Antibody data
- Antibody Data
- Antigen structure
- References [5]
- Comments [0]
- Validations
- Immunocytochemistry [3]
- Flow cytometry [1]
- Other assay [5]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-9032 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- COX2 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- This antibody is predicted to react with bovine, canine and porcine based on sequence homology. This antibody is tested in Peptide ELISA: antibody detection limit dilution 32,000.
- Reactivity
- Human, Mouse
- Host
- Goat
- Isotype
- IgG
- Vial size
- 100 µg
- Concentration
- 0.5 mg/mL
- Storage
- -20° C, Avoid Freeze/Thaw Cycles
Submitted references Sulindac acetohydrazide derivative attenuates against cisplatin induced organ damage by modulation of antioxidant and inflammatory signaling pathways.
Molecular docking, pharmacokinetic studies, and in vivo pharmacological study of indole derivative 2-(5-methoxy-2-methyl-1H-indole-3-yl)-N'-[(E)-(3-nitrophenyl) methylidene] acetohydrazide as a promising chemoprotective agent against cisplatin induced organ damage.
Korean Red Ginseng affects ovalbumin-induced asthma by modulating IL-12, IL-4, and IL-6 levels and the NF-κB/COX-2 and PGE(2) pathways.
Saururus chinensis-controlled allergic pulmonary disease through NF-κB/COX-2 and PGE(2) pathways.
Anti-inflammatory Effect of Curcuma longa and Allium hookeri Co-treatment via NF-κB and COX-2 Pathways.
Razak S, Afsar T, Bibi N, Abulmeaty M, Bhat MA, Inam A, Trembley JH, Almajwal A, Shabbir M, Alruwaili NW, Algarni A
Scientific reports 2022 Jul 11;12(1):11749
Scientific reports 2022 Jul 11;12(1):11749
Molecular docking, pharmacokinetic studies, and in vivo pharmacological study of indole derivative 2-(5-methoxy-2-methyl-1H-indole-3-yl)-N'-[(E)-(3-nitrophenyl) methylidene] acetohydrazide as a promising chemoprotective agent against cisplatin induced organ damage.
Razak S, Afsar T, Bibi N, Abulmeaty M, Qamar W, Almajwal A, Inam A, Al Disi D, Shabbir M, Bhat MA
Scientific reports 2021 Mar 18;11(1):6245
Scientific reports 2021 Mar 18;11(1):6245
Korean Red Ginseng affects ovalbumin-induced asthma by modulating IL-12, IL-4, and IL-6 levels and the NF-κB/COX-2 and PGE(2) pathways.
Lee SY, Kim MH, Kim SH, Ahn T, Kim SW, Kwak YS, Cho IH, Nah SY, Cho SS, Park KM, Park DH, Bae CS
Journal of ginseng research 2021 Jul;45(4):482-489
Journal of ginseng research 2021 Jul;45(4):482-489
Saururus chinensis-controlled allergic pulmonary disease through NF-κB/COX-2 and PGE(2) pathways.
Song M, Lee SY, Kim M, Park S, Park J, Kwon Y, Park DH
PeerJ 2020;8:e10043
PeerJ 2020;8:e10043
Anti-inflammatory Effect of Curcuma longa and Allium hookeri Co-treatment via NF-κB and COX-2 Pathways.
Lee SY, Cho SS, Li Y, Bae CS, Park KM, Park DH
Scientific reports 2020 Mar 31;10(1):5718
Scientific reports 2020 Mar 31;10(1):5718
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
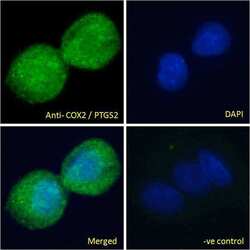
- Experimental details
- Immunofluorescence analysis of COX2 in HepG2 cells using a COX2 monoclonal antibody (Product # PA1-9032) at 10 µg/mL for1hr. The cells were paraformaldehyde fixed and permeabilized with 0.15% Triton. Primary incubation was followed by Alexa Fluor 488 secondary antibody (2 µg/mL) showing cytoplasmic/vesicle staining. The nuclear stain is DAPI (blue). Negative control: Unimmunized goat IgG (10 µg/mL)followed by Alexa Fluor 488 secondary antibody (2 µg/mL).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
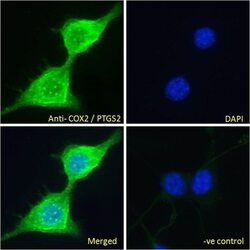
- Experimental details
- Immunofluorescence analysis of COX2 in NIH3T3 cells using a COX2 monoclonal antibody (Product # PA1-9032) at 10 µg/mL for1hr. The cells were paraformaldehyde fixed and permeabilized with 0.15% Triton. Primary incubation was followed by Alexa Fluor 488 secondary antibody (2 µg/mL) showing cytoplasm and vesicle staining. The nuclear stain is DAPI (blue).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
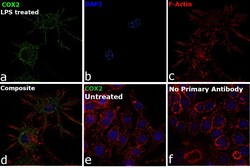
- Experimental details
- Immunofluorescence analysis of COX2 was performed using 70% confluent log phase RAW 264.7 cells treated with 100 ng/mL of Lipopolysaccharide for 24 Hrs. The cells were fixed with 4% Paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 2% BSA for 10 minutes at room temperature. The cells were labeled with COX2 Polyclonal Antibody (Product # PA1-9032) at 5 µg/mL in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Rabbit anti-Goat IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (Product # A-11078), (1:2000 dilution) for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing Cytoplasmic and nuclear membrane localization. Panel e represents untreated cells with reduced signal. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
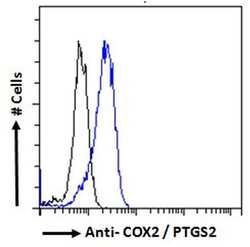
- Experimental details
- Flow cytometric analysis of COX2 in HeLa cells using a COX2 monoclonal antibody (Product # PA1-9032) at 10 µg/mL for 1hr, depicted by the blue line. The cells were paraformaldehyde fixed and permeabilized with 0.5% Triton. Primary incubation followed by Alexa Fluor 488 secondary antibody (2 µg/mL). IgG control: Unimmunized goat IgG (black line) followed by Alexa Fluor 488 secondary antibody.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
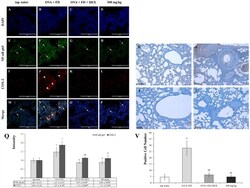
- Experimental details
- Figure 6 Saururus chinensis (SC) water-extract controlled inflammation through the NF-kappa B/COX-2 and PGE 2 axis. (A-P) Saururus chinensis (SC) water-extract inhibited COX-2 expression via preventing NF-kappa B translocation. Compared to the results in ovalbumin and fine dust treatment group in tap water treatment group the expression of COX-2 protein (red spot) was decreased significantly. In ovalbumin and fine dust treatment group NF-kappa B proteins were translocated from cytoplasm to nucleus and the expression of COX-2 protein was increased effectively. Both in dexamethasone treatment group and in 300 mg/kg SC treatment group, the proteins expression were down-regulated, both of NF-kappa B and COX-2. (R-I) SC effectively inhibited the PGE 2 expression. R, tap water treatment (control) group; S, ovalbumin and fine dust treatment (induction) group; T, ovalbumin, fine dust, and dexamethasone treatment (positive control) group; I, ovalbumin, fine dust, and 300 mg/kg SC treatment group. Arrow, NF-kappa B; arrowhead, COX-2. Magnification, 1000 x. Scale bar, 50 mu m. (Q) The quantitative score of NF- kappa B p65 vs. nucleus or COX-2 vs. nucleus. All the values are presented as mean +- standard deviation. n = 8 per each group. * p < 0.05 vs. tap water treatment group; $ p < 0.05 vs. ovalbumin and fine dust treatment group; $$ p < 0.001 vs. ovalbumin and fine dust treatment group. (V) The number of PGE 2 positive cells in the lung tissue. All values are presented as mean +- stan
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
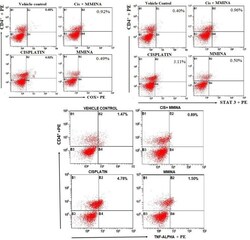
- Experimental details
- Figure 2 Effect of MMINA on CD4 + COX2, CD4 + STAT3, and CD4 + TNF-alpha population. Flow cytometric analysis of CD4 + COX2, STAT3, TNF-alpha and CD4 + expressing cells in whole bllod from one rat from each group. Statistical analysis was performed using a one way ANOVA followed by the Tukey-Kramer post-test. Each value indicates the mean +- SEM of six animals.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
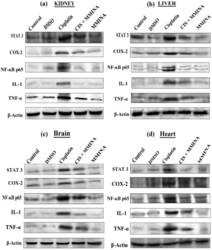
- Experimental details
- Figure 5 Western blot analysis of STAT3, COX2, NFkB p65, IL1 and TNF-alpha protein expression in ( a ) Kidney, ( b ) Liver, ( c ) Brain and ( d ) Heart tissue lysates from different treatment groups. Treatments are indicated above the blots. Full sized blots are included in the Supplementary file S3 . All gels and blots were run under the same experimental conditions as described in "" Methods "".
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
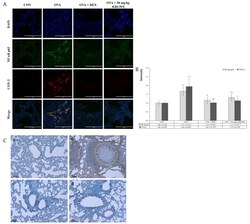
- Experimental details
- Fig. 4 Effect of Korean Red Ginseng water extract (KRGWE) on translocation of NF-kappaBp65 from the cytoplasm to the nucleus . (A) KRGWE significantly inhibited the level of COX-2 expression in the cytoplasm through NF-kappaB translocation from the cytoplasm to the nucleus where it would act as a transcription factor of COX-2. Magnification 1000-fold. Scale bar indicates 50 mum. (B) According to the respective fluorescence, KRGWE downregulated NF-kappaB translocation and COX-2 expression. (C) KRGWE significantly suppressed PGE 2 expression, which was elicited by ovalbumin treatments. Magnification 200-fold. Scale bar indicates 100 mum; a: control group; b: ovalbumin-treated group; c: 1 mg/kg/day DEX treated group after ovalbumin treatment; d: 50 mg/kg/day KRGWE-treated group after ovalbumin treatment. Statistical significance is indicated as follows: * p < 0.05 vs. control group; ** p < 0.01 vs. control group; $ p < 0.05 vs. ovalbumin-treated group; $$ p < 0.01 vs. ovalbumin-treated group Fig. 4
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
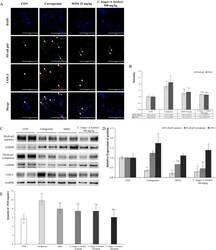
- Experimental details
- Figure 4 C. longa and A. hookeri co-treatment inhibits NF-kappaB and COX-2 expression. ( A ) NF-kappaB is downregulated in the nucleus and COX-2 in the cytoplasm. NF-kappaB and COX-2 are not expressed in the control group and only the nucleus is stained with DAPI (blue spots). Carrageenan stimulates the expression of NF-kappaB in the nucleus and that of COX-2 in the cytoplasm. MSM suppresses the expression of NF-kappaB and COX-2 induced by carrageenan, but the expression of NF-kappaB (arrow) and COX-2 (arrowhead) observed is minimal. C. longa and A. hookeri co-treatment significantly inhibits the expression of NF-kappaB and COX-2. ( B ) The graph and scores present the immunofluorescent results using the image analyzing program in the K1-Fluo confocal microscope. MSM and C. longa and A. hookeri co-treatment statistically significantly control NF-kappaB and COX-2 expression effectively. ( C ) Compared to the density of NF-kappaB in the nucleus and cytoplasm, the pattern is similar to that observed in the immunofluorescence assay. C. longa and A. hookeri co-treatment blocks the translocation of NF-kappaB into the nucleus from the cytoplasm and inhibits the expression of COX-2 in the cytoplasm. ( D ) Depending on the image analysis of the western blotting of NF-kappaB (in nucleus and in cytoplasm) and COX-2 (in the skin tissue), the density graph based on each band per beta-actin was presented using the Image J program. ( E ) MSM and C. longa and A. hookeri co
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry