonomous bergmann glia degeneration.
Posttranslational modification of ataxin-7 at lysine 257 prevents autophagy-mediated turnover of an N-terminal caspase-7 cleavage fragment.
Proteolytic cleavage of ataxin-7 by caspase-7 modulates cellular toxicity and transcriptional dysregulation.
A conditional pan-neuronal Drosophila model of spinocerebellar ataxia 7 with a reversible adult phenotype suitable for identifying modifier genes.
PML clastosomes prevent nuclear accumulation of mutant ataxin-7 and other polyglutamine proteins.
Niewiadomska-Cimicka A, Doussau F, Perot JB, Roux MJ, Keime C, Hache A, Piguet F, Novati A, Weber C, Yalcin B, Meziane H, Champy MF, Grandgirard E, Karam A, Messaddeq N, Eisenmann A, Brouillet E, Nguyen HHP, Flament J, Isope P, Trottier Y
The Journal of neuroscience : the official journal of the Society for Neuroscience 2021 Jun 2;41(22):4910-4936
The Journal of neuroscience : the official journal of the Society for Neuroscience 2021 Jun 2;41(22):4910-4936
DNAzyme Cleavage of CAG Repeat RNA in Polyglutamine Diseases.
Zhang N, Bewick B, Schultz J, Tiwari A, Krencik R, Zhang A, Adachi K, Xia G, Yun K, Sarkar P, Ashizawa T
Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 2021 Jul;18(3):1710-1728
Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 2021 Jul;18(3):1710-1728
Respiratory dysfunction in a mouse model of spinocerebellar ataxia type 7.
Fusco AF, Pucci LA, Switonski PM, Biswas DD, McCall AL, Kahn AF, Dhindsa JS, Strickland LM, La Spada AR, ElMallah MK
Disease models & mechanisms 2021 Jul 1;14(7)
Disease models & mechanisms 2021 Jul 1;14(7)
Metabolic and Organelle Morphology Defects in Mice and Human Patients Define Spinocerebellar Ataxia Type 7 as a Mitochondrial Disease.
Ward JM, Stoyas CA, Switonski PM, Ichou F, Fan W, Collins B, Wall CE, Adanyeguh I, Niu C, Sopher BL, Kinoshita C, Morrison RS, Durr A, Muotri AR, Evans RM, Mochel F, La Spada AR
Cell reports 2019 Jan 29;26(5):1189-1202.e6
Cell reports 2019 Jan 29;26(5):1189-1202.e6
Lentiviral vector-mediated overexpression of mutant ataxin-7 recapitulates SCA7 pathology and promotes accumulation of the FUS/TLS and MBNL1 RNA-binding proteins.
Alves S, Marais T, Biferi MG, Furling D, Marinello M, El Hachimi K, Cartier N, Ruberg M, Stevanin G, Brice A, Barkats M, Sittler A
Molecular neurodegeneration 2016 Jul 28;11(1):58
Molecular neurodegeneration 2016 Jul 28;11(1):58
Mutant CAG Repeats Effectively Targeted by RNA Interference in SCA7 Cells.
Fiszer A, Wroblewska JP, Nowak BM, Krzyzosiak WJ
Genes 2016 Dec 17;7(12)
Genes 2016 Dec 17;7(12)
Proteolytic cleavage of ataxin-7 promotes SCA7 retinal degeneration and neurological dysfunction.
Guyenet SJ, Mookerjee SS, Lin A, Custer SK, Chen SF, Sopher BL, La Spada AR, Ellerby LM
Human molecular genetics 2015 Jul 15;24(14):3908-17
Human molecular genetics 2015 Jul 15;24(14):3908-17
RNA interference-based therapy for spinocerebellar ataxia type 7 retinal degeneration.
Ramachandran PS, Bhattarai S, Singh P, Boudreau RL, Thompson S, Laspada AR, Drack AV, Davidson BL
PloS one 2014;9(4):e95362
PloS one 2014;9(4):e95362
Nonallele specific silencing of ataxin-7 improves disease phenotypes in a mouse model of SCA7.
Ramachandran PS, Boudreau RL, Schaefer KA, La Spada AR, Davidson BL
Molecular therapy : the journal of the American Society of Gene Therapy 2014 Sep;22(9):1635-42
Molecular therapy : the journal of the American Society of Gene Therapy 2014 Sep;22(9):1635-42
Histone deacetylase-3 interacts with ataxin-7 and is altered in a spinocerebellar ataxia type 7 mouse model.
Duncan CE, An MC, Papanikolaou T, Rugani C, Vitelli C, Ellerby LM
Molecular neurodegeneration 2013 Oct 27;8:42
Molecular neurodegeneration 2013 Oct 27;8:42
Reduction of mutant ataxin-7 expression restores motor function and prevents cerebellar synaptic reorganization in a conditional mouse model of SCA7.
Furrer SA, Waldherr SM, Mohanachandran MS, Baughn TD, Nguyen KT, Sopher BL, Damian VA, Garden GA, La Spada AR
Human molecular genetics 2013 Mar 1;22(5):890-903
Human molecular genetics 2013 Mar 1;22(5):890-903
Interferon β induces clearance of mutant ataxin 7 and improves locomotion in SCA7 knock-in mice.
Chort A, Alves S, Marinello M, Dufresnois B, Dornbierer JG, Tesson C, Latouche M, Baker DP, Barkats M, El Hachimi KH, Ruberg M, Janer A, Stevanin G, Brice A, Sittler A
Brain : a journal of neurology 2013 Jun;136(Pt 6):1732-45
Brain : a journal of neurology 2013 Jun;136(Pt 6):1732-45
Spinocerebellar ataxia type 7 cerebellar disease requires the coordinated action of mutant ataxin-7 in neurons and glia, and displays non-cell-autonomous bergmann glia degeneration.
Furrer SA, Mohanachandran MS, Waldherr SM, Chang C, Damian VA, Sopher BL, Garden GA, La Spada AR
The Journal of neuroscience : the official journal of the Society for Neuroscience 2011 Nov 9;31(45):16269-78
The Journal of neuroscience : the official journal of the Society for Neuroscience 2011 Nov 9;31(45):16269-78
Posttranslational modification of ataxin-7 at lysine 257 prevents autophagy-mediated turnover of an N-terminal caspase-7 cleavage fragment.
Mookerjee S, Papanikolaou T, Guyenet SJ, Sampath V, Lin A, Vitelli C, DeGiacomo F, Sopher BL, Chen SF, La Spada AR, Ellerby LM
The Journal of neuroscience : the official journal of the Society for Neuroscience 2009 Dec 2;29(48):15134-44
The Journal of neuroscience : the official journal of the Society for Neuroscience 2009 Dec 2;29(48):15134-44
Proteolytic cleavage of ataxin-7 by caspase-7 modulates cellular toxicity and transcriptional dysregulation.
Young JE, Gouw L, Propp S, Sopher BL, Taylor J, Lin A, Hermel E, Logvinova A, Chen SF, Chen S, Bredesen DE, Truant R, Ptacek LJ, La Spada AR, Ellerby LM
The Journal of biological chemistry 2007 Oct 12;282(41):30150-60
The Journal of biological chemistry 2007 Oct 12;282(41):30150-60
A conditional pan-neuronal Drosophila model of spinocerebellar ataxia 7 with a reversible adult phenotype suitable for identifying modifier genes.
Latouche M, Lasbleiz C, Martin E, Monnier V, Debeir T, Mouatt-Prigent A, Muriel MP, Morel L, Ruberg M, Brice A, Stevanin G, Tricoire H
The Journal of neuroscience : the official journal of the Society for Neuroscience 2007 Mar 7;27(10):2483-92
The Journal of neuroscience : the official journal of the Society for Neuroscience 2007 Mar 7;27(10):2483-92
PML clastosomes prevent nuclear accumulation of mutant ataxin-7 and other polyglutamine proteins.
Janer A, Martin E, Muriel MP, Latouche M, Fujigasaki H, Ruberg M, Brice A, Trottier Y, Sittler A
The Journal of cell biology 2006 Jul 3;174(1):65-76
The Journal of cell biology 2006 Jul 3;174(1):65-76
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
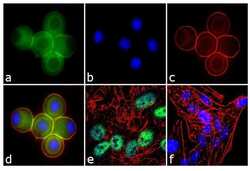
- Experimental details
- Immunofluorescence analysis of Ataxin 7 was performed using 70% confluent log phase U-87 MG cells treated with 100 ng of Nocodazole for 16 hours. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with Ataxin 7 Rabbit Polyclonal Antibody (Product # PA1-749) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e is untreated cell with nuclear localization. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
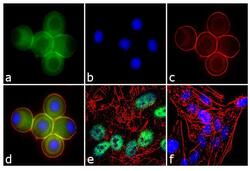
- Experimental details
- Immunofluorescence analysis of Ataxin 7 was performed using 70% confluent log phase U-87 MG cells treated with 100 ng of Nocodazole for 16 hours. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with Ataxin 7 Rabbit Polyclonal Antibody (Product # PA1-749) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e is untreated cell with nuclear localization. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
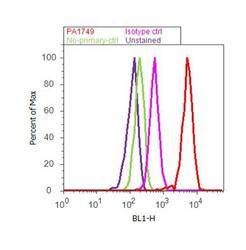
- Experimental details
- Flow cytometry analysis of Ataxin 7 was performed using SH-SY5Y cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with Ataxin 7 Rabbit Polyclonal Antibody (PA1-749, red histogram) or with rabbit isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control..
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
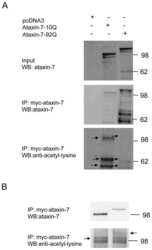
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
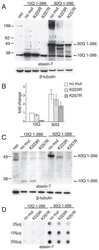
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
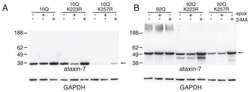
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
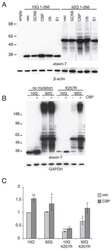
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
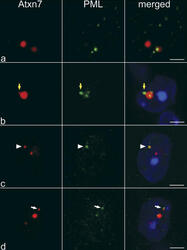
- Experimental details
- Figure 1. ATXN7 aggregates colocalize with PML bodies in SCA7 transgenic mice. Immunofluorescence analysis of cerebellar Purkinje cells (a) and retinal ganglion neurons (b-d) using antibodies against ATXN7 (affinity-purified polyclonal antibody 1261) and PML (chicken anti-PML antibody). Large ATXN7 aggregates sequestered PML bodies (a and b). In ganglion neurons, PML bodies were also surrounded by (b, yellow arrows), colocalized with (c, arrowheads), or juxtaposed to (d, white arrows) small ATXN7 aggregates. The same ganglion neuron on two different focal plans, shown in panels c and d, displayed distinct colocalization between PML bodies and ATXN7 aggregates. Bars, 5 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
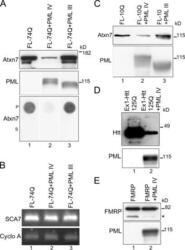
- Experimental details
- Figure 5. ATXN7 and Htt-exon1 are efficiently degraded in the presence of PML IV. (A) Western blot and filter assay of FL-74Q alone or coexpressed with PML IV or III in COS-7 cells. (top) Western blot (30 mug protein, anti-ATXN7 1C1, and anti-PML polyclonal). ATXN7 levels strongly decreased in the presence of PML IV (lane 2) but slightly increased with PML III (lane 3). (bottom) Pellet (P) or supernatant (S) fractions of cell extracts (30 mug protein) were filtered, and SDS-insoluble ATXN7 was revealed by anti-ATXN7 1C1. Coexpression of PML IV led to the disappearance of SDS-insoluble aggregates of mutant ATXN7 (lane 2), whereas PML III slightly increased ATXN7 (lane 3 compared with lane 1). (B) RT-PCR. mRNAs were extracted from cells expressing FL-74Q alone (lane 1) or combined with PML IV (lane 2) or III (lane 3). Primers specific to the SCA7 cDNA and the cyclophilin A (Cyclo A) reference cDNA were used for PCR. Coexpression of PML IV or III had no effect on mutant ATXN7 mRNA. (C) Western blot of cells expressing FL-10Q alone (lane 1) or coexpressed with PML IV (lane 2) or III (lane 3). PML IV decreased and PML III slightly increased ATXN7 levels (same antibodies as in A). (D) Western blot of mutant Htt-exon1 expressed in HeLa cells; Htt-exon1 (Ex1) with 125Q alone (lane 1) or with PML IV (lane 2). Levels of Htt-exon1 (detected with antibody 1C2) were strongly decreased when PML IV was coexpressed. (E) Western blot of FMRP expressed in COS-7 cells alone (lane 1) or with PML
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
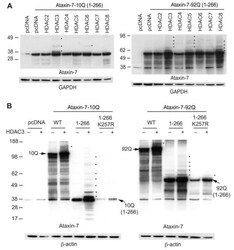
- Experimental details
- Figure 1 HDAC3 stabilizes ataxin-7 and enhances post-translational modifications. (A) Ataxin-7-10Q (1-266) and ataxin-7-92Q (1-266) were transiently transfected with HDAC constructs [ 2 - 8 ] in HEK293T cells for 72 hrs. Lysates were collected and separated via SDS-PAGE on 4-12% bis-tris gels. Western blot transfer and analysis with an antibody to ataxin-7 (PA1-749) revealed the presence of bands of expected molecular weight for the ataxin-7 stop constructs as well as bands of higher molecular weight indicated by asterisks, particularly evident in the HDAC3 co-tranfection. GAPDH was used as a loading control. (B) Ataxin-7-10Q, ataxin-7-10Q (1-266), ataxin-7-10Q (1-266) K257R, ataxin-7-92Q, ataxin-7-92Q (1-266) or ataxin-7-92Q (1-266) K257R were co-transfected with vector control or HDAC3 in HEK293T cells. Western blot analysis of cellular lysates with ataxin-7 (PA1-749) antibody revealed the presence of bands corresponding to the wild type, expanded and ataxin-7 1-266 stop mutant. Bands of higher molecular weight were dominant when ataxin-7 was co-transfected with HDAC3 (indicated by asterisks). Note that these bands are diminished in the SUMOylation-deficient ataxin-7 mutant (K257R). beta-actin was used as a loading control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
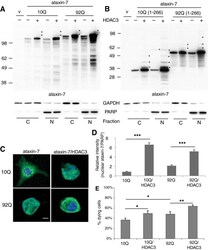
- Experimental details
- Figure 3 HDAC3 alters ataxin-7 subcellular localization. (A,B) Expression of ataxin-7 in cytoplasmic and nuclear fractions of HEK293T cells. Cells were transiently transfected with ataxin-7-10Q (wild type), ataxin-7-92Q (expanded) (A) , or the 1-266 N-terminal fragment of wild type and expanded ataxin-7 (B) and cotransfected with vector control (v) or HDAC3. Lysates were subjected to subcellular fractionation followed by western blot analysis of nuclear and cytoplasmic fractions. Ataxin-7 antibody revealed the presence of bands corresponding to the wild type, expanded and stop mutant ataxin-7. Bands of higher molecular weight of ataxin-7 are evident when ataxin-7 is co-expressed with HDAC3 (indicated by asterisks) and present in both nuclear and cytoplasmic fractions. Co-transfection of HDAC3 was confirmed by immunoblotting (data not shown). To ensure equal loading, immunoblotting with GAPDH was used for the cytoplasmic fractions and poly-ADP ribose (PARP) for the nuclear fraction. (C) Immunocytochemistry of transfected HEK293T cells. Cells were fixed and stained with antibodies to ataxin-7 conjugated to Alexa 488 (green), HDAC3 conjugated to Alexa 555 (red), and counterstained with DAPI (blue). Only the ataxin-7 and DAPI expression are shown, although co-transfection of HDAC3 was determined by visualization of the red track when appropriate. Cells were imaged using confocal microscopy to elucidate colocalization of ataxin-7 and DAPI. Scale bar represents 5 mum. (D) Quantific
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
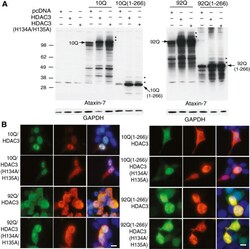
- Figure 4 Role of HDAC3 deacetylase activity in ataxin-7 post-translational modification and subcellular localization. (A) Ataxin-7-10Q, ataxin-7-10Q (1-266), ataxin-7-92Q or ataxin-7-92Q (1-266) were cotransfected with vector control or HDAC3 or a catalytically-inactive mutant of HDAC3, HDAC3 (H134A/H135A), in HEK293T cells. Western blot analysis of cellular lysates with ataxin-7 (PA1-749) antibody revealed the presence of bands corresponding to the wild type, expanded and stop mutant ataxin-7. Bands of higher molecular weight that are detected when ataxin-7 is co-expressed with HDAC3 (indicated by asterisks) are also present when ataxin-7 is co-expressed with the catalytically-inactive HDAC3 mutant. Co-expression of HDAC3 was confirmed by immunoblotting (HDAC3 antibody sc17795; data not shown) and GAPDH was used as a loading control. (B) Immunocytochemistry of HEK293T cells transiently transfected with ataxin-7-10Q (wild type) or ataxin-7-92Q (expanded), and co-transfected with HDAC3 or the catalytically-inactive mutant HDAC3 (H134A/H135A). Cells were fixed and stained with antibodies to ataxin-7 conjugated to Alexa 488 (green), HDAC3 conjugated to Alexa 555 (red), and counterstained with DAPI (blue). Cells were imaged using confocal microscopy to elucidate colocalization of ataxin-7 and HDAC3, which can be detected by yellow co-staining. Scale bar represents 5 mum wild type.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
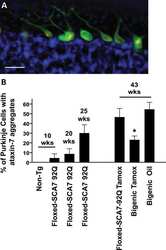
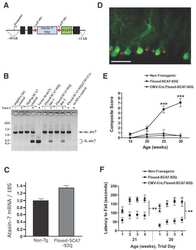
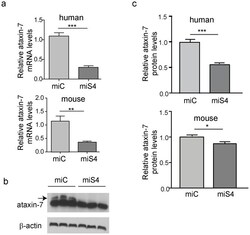
- Figure 1 Reduction of ataxin-7 mRNA in the SCA7 mouse retina. (a) Relative levels of human (top panel) or mouse (bottom panel) ataxin-7 mRNA levels one month post-injection with AAV2/1 miC or miS4. (b) Representative western blot demonstrating mutant human (upper band, denoted by arrow) and mouse ataxin-7 (lower band) protein levels one month post injection of AAV2/1 miC or miS4. For quantification (c), ataxin-7 protein levels were normalized to beta-actin (upper panel, human protein; lower panel, mouse protein). For all panels, results are represented as mean +-SEM (n = 3), ***p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
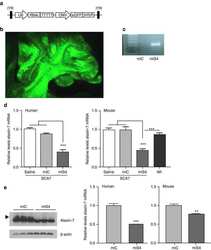
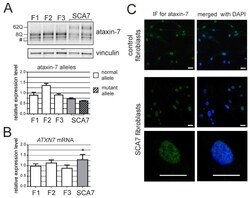
- Figure 1 ATXN7 expression in human fibroblasts. ( A ) Western blot analysis of ataxin-7 levels in control (F1, F2 and F3) and spinocerebellar ataxia type 7 (SCA7) fibroblasts. Representative blot is shown and a graph presenting quantitation based on analyses from three separate protein isolations. In the case where the expression level of individual alleles was analyzed separately, clear bars represent normal allele and hatched bars represent mutant allele; ( B ) Quantitative Reverse transcription polymerase chain reaction (qRT-PCR) analysis of total ATXN7 mRNA levels in control and SCA7 fibroblasts; ( C ) Representative images of anti-ataxin-7 immunofluorescence (IF) in fibroblast cell lines (control: GM07492 and SCA7). Scale bar = 25 mum. 4',6-diamidino-2-phenylindol (DAPI) staining of the nuclei is in blue.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
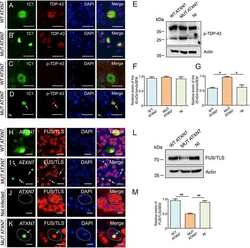
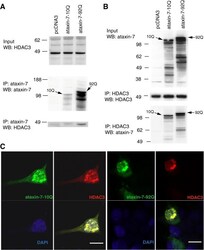
- Figure 2 HDAC3 and ataxin-7 physically interact and colocalize. (A) Immunoprecipitation of HEK293T cells transiently transfected with ataxin-7-10Q (wild type) or ataxin-7-92Q (expanded), or vector. Whole cell lysates were subject to immunoprecipitation with an antibody to ataxin-7 (PA1-749), separated by SDS-PAGE and immunoblotted with an antibody to HDAC3 (N-19). (B) Reverse immunoprecipation with HDAC3 (B-12) and western blotting with ataxin-7 (K) supports the binding of ataxin-7 to HDAC3. (C) Immunocytochemistry of HEK293T cells transiently transfected with ataxin-7-10Q (wild type) or ataxin-7-92Q (expanded), and co-transfected with HDAC3. Cells were fixed and stained with antibodies to ataxin-7 conjugated to Alexa 488 (green), HDAC3 conjugated to Alexa 555 (red), and counterstained with DAPI (blue). Cells were imaged using confocal microscopy to elucidate colocalization of ataxin-7 and HDAC3. Scale bar represents 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
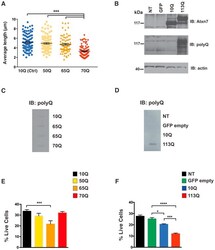
- Experimental details
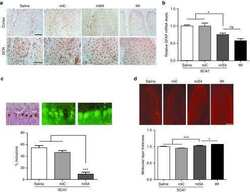
- Figure 4. SCA7 Stem Cell Neural Derivatives Exhibit PolyQ Length-Dependent Disease Phenotypes (A) Graph of average mitochondrial length of control (10Q) and SCA7 patient (50Q, 65Q, 70Q) neuronal progenitor cells (NPCs) on the basis of Tom20 immunostaining. Quantification of average mitochondrial length analyzed using ImageJ mitochondrial morphometry plugin. Each dot represents the average mitochondrial length from an individual cell, and data are represented as mean +- SEM. Two control patient lines (one or two clones per line) and two SCA7 patient lines (two clones per line); n = 70-100 cells/genotype. ***p < 0.001 (one-way ANOVA with post hoc Tukey's multiple-comparison test). (B) Ataxin-7-knockout NPCs were transduced with empty vector, ataxin-7-10Q, or ataxin-7-113Q lentivirus vector. Non-transduced (NT) cells serve as a negative control for transfection. Protein lysates were immunoblotted with anti-ataxin-7 or antiexpanded polyQ tract antibody, as indicated. beta-Actin immunoblotting analysis was performed as a loading control. (C) Protein lysates from control and SCA7 patient NPCs of the indicated CAG repeat allele size were filtered through nitrocellulose and then immuno-blotted with anti-expanded polyQ tract antibody. (D) Protein lysates from non-transduced ataxin-7-knockout NPCs or from ataxin-7-knockout NPCs transduced with empty vector, ataxin-7-10Q, or ataxin-7-113Q lentivirus vector were filtered through nitrocellulose and then immunoblotted with anti-expanded po
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry