Antibody data
- Antibody Data
- Antigen structure
- References [60]
- Comments [0]
- Validations
- Immunocytochemistry [1]
- Other assay [41]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 40-4700 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Occludin Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- This antibody is specific for the human occludin protein. On western blots, it identifies the target band at ~65 kDa. Reactivity has been confirmed with human Caco-2 and HT29, dog MDCK, mouse TCMK, and rat KNRK cell lysates, and mouse kidney, liver, and rat liver homogenates by western blotting, Caco-2 cells by immunofluorescence, and paraffin-embedded human small intestine tissue by immunohistochemistry. For immunofluorescence using Caco-2 cells, fixation with cold ethanol is recommended. For immunohistochemistry in paraffin-embedded tissues, enzyme digestion with pepsin is required prior to staining.
- Reactivity
- Human, Mouse, Rat, Canine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 µg
- Concentration
- 0.25 mg/mL
- Storage
- -20°C
Submitted references Diprotin A TFA Exerts Neurovascular Protection in Ischemic Cerebral Stroke.
The Host CYP1A1-Microbiota Metabolic Axis Promotes Gut Barrier Disruption in Methicillin-Resistant Staphylococcus aureus-Induced Abdominal Sepsis.
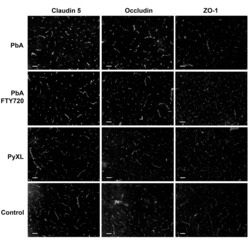
Claudin-5 binder enhances focused ultrasound-mediated opening in an in vitro blood-brain barrier model.
Short-term exposure to JUUL electronic cigarettes can worsen ischemic stroke outcome.
The protective effect and potential mechanisms of eugenol against Salmonella in vivo and in vitro.
Heat stress-induced mucosal barrier dysfunction is potentially associated with gut microbiota dysbiosis in pigs.
Glycine represses endoplasmic reticulum stress-related apoptosis and improves intestinal barrier by activating mammalian target of rapamycin complex 1 signaling.
Inactivation of mouse transmembrane prolyl 4-hydroxylase increases blood brain barrier permeability and ischemia-induced cerebral neuroinflammation.
Fecal Microbiota Transplant in a Pre-Clinical Model of Type 2 Diabetes Mellitus, Obesity and Diabetic Kidney Disease.
Benzophenone-3 breaches mouse Sertoli cell barrier and alters F-actin organization without evoking apoptosis.
Mucin O-glycan-microbiota axis orchestrates gut homeostasis in a diarrheal pig model.
Development of a novel human intestinal model to elucidate the effect of anaerobic commensals on Escherichia coli infection.
Dietary Fish Hydrolysate Improves Memory Performance Through Microglial Signature Remodeling During Aging.
Development of Anti-inflammatory Probiotic Limosilactobacillus reuteri EFEL6901 as Kimchi Starter: in vitro and In vivo Evidence.
Annexin A1 protects against cerebral ischemia-reperfusion injury by modulating microglia/macrophage polarization via FPR2/ALX-dependent AMPK-mTOR pathway.
Cystine reduces tight junction permeability and intestinal inflammation induced by oxidative stress in Caco-2 cells.
Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes.
Design and Validation of a Human Brain Endothelial Microvessel-on-a-Chip Open Microfluidic Model Enabling Advanced Optical Imaging.
The impact of synthetic amorphous silica (E 551) on differentiated Caco-2 cells, a model for the human intestinal epithelium.
TGF-β1 relieves epithelial-mesenchymal transition reduction in hypospadias induced by DEHP in rats.
Hyaluronan 35 kDa enhances epithelial barrier function and protects against the development of murine necrotizing enterocolitis.
The JAK-Inhibitor Tofacitinib Rescues Human Intestinal Epithelial Cells and Colonoids from Cytokine-Induced Barrier Dysfunction.
Prevention of excessive exercise-induced adverse effects in rats with Bacillus subtilis BSB3.
MyD88 regulates a prolonged adaptation response to environmental dust exposure-induced lung disease.
Intestinal Permeability and IgA Provoke Immune Vasculitis Linked to Cardiovascular Inflammation.
Yeast fermentate prebiotic improves intestinal barrier integrity during heat stress by modulation of the gut microbiota in rats.
Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis.
Gliclazide alone or in combination with atorvastatin ameliorated reproductive damage in streptozotocin-induced type 2 diabetic male rats.
Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells.
Geniposide and Chlorogenic Acid Combination Ameliorates Non-alcoholic Steatohepatitis Involving the Protection on the Gut Barrier Function in Mouse Induced by High-Fat Diet.
Involvement of CYP2E1 in the Course of Brain Edema Induced by Subacute Poisoning With 1,2-Dichloroethane in Mice.
HIV induces production of IL-18 from intestinal epithelial cells that increases intestinal permeability and microbial translocation.
ZO-1 protein is required for hydrogen peroxide to increase MDCK cell paracellular permeability in an ERK 1/2-dependent manner.
Urban fine particulate matter (PM2.5) exposure destroys blood-testis barrier (BTB) integrity through excessive ROS-mediated autophagy.
Immunohistochemistry in Investigative and Toxicologic Pathology.
Obesogenic diet-induced gut barrier dysfunction and pathobiont expansion aggravate experimental colitis.
Chronic depletion of gonadal testosterone leads to blood-brain barrier dysfunction and inflammation in male mice.
Chronic sleep restriction disrupts interendothelial junctions in the hippocampus and increases blood-brain barrier permeability.
Urban fine particulate matter exposure causes male reproductive injury through destroying blood-testis barrier (BTB) integrity.
Social stress induces neurovascular pathology promoting depression.
Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs).
Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior.
A2A Adenosine Receptor Antagonism Reverts the Blood-Brain Barrier Dysfunction Induced by Sleep Restriction.
Occludin Content Modulates Hydrogen Peroxide-Induced Increase in Renal Epithelial Paracellular Permeability.
17β-estradiol inhibits MMP-9 and SUR1/TrpM4 expression and activation and thereby attenuates BSCB disruption/hemorrhage after spinal cord injury in male rats.
LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation.
Assessment of an in vitro model of pulmonary barrier to study the translocation of nanoparticles.
Diets high in fermentable protein and fibre alter tight junction protein composition with minor effects on barrier function in piglet colon.
Experimental cerebral malaria pathogenesis--hemodynamics at the blood brain barrier.
Estrogen receptor alpha deficiency protects against development of cognitive impairment in murine lupus.
Mast cells protect against Pseudomonas aeruginosa-induced lung injury.
Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain.
Functional tight junction barrier localizes in the second layer of the stratum granulosum of human epidermis.
Bile acid-induced expression of farnesoid X receptor as the basis for superiority of internal biliary drainage in experimental biliary obstruction.
Evidence for phenotypic plasticity in aggressive triple-negative breast cancer: human biology is recapitulated by a novel model system.
Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension.
Role of caspases in cytokine-induced barrier breakdown in human brain endothelial cells.
Modeling hepatitis C virus infection using human induced pluripotent stem cells.
Isolation and properties of an in vitro human outer blood-retinal barrier model.
Na+/K+-ATPase α1 identified as an abundant protein in the blood-labyrinth barrier that plays an essential role in the barrier integrity.
Zhou MY, Zhang YJ, Ding HM, Wu WF, Cai WW, Wang YQ, Geng DQ
Frontiers in neuroscience 2022;16:861059
Frontiers in neuroscience 2022;16:861059
The Host CYP1A1-Microbiota Metabolic Axis Promotes Gut Barrier Disruption in Methicillin-Resistant Staphylococcus aureus-Induced Abdominal Sepsis.
Ma X, Jin H, Chu X, Dai W, Tang W, Zhu J, Wang F, Yang X, Li W, Liu G, Yang X, Liang H
Frontiers in microbiology 2022;13:802409
Frontiers in microbiology 2022;13:802409
Claudin-5 binder enhances focused ultrasound-mediated opening in an in vitro blood-brain barrier model.
Chen L, Sutharsan R, Lee JL, Cruz E, Asnicar B, Palliyaguru T, Wasielewska JM, Gaudin A, Song J, Leinenga G, Götz J
Theranostics 2022;12(5):1952-1970
Theranostics 2022;12(5):1952-1970
Short-term exposure to JUUL electronic cigarettes can worsen ischemic stroke outcome.
Sifat AE, Archie SR, Nozohouri S, Villalba H, Zhang Y, Sharma S, Ghanwatkar Y, Vaidya B, Mara D, Cucullo L, Abbruscato TJ
Fluids and barriers of the CNS 2022 Sep 9;19(1):74
Fluids and barriers of the CNS 2022 Sep 9;19(1):74
The protective effect and potential mechanisms of eugenol against Salmonella in vivo and in vitro.
Zhao X, Zheng S, Wei S, Tian Q, Tao Y, Bo R, Liu M, Li J
Poultry science 2022 May;101(5):101801
Poultry science 2022 May;101(5):101801
Heat stress-induced mucosal barrier dysfunction is potentially associated with gut microbiota dysbiosis in pigs.
Xia B, Wu W, Fang W, Wen X, Xie J, Zhang H
Animal nutrition (Zhongguo xu mu shou yi xue hui) 2022 Mar;8(1):289-299
Animal nutrition (Zhongguo xu mu shou yi xue hui) 2022 Mar;8(1):289-299
Glycine represses endoplasmic reticulum stress-related apoptosis and improves intestinal barrier by activating mammalian target of rapamycin complex 1 signaling.
Yang Y, Fan X, Ji Y, Li J, Dai Z, Wu Z
Animal nutrition (Zhongguo xu mu shou yi xue hui) 2022 Mar;8(1):1-9
Animal nutrition (Zhongguo xu mu shou yi xue hui) 2022 Mar;8(1):1-9
Inactivation of mouse transmembrane prolyl 4-hydroxylase increases blood brain barrier permeability and ischemia-induced cerebral neuroinflammation.
Byts N, Sharma S, Malm T, Kaakinen M, Korhonen P, Jaakkonen L, Keuters M, Huuskonen M, Pietilä I, Koistinaho J, Koivunen P, Myllyharju J
The Journal of biological chemistry 2022 Mar;298(3):101721
The Journal of biological chemistry 2022 Mar;298(3):101721
Fecal Microbiota Transplant in a Pre-Clinical Model of Type 2 Diabetes Mellitus, Obesity and Diabetic Kidney Disease.
Bastos RMC, Simplício-Filho A, Sávio-Silva C, Oliveira LFV, Cruz GNF, Sousa EH, Noronha IL, Mangueira CLP, Quaglierini-Ribeiro H, Josefi-Rocha GR, Rangel ÉB
International journal of molecular sciences 2022 Mar 31;23(7)
International journal of molecular sciences 2022 Mar 31;23(7)
Benzophenone-3 breaches mouse Sertoli cell barrier and alters F-actin organization without evoking apoptosis.
Zhang XY, Jiao XF, Wu D, Chen F, Ding ZM, Wang YS, Meng F, Duan ZQ, Xiong JJ, Yang CX, Huo LJ
Environmental toxicology 2022 Jan;37(1):28-40
Environmental toxicology 2022 Jan;37(1):28-40
Mucin O-glycan-microbiota axis orchestrates gut homeostasis in a diarrheal pig model.
Xia B, Zhong R, Wu W, Luo C, Meng Q, Gao Q, Zhao Y, Chen L, Zhang S, Zhao X, Zhang H
Microbiome 2022 Aug 31;10(1):139
Microbiome 2022 Aug 31;10(1):139
Development of a novel human intestinal model to elucidate the effect of anaerobic commensals on Escherichia coli infection.
McGrath CJ, Laveckis E, Bell A, Crost E, Juge N, Schüller S
Disease models & mechanisms 2022 Apr 1;15(4)
Disease models & mechanisms 2022 Apr 1;15(4)
Dietary Fish Hydrolysate Improves Memory Performance Through Microglial Signature Remodeling During Aging.
Chataigner M, Lucas C, Di Miceli M, Pallet V, Laye S, Mehaignerie A, Bouvret E, Dinel AL, Joffre C
Frontiers in nutrition 2021;8:750292
Frontiers in nutrition 2021;8:750292
Development of Anti-inflammatory Probiotic Limosilactobacillus reuteri EFEL6901 as Kimchi Starter: in vitro and In vivo Evidence.
Seo H, Seong H, Kim GY, Jo YM, Cheon SW, Song Y, Ryu BH, Kang H, Han NS
Frontiers in microbiology 2021;12:760476
Frontiers in microbiology 2021;12:760476
Annexin A1 protects against cerebral ischemia-reperfusion injury by modulating microglia/macrophage polarization via FPR2/ALX-dependent AMPK-mTOR pathway.
Xu X, Gao W, Li L, Hao J, Yang B, Wang T, Li L, Bai X, Li F, Ren H, Zhang M, Zhang L, Wang J, Wang D, Zhang J, Jiao L
Journal of neuroinflammation 2021 May 22;18(1):119
Journal of neuroinflammation 2021 May 22;18(1):119
Cystine reduces tight junction permeability and intestinal inflammation induced by oxidative stress in Caco-2 cells.
Hasegawa T, Mizugaki A, Inoue Y, Kato H, Murakami H
Amino acids 2021 Jul;53(7):1021-1032
Amino acids 2021 Jul;53(7):1021-1032
Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes.
Jia H, Zhang Y, Si X, Jin Y, Jiang D, Dai Z, Wu Z
Nutrients 2021 Jan 26;13(2)
Nutrients 2021 Jan 26;13(2)
Design and Validation of a Human Brain Endothelial Microvessel-on-a-Chip Open Microfluidic Model Enabling Advanced Optical Imaging.
Salman MM, Marsh G, Kusters I, Delincé M, Di Caprio G, Upadhyayula S, de Nola G, Hunt R, Ohashi KG, Gray T, Shimizu F, Sano Y, Kanda T, Obermeier B, Kirchhausen T
Frontiers in bioengineering and biotechnology 2020;8:573775
Frontiers in bioengineering and biotechnology 2020;8:573775
The impact of synthetic amorphous silica (E 551) on differentiated Caco-2 cells, a model for the human intestinal epithelium.
Hempt C, Kaiser JP, Scholder O, Buerki-Thurnherr T, Hofmann H, Rippl A, Schuster TB, Wick P, Hirsch C
Toxicology in vitro : an international journal published in association with BIBRA 2020 Sep;67:104903
Toxicology in vitro : an international journal published in association with BIBRA 2020 Sep;67:104903
TGF-β1 relieves epithelial-mesenchymal transition reduction in hypospadias induced by DEHP in rats.
Zhou Y, Huang F, Liu Y, Li D, Zhou Y, Shen L, Long C, Liu X, Wei G
Pediatric research 2020 Mar;87(4):639-646
Pediatric research 2020 Mar;87(4):639-646
Hyaluronan 35 kDa enhances epithelial barrier function and protects against the development of murine necrotizing enterocolitis.
Gunasekaran A, Eckert J, Burge K, Zheng W, Yu Z, Kessler S, de la Motte C, Chaaban H
Pediatric research 2020 Jun;87(7):1177-1184
Pediatric research 2020 Jun;87(7):1177-1184
The JAK-Inhibitor Tofacitinib Rescues Human Intestinal Epithelial Cells and Colonoids from Cytokine-Induced Barrier Dysfunction.
Sayoc-Becerra A, Krishnan M, Fan S, Jimenez J, Hernandez R, Gibson K, Preciado R, Butt G, McCole DF
Inflammatory bowel diseases 2020 Feb 11;26(3):407-422
Inflammatory bowel diseases 2020 Feb 11;26(3):407-422
Prevention of excessive exercise-induced adverse effects in rats with Bacillus subtilis BSB3.
Ducray HAG, Globa L, Pustovyy O, Roberts MD, Rudisill M, Vodyanoy V, Sorokulova I
Journal of applied microbiology 2020 Apr;128(4):1163-1178
Journal of applied microbiology 2020 Apr;128(4):1163-1178
MyD88 regulates a prolonged adaptation response to environmental dust exposure-induced lung disease.
Johnson AN, Harkema JR, Nelson AJ, Dickinson JD, Kalil J, Duryee MJ, Thiele GM, Kumar B, Singh AB, Gaurav R, Glover SC, Tang Y, Romberger DJ, Kielian T, Poole JA
Respiratory research 2020 Apr 22;21(1):97
Respiratory research 2020 Apr 22;21(1):97
Intestinal Permeability and IgA Provoke Immune Vasculitis Linked to Cardiovascular Inflammation.
Noval Rivas M, Wakita D, Franklin MK, Carvalho TT, Abolhesn A, Gomez AC, Fishbein MC, Chen S, Lehman TJ, Sato K, Shibuya A, Fasano A, Kiyono H, Abe M, Tatsumoto N, Yamashita M, Crother TR, Shimada K, Arditi M
Immunity 2019 Sep 17;51(3):508-521.e6
Immunity 2019 Sep 17;51(3):508-521.e6
Yeast fermentate prebiotic improves intestinal barrier integrity during heat stress by modulation of the gut microbiota in rats.
Ducray HAG, Globa L, Pustovyy O, Morrison E, Vodyanoy V, Sorokulova I
Journal of applied microbiology 2019 Oct;127(4):1192-1206
Journal of applied microbiology 2019 Oct;127(4):1192-1206
Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis.
Chen L, Li H, Li J, Chen Y, Yang Y
International journal of molecular medicine 2019 Mar;43(3):1139-1148
International journal of molecular medicine 2019 Mar;43(3):1139-1148
Gliclazide alone or in combination with atorvastatin ameliorated reproductive damage in streptozotocin-induced type 2 diabetic male rats.
Öztaş E, Yılmaz TE, Güzel E, Sezer Z, Okyar A, Özhan G
Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society 2019 Mar;27(3):422-431
Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society 2019 Mar;27(3):422-431
Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells.
Zhou Z, Bian C, Luo Z, Guille C, Ogunrinde E, Wu J, Zhao M, Fitting S, Kamen DL, Oates JC, Gilkeson G, Jiang W
Scientific reports 2019 Jun 10;9(1):8367
Scientific reports 2019 Jun 10;9(1):8367
Geniposide and Chlorogenic Acid Combination Ameliorates Non-alcoholic Steatohepatitis Involving the Protection on the Gut Barrier Function in Mouse Induced by High-Fat Diet.
Peng JH, Leng J, Tian HJ, Yang T, Fang Y, Feng Q, Zhao Y, Hu YY
Frontiers in pharmacology 2018;9:1399
Frontiers in pharmacology 2018;9:1399
Involvement of CYP2E1 in the Course of Brain Edema Induced by Subacute Poisoning With 1,2-Dichloroethane in Mice.
Jin X, Liao Y, Tan X, Wang G, Zhao F, Jin Y
Frontiers in pharmacology 2018;9:1317
Frontiers in pharmacology 2018;9:1317
HIV induces production of IL-18 from intestinal epithelial cells that increases intestinal permeability and microbial translocation.
Allam O, Samarani S, Mehraj V, Jenabian MA, Tremblay C, Routy JP, Amre D, Ahmad A
PloS one 2018;13(3):e0194185
PloS one 2018;13(3):e0194185
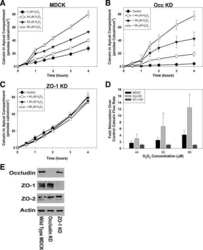
ZO-1 protein is required for hydrogen peroxide to increase MDCK cell paracellular permeability in an ERK 1/2-dependent manner.
Bilal S, Jaggi S, Janosevic D, Shah N, Teymour S, Voronina A, Watari J, Axis J, Amsler K
American journal of physiology. Cell physiology 2018 Sep 1;315(3):C422-C431
American journal of physiology. Cell physiology 2018 Sep 1;315(3):C422-C431
Urban fine particulate matter (PM2.5) exposure destroys blood-testis barrier (BTB) integrity through excessive ROS-mediated autophagy.
Wei Y, Cao XN, Tang XL, Shen LJ, Lin T, He DW, Wu SD, Wei GH
Toxicology mechanisms and methods 2018 May;28(4):302-319
Toxicology mechanisms and methods 2018 May;28(4):302-319
Immunohistochemistry in Investigative and Toxicologic Pathology.
Janardhan KS, Jensen H, Clayton NP, Herbert RA
Toxicologic pathology 2018 Jul;46(5):488-510
Toxicologic pathology 2018 Jul;46(5):488-510
Obesogenic diet-induced gut barrier dysfunction and pathobiont expansion aggravate experimental colitis.
Lee JC, Lee HY, Kim TK, Kim MS, Park YM, Kim J, Park K, Kweon MN, Kim SH, Bae JW, Hur KY, Lee MS
PloS one 2017;12(11):e0187515
PloS one 2017;12(11):e0187515
Chronic depletion of gonadal testosterone leads to blood-brain barrier dysfunction and inflammation in male mice.
Atallah A, Mhaouty-Kodja S, Grange-Messent V
Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2017 Sep;37(9):3161-3175
Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2017 Sep;37(9):3161-3175
Chronic sleep restriction disrupts interendothelial junctions in the hippocampus and increases blood-brain barrier permeability.
Hurtado-Alvarado G, Velázquez-Moctezuma J, Gómez-González B
Journal of microscopy 2017 Oct;268(1):28-38
Journal of microscopy 2017 Oct;268(1):28-38
Urban fine particulate matter exposure causes male reproductive injury through destroying blood-testis barrier (BTB) integrity.
Cao XN, Shen LJ, Wu SD, Yan C, Zhou Y, Xiong G, Wang YC, Liu Y, Liu B, Tang XL, Guo M, Liu DY, Long CL, Sun M, He DW, Lin T, Wei GH
Toxicology letters 2017 Jan 15;266:1-12
Toxicology letters 2017 Jan 15;266:1-12
Social stress induces neurovascular pathology promoting depression.
Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB, Janssen WG, Labonté B, Parise EM, Lorsch ZS, Golden SA, Heshmati M, Tamminga C, Turecki G, Campbell M, Fayad ZA, Tang CY, Merad M, Russo SJ
Nature neuroscience 2017 Dec;20(12):1752-1760
Nature neuroscience 2017 Dec;20(12):1752-1760
Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs).
DeStefano JG, Xu ZS, Williams AJ, Yimam N, Searson PC
Fluids and barriers of the CNS 2017 Aug 4;14(1):20
Fluids and barriers of the CNS 2017 Aug 4;14(1):20
Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior.
Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E, Ben-Amram H, Koren O, Forsythe P, Bienenstock J
Nature communications 2017 Apr 4;8:15062
Nature communications 2017 Apr 4;8:15062
A2A Adenosine Receptor Antagonism Reverts the Blood-Brain Barrier Dysfunction Induced by Sleep Restriction.
Hurtado-Alvarado G, Domínguez-Salazar E, Velázquez-Moctezuma J, Gómez-González B
PloS one 2016;11(11):e0167236
PloS one 2016;11(11):e0167236
Occludin Content Modulates Hydrogen Peroxide-Induced Increase in Renal Epithelial Paracellular Permeability.
Janosevic D, Axis J, Bacallao RL, Amsler K
Journal of cellular biochemistry 2016 Mar;117(3):769-79
Journal of cellular biochemistry 2016 Mar;117(3):769-79
17β-estradiol inhibits MMP-9 and SUR1/TrpM4 expression and activation and thereby attenuates BSCB disruption/hemorrhage after spinal cord injury in male rats.
Lee JY, Choi HY, Na WH, Ju BG, Yune TY
Endocrinology 2015 May;156(5):1838-50
Endocrinology 2015 May;156(5):1838-50
LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation.
Sohet F, Lin C, Munji RN, Lee SY, Ruderisch N, Soung A, Arnold TD, Derugin N, Vexler ZS, Yen FT, Daneman R
The Journal of cell biology 2015 Mar 16;208(6):703-11
The Journal of cell biology 2015 Mar 16;208(6):703-11
Assessment of an in vitro model of pulmonary barrier to study the translocation of nanoparticles.
Dekali S, Gamez C, Kortulewski T, Blazy K, Rat P, Lacroix G
Toxicology reports 2014;1:157-171
Toxicology reports 2014;1:157-171
Diets high in fermentable protein and fibre alter tight junction protein composition with minor effects on barrier function in piglet colon.
Richter JF, Pieper R, Zakrzewski SS, Günzel D, Schulzke JD, Van Kessel AG
The British journal of nutrition 2014 Mar 28;111(6):1040-9
The British journal of nutrition 2014 Mar 28;111(6):1040-9
Experimental cerebral malaria pathogenesis--hemodynamics at the blood brain barrier.
Nacer A, Movila A, Sohet F, Girgis NM, Gundra UM, Loke P, Daneman R, Frevert U
PLoS pathogens 2014 Dec;10(12):e1004528
PLoS pathogens 2014 Dec;10(12):e1004528
Estrogen receptor alpha deficiency protects against development of cognitive impairment in murine lupus.
Cunningham MA, Wirth JR, Freeman LR, Boger HA, Granholm AC, Gilkeson GS
Journal of neuroinflammation 2014 Dec 16;11:171
Journal of neuroinflammation 2014 Dec 16;11:171
Mast cells protect against Pseudomonas aeruginosa-induced lung injury.
Junkins RD, Carrigan SO, Wu Z, Stadnyk AW, Cowley E, Issekutz T, Berman J, Lin TJ
The American journal of pathology 2014 Aug;184(8):2310-21
The American journal of pathology 2014 Aug;184(8):2310-21
Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain.
Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B
The Journal of comparative neurology 2013 Oct 15;521(15):3389-405
The Journal of comparative neurology 2013 Oct 15;521(15):3389-405
Functional tight junction barrier localizes in the second layer of the stratum granulosum of human epidermis.
Yoshida K, Yokouchi M, Nagao K, Ishii K, Amagai M, Kubo A
Journal of dermatological science 2013 Aug;71(2):89-99
Journal of dermatological science 2013 Aug;71(2):89-99
Bile acid-induced expression of farnesoid X receptor as the basis for superiority of internal biliary drainage in experimental biliary obstruction.
Wu L, Li W, Wang Z, Yuan Z, Hyder Q
Scandinavian journal of gastroenterology 2013 Apr;48(4):496-503
Scandinavian journal of gastroenterology 2013 Apr;48(4):496-503
Evidence for phenotypic plasticity in aggressive triple-negative breast cancer: human biology is recapitulated by a novel model system.
D'Amato NC, Ostrander JH, Bowie ML, Sistrunk C, Borowsky A, Cardiff RD, Bell K, Young LJ, Simin K, Bachelder RE, Delrow J, Dawson A, Yee LD, Mrózek K, Clay TM, Osada T, Seewaldt VL
PloS one 2012;7(9):e45684
PloS one 2012;7(9):e45684
Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension.
Mi XS, Feng Q, Lo AC, Chang RC, Lin B, Chung SK, So KF
PloS one 2012;7(10):e45469
PloS one 2012;7(10):e45469
Role of caspases in cytokine-induced barrier breakdown in human brain endothelial cells.
Lopez-Ramirez MA, Fischer R, Torres-Badillo CC, Davies HA, Logan K, Pfizenmaier K, Male DK, Sharrack B, Romero IA
Journal of immunology (Baltimore, Md. : 1950) 2012 Sep 15;189(6):3130-9
Journal of immunology (Baltimore, Md. : 1950) 2012 Sep 15;189(6):3130-9
Modeling hepatitis C virus infection using human induced pluripotent stem cells.
Schwartz RE, Trehan K, Andrus L, Sheahan TP, Ploss A, Duncan SA, Rice CM, Bhatia SN
Proceedings of the National Academy of Sciences of the United States of America 2012 Feb 14;109(7):2544-8
Proceedings of the National Academy of Sciences of the United States of America 2012 Feb 14;109(7):2544-8
Isolation and properties of an in vitro human outer blood-retinal barrier model.
Hamilton RD, Leach L
Methods in molecular biology (Clifton, N.J.) 2011;686:401-16
Methods in molecular biology (Clifton, N.J.) 2011;686:401-16
Na+/K+-ATPase α1 identified as an abundant protein in the blood-labyrinth barrier that plays an essential role in the barrier integrity.
Yang Y, Dai M, Wilson TM, Omelchenko I, Klimek JE, Wilmarth PA, David LL, Nuttall AL, Gillespie PG, Shi X
PloS one 2011 Jan 31;6(1):e16547
PloS one 2011 Jan 31;6(1):e16547
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
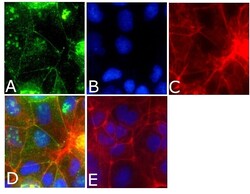
- Immunofluorescent analysis of occludin Antibody was done on 90% confluent log phase CACO2 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with Occludin Antibody (Product # 40-4700) at 1µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing cell junctional localization. Panel e is a no primary antibody control. The images were captured at 40X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
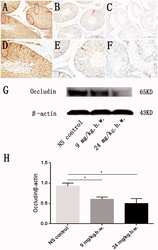
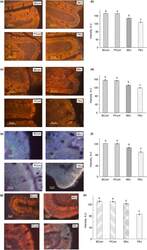
- Figure 3. Alteration of occludin in rat testis after PM2.5 treatment. Immuno-histochemical staining of occludin in testicular tissues from representative rats was performed. An enhanced occludin staining was observed in normal saline-treated group and located between germ cells of testicular seminiferous epithelium, especially basal compartment (A, D) (circled and arrow). Occludin expression lowered saliently in both low-dose PM2.5-treated group (B, E) and high-dose PM2.5-treated group (C, F) compared with control group. In support of this, Western blotting analysis demonstrated that occludin levels was downregulated in testicular tissues from representative rats that treated with different concentration PM2.5 (9 mg/kg b.w. and 24 mg/kg b.w. PM2.5) compared with control group (G). Densitometry values of occludin was normalized to b-actin. Quantitative analysis for the immune-reactive bands in the results of Western blotting analysis indicated that occludin expression decreased significantly in PM2.5-treated group, when compared with normal saline group ( p < 0.05) (H). Three sections were analyzed for each sample. *Significantly different from control, p < 0.05. Stained cells were indicated by arrows in the figure.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
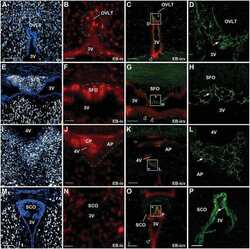
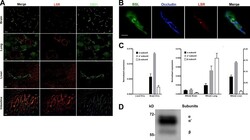
- Figure 1. LSR expression in adult mice. (A) Tissue sections of brain, lung, liver, and intestine were colabeled with antibodies directed against LSR (red) and CD31 (green, to label blood vessels). Expression of LSR in CD31 + ECs is only observed in the brain. Bar, 100 um. (B) Tissue sections from adult mice were labeled with the blood vessel marker BSL I (green), and antibodies against occludin (blue, bicellular TJ) and LSR (red). The merged image indicates that in CNS blood vessels LSR is localized at the tricellular TJ where two bicellular TJ meet. Bar, 5 um. (C, left) Quantitative real-time PCR analysis of LSR isoforms from purified brain ECs and liver ECs. Analysis indicates no detectable LSR expression in liver ECs but high expression of the alpha' variant followed by alpha and beta variants in brain ECs. (C, middle and right) Quantitative real-time PCR analysis of LSR variants from whole brain, lung, and liver. The expression in liver is much higher than brain or lung, and thus was given its own y axis (right). Splice variant composition is similar between brain and liver (alpha' variant followed by alpha and beta variants) but different in the lung (beta variant followed by alpha' and alpha variants). Data represent means +- SD (error bars) from three indipendent experiments. (D) Western blot profile of LSR from purified brain capillaries shows a stronger band for the alpha' splice variant followed by alpha and beta.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
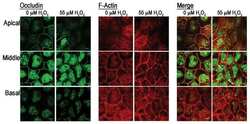
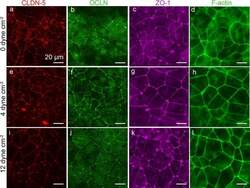
- Fig. 8 Representative immunofluorescence images of dhBMEC monolayers fixed and stained after 40 h at 0, 4, and 12 dyne cm -2 . a , e , i CLDN-5. b , f , j OCLN. c , g , k ZO-1. d , h , l F-actin. Note that CLDN-5/OCLN and ZO-1/f-actin were obtained for different monolayers
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
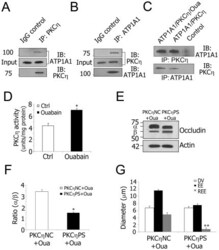
- Figure 5 Decreased Na + /K + -ATPase activity increases TJ permeability via PKCeta phosphorylated occludin. A and B , Co-immunoprecipitation using isolated stria vascularis capillary lysates shows that PKCeta is in a complex with ATP1A1. Goat IgG served as a negative control. C , Protein-protein interaction analysis with purified ATP1A1 (250 ng) and PKCeta (250 ng). Ouabain (10 uM) was added where indicated. Control lanes consisted of either anti-ATP1A1 antibody and purified PKCeta or anti-PKCeta antibody and purified ATP1A1. D , Ouabain inhibition of Na + /K + -ATPase activity causes increased PKCeta activity in isolated stria vascularis capillaries (*P = 0.013
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
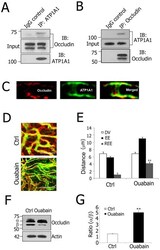
- Figure 4 ATP1A1 regulation of TJ permeability involves occludin phosphorylation in the blood-labyrinth barrier. A and B , Co-immunoprecipitation using isolated stria vascularis capillary lysates demonstrates that ATP1A1 is in a complex with occludin. Preimmune goat IgG served as a negative control. C , Immunolabeling of stria vascularis capillaries for occludin (Left, red) and ATP1A1 (Middle, green). The merged image (Right) shows that occludin and ATP1A1 co-localization. D , Confocal images show increased TJ permeability in ouabain-treated tissues (Lower) compared to control tissues (Upper). Serum protein IgG (green, antibody for IgG (H+L) is outside the lumen of the capillary (red, antibody for collagen type IV). E , REE analysis confirmed the significantly increased permeability of ouabain-treated tissues (**P = 0.005
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
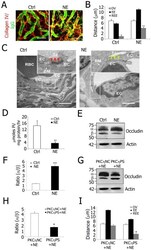
- Experimental details
- Figure 6 PKCeta phosphorylated occludin from decreased Na + /K + -ATPase activity is a mechanism for noise-induced blood-labyrinth barrier disruption. A, Confocal images of IgG leakage from capillaries in disrupted blood-labyrinth barrier of noise-exposed (NE) animals. Stria vascularis capillary networks were labeled with anti-collagen type IV (red) and anti-serum protein IgG (H+L, green) antibodies. IgG was confined in normal stria vascularis capillaries (Left) but not in NE animals (Right). B, REE analysis shows significantly increased blood-labyrinth barrier permeability in NE mice (**P = 0.005
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
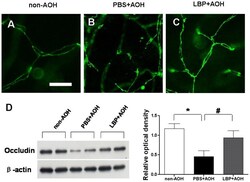
- Experimental details
- Figure 4 Protection of tight junctions between endothelial cells in LBP-fed retina at day 4 after AOH. (A-C) Representative photos of occludin-stained tight junctions in the network of capillaries on retinal flat-mounts, in the non-AOH control group, PBS-fed-AOH group and LBP-fed-AOH group. Note that the broken chains of tight junctions in (B). Scale bar: 15 um. PBS, phosphate-buffered saline vehicle; AOH, acute ocular hypertension; LBP, Lycium Barbarum Polysaccharides solution.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
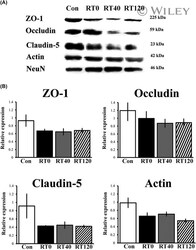
- Experimental details
- Chronic sleep restriction decreases the expression of tight junction proteins. (A) Representative western blots of the expression of tight junction proteins ZO-1, occludin, claudin-5 and the cytoskeletal protein actin in intact controls and sleep restricted rats with sleep recovery times of 0 min (RT0), 40 min (RT40) and 120 min (RT120). (B) The graph shows the expression of tight junction and cytoskeleton proteins in Con, RT0, RT40 and RT120 groups. Data were normalised against NeuN and are expressed as the mean +- SEM of three independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
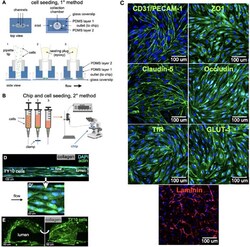
- Experimental details
- FIGURE 2 Cell seeding procedures and formation of a human brainmicrovessel using TY10 cells. (A) Cell seeding, 1st method. Representation of the steps used to increase the cell concentration before injection into the chip. Cells are allowed to settle by gravity (left and central panels) in the cell seeder and then injected as a bolus into the hollow lumen. Prior to injection, the chamber is capped with a plugged pipette tip (central panel). (B) Cell seeding, 2nd method. Representation of the steps used to inject cells into the chip in a more controlled and uniform manner compared to the 1st method. Cells are allowed to settle at the bottom of a syringe, and are then delivered into the hollow lumen at constant flow controlled by a syringe pump. (C) Immunostaining of TY10 monolayers: endothelial cell marker PECAM-1/CD31, transporters Glut-1 and transferrin receptor (TfR), and tight junction proteins ZO-1, Occludin and Claudin-5. Staining for laminin provides evidence that TY10 cells deposit extracellular matrix while in culture. Scale bar, 100 mum. (D) Representative image of a chemically fixed sample of TY10 cells after they were grown in the brain microvessel-on-a-chip with medium flowing from left to right at 1 mµL/min for 7 days. Volumetric image was obtained using a spinning disk confocal microscope. Maximum z-projection is shown for a sample stained with DAPI (nuclei, blue) and phalloidin (actin, green). Scale bar, 100 mum. (D') Representative image of a chemically
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
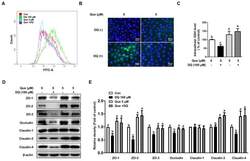
- Figure 3 Quercetin attenuated diquat-induced ROS accumulation and reduced protein abundance of tight junction proteins in IPEC-1 cells. IPEC-1 cells were treated as described in Figure 2 . ( A ) DCFH-DA-positive populations were detected by flow cytometry analysis. ( B ) Intracellular ROS levels were determined by fluorescence microscope (magnification x200). ( C ) Intracellular GSH levels were measured. ( D , E ) Protein abundance for ZO-1, ZO-2, ZO-3, occludin, claudin-1, claudin-3, and claudin-4 were determined and analyzed by the Western blot assay. beta-actin was used as the loading control. Representative experimental results were repeated in three independent experiments and values are means +- SEMs, n = 3. Means without a common letter are considered as a significant difference, p < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
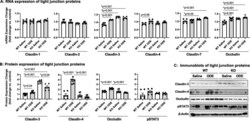
- Fig. 6 Repetitive ODE exposure increases expression of several tight junction proteins known to be upregulated in inflamed/injured lung in WT mice but not in MyD88 KO mice. WT and MyD88 KO mice were treated i.n. daily for 3 weeks with saline or ODE. Panel a, Expression of tight junction mRNA was measured by real-time quantitative PCR and are reported as fold-changes normalized to control. Panel b , Quantification of tight junction protein expression in ODE-treated mice compared to control mice as determined by the immunoblot presented in Panel c . Scatter plots demonstrate mean with standard error bars of N = 3 animals per group with 2 replicates per sample
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
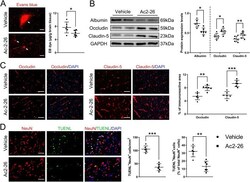
- Fig. 4 Effect of Ac2-26 on BBB disruption and neuronal apoptosis in the peri-infarct cortex at 3 days post-tMCAO/R. a Representative photographs of immunostaining and quantitative analyses of EB dye extravasation. White arrows indicated exudative EB dye. Scale bar = 100 mum. b Representative western blotting bands and densitometric quantifications of albumin extravasation and TJ (occludin and claudin-5) expressions. c-d Representative photographs of immunostaining and quantitative analyses of TJ (occludin and claudin-5; red) expression and neuronal apoptosis (NeuN, red; TUNEL, green). Scale bar = 200 mum. Data were presented as the mean +- SD ( n = 6/group), and were analyzed by independent samples t test. * p < 0.05, ** p < 0.01, and *** p < 0.001
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
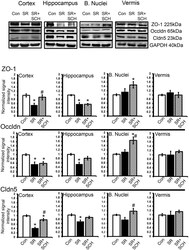
- Fig 4 A 2A adenosine receptor antagonism reverts the effect of sleep restriction on tight junction protein expression. At the top are depicted representative western blots of tight junction protein expression in the cortex, hippocampus, basal nuclei and vermis. Graphs show the relative optical density of tight junction proteins on the following groups: control plus DMSO (Con), sleep restriction plus DMSO (SR), and sleep restriction plus SCH58261 at 0.1mg/kg (SR+SCH) (n = 6 per group). GAPDH was used for normalization. Abbreviations are as follow: Cldn5: claudin-5; Occldn: occludin; ZO-1: Zonula occludens-1. Mean +- s.e.m. Two-way ANOVA test, post hoc test orthogonal contrast codes, *p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
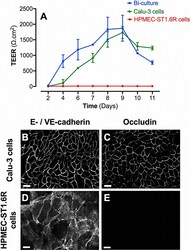
- Experimental details
- Fig. 1 Barrier properties of mono- and bi-cultures. A represents the time course of TEER development in mono- and bi-cultures of Calu-3 epithelial cells and HPMEC-ST1.6R endothelial cells. All cells were cultured in 12-Transwell (r) filter plates using RPMI 1640 media supplemented with 10% (v/v) FCS and 1% (v/v) penicillin/streptomycin. Mono-cultures of Calu-3 and HPMEC-ST1.6R cells are represented by green and red curves, respectively, and bi-cultures by the blue curve. Data represent the mean +- SD of four independent experiments. Immunofluorescent labelling of adherent (E-/VE-cadherin) and tight (occludin) junction proteins was performed in bi-cultures on Calu-3 and HPMEC-ST1.6R cells (in white, B-E), at day 8 post-seeding (scale bars = 20 mum).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
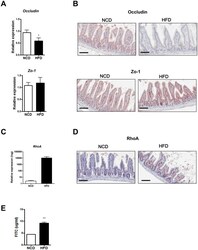
- Experimental details
- Fig 4 Disruption of the gut barrier in high-fat diet (HFD)-fed mice. (A) RT-qPCR analysis of Occludin and Zo-1 using RNA from the distal ileum of normal chow diet (NCD)- or HFD-fed mice ( n = 5 each). (B) Representative immunohistochemistry of distal ileal sections using anti-Occludin or -Zo-1 mAb. (C and D) RhoA expression in the intestine of mice fed NCD or HFD. mRNA expression (C) and representative immunohistochemistry (D) of RhoA in the small intestine. (E) Serum levels of FITC 4 h after oral FITC-dextran administration to NCD- or HFD-fed mice by oral gavage ( n = 5 each). Original magnification x200 for all images. Scale bar, 100 mum. All values are expressed as the means +- SEM. A student's t -test was used to compare values between two groups. * P < 0.05, ** P < 0.01.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
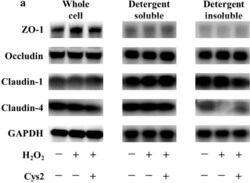
- Experimental details
- Fig. 3 Cystine (Cys2) improved the decrease of the ratio of claudin-4 in the detergent-insoluble fractions (IS) to soluble fractions (S). Caco-2 cells were separated the detergent-insoluble fractions and soluble fractions after 2 h of incubation with 0.5 mM H 2 O 2 . a Whole cell extracts, detergent-soluble fractions and insoluble fractions of Caco-2 cells were immunoblotted for ZO-1, occludin, claudin-1, claudin-4, and GAPDH. The expression level of the TJ proteins zonula occludens-1 (ZO-1) ( b ), occludin ( c ), claudin-1 ( d ), and claudin-4 ( e ) in the whole cell extracts was measured and the ratio of TJ proteins in the detergent-insoluble fractions to soluble fractions was calculated. Control cells (Con) were monolayers pretreated with DMEM and not incubated with 0.5 mM H 2 O 2 . Protein expression was normalized to GAPDH levels and expressed as mean fold change relative to the Con group. Values are represented as mean +- SEM ( n = 6)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
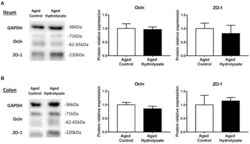
- Experimental details
- Figure 10 Effects of the fish hydrolysate supplementation on protein expression of intestinal permeability markers. Intestinal permeability markers ocln and ZO-1 protein expressions in (A) the ileum and (B) the colon of aged mice fed with the control diet or the hydrolysate-enriched diet for 10 weeks. n = 8 per group. Data are presented as mean +- SEM.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
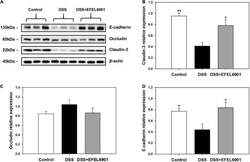
- FIGURE 5 Effects of Limosilactobacillus reuteri EFEL6901 strain on tight junction protein levels in mouse colon. (A) Representative western blotting of E-cadherin, occludin, claudin-3, and beta-actin (loading control). Quantification of E-cadherin (B) , occludin (C) , and claudin-3 (D) protein expression levels in the colon. Results are expressed as means +- standard error ( n = 7). Significant differences are presented with the DSS group: * p < 0.05; ** p < 0.01.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
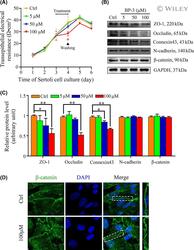
- 3 FIGURE The effects of BP-3 on Sertoli cell barrier function and cell junction proteins. (A) A typical result of transepithelial electrical resistance (TER) in the control group (0.1% DMSO) versus different treatment groups. Sertoli cells were cultured in transwell inserts in 24-well plate for continuous 6 days and TER values were daily detected. TER in all groups presented a similar growth in the former 3 days and after the treatment on Day 3, a significant decrease of TER in 100 muM group occurred. Besides, after removing BP-3 on Day 4, TER in 100 muM group began to rise again. Every single point on the curve for each day was a mean +- SD of triplicate units in one experiment. * p < .05, ** p < .01. (B) Representative images of Western blot analysis to assess the expression of tight junction proteins ZO-1 and Occludin, basal ES proteins N-cadherin, and beta-catenin and gap junction protein Connexin43. GAPDH was served as a loading control. Sertoli cells were cultured in 6-well plates for 3 days and then treated with BP-3 for 24 h. After the treatment, all cells were used to obtain protein lysates used in western blot analysis. (C) Relative level of proteins from western blot analysis was determined by densitometric scans. GAPDH was used to standardize the amount of related protein. The level of ZO-1, Occludin, and Connexin43 presented a significant decrease after BP-3 treatment in 100 muM group, but the level of basal ES proteins (N-cadherin and beta-catenin) was stable. *
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
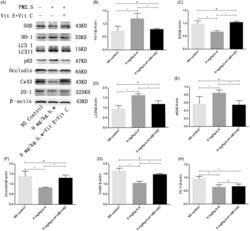
- Figure 11. The expression of SOD, HO-1, LC3II/I, p62, occludin, Cx43 and ZO-1 after vitamin C + E treatment. To investigate the relationship between oxidative stress and autophagy, the expression of LC3II/I and p62 was detected after intervention of ROS. The protein expression of occludin, Cx43 and ZO-1 was also detected by Western blot to investigate whether PM2.5-induced BTB impairment could be rescued after intervention of vitamin C + vitamin E. Western blotting analysis demonstrated that the HO-1 expression was downregulated after vitamin C + E treatment ( p < 0.05). The expression levels of SOD were upregulated after treatment with vitamin C + E ( p < 0.05) (A). All of these proteins in group PM2.5 + vitamin C + vitamin E were normally expressed compared to the NS control group ( p > 0.05) (B and C). In PM2.5 + vitamin C + E group, the expression of LC3II/I and p62 was markedly decreased compared with PM2.5 group. Although the expression of tight junction (ZO-1) was not obviously influenced following antioxidants treatment ( p > 0.05) (E), Western blotting analysis demonstrated that the occludin and Cx43 expressions were significantly increased after vitamin E + C supplement compared with PM2.5 group ( p < 0.05) (F and G). Densitometry value analysis for each independent experiment was performed at least in triplicate. Densitometry values were normalized to b-actin. * p < 0.05 versus control group. # p > 0.05 versus PM2.5 + vitamin C + vitamin E group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
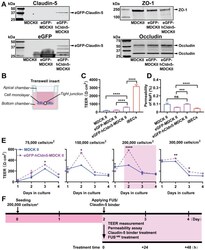
- Figure 2 eGFP-hCldn5-MDCK II cells exhibit a tight monolayer as determined by TEER and cargo leakage. (A) Western blotting with either a claudin-5 or GFP antibody reveals expression of the eGFP-hClaudin5 fusion protein in eGFP-hCldn5-MDCK II but not eGFP-MDCK II cells. Expression of the tight junction proteins ZO-1 and occludin is also shown. (B) Scheme of Transwell insert to measure TEER and permeability. (C) eGFP-hCldn5-MDCK II cells display a four-fold higher TEER than MDCK II cells. iBEC cells are included for comparison. (D) All three MDCK II cell lines show a < 0.2% permeability for sodium fluorescein (NaFl), indicating a tight BBB. (E) TEER of eGFP-hCldn5-MDCK II and MDCK II cells shown as a function of cell density and days in culture. Asterisks refer to TEER differences between eGFP-hCldn5-MDCK II and MDCK II for a given time point. TEER values are shown as Omega*cm 2 and results are expressed as mean +- SEM. N>10 per condition. ( F ) Schematic illustration of the experimental workflow. Statistical significance was determined as unpaired Student's t-test (*p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
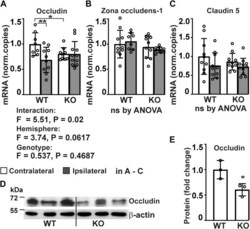
- Experimental details
- Analysis of expression of tight junction proteins in P4htm -/- (KO) and WT cortex. A - E , mice were sacrificed 24 h after the onset of permanent MCAO and injured ipsilateral and noninjured contralateral cortexes were collected for mRNA extraction ( A - C ) and protein isolation ( D and E ). A - C , expression of tight junction proteins was assessed by qRT-PCR, n = 8 to 11 mice per group, * p < 0.05 ** p < 0.01 by procedure of Benjamini, Krieger, and Yekutieli after two-way ANOVA for multiple comparisons. D and E , expression of occludin protein was analyzed by Western blotting of cortex tissue lysates from unoperated WT and KO mice, n = 3 mice per genotype. * p < 0.05 by unpaired t test. MCAO, middle cerebral artery occlusion.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
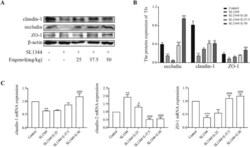
- Experimental details
- Eugenol improved the expressions of the duodenal tight junction proteins ZO-1, claudin-1 and occludin in Salmonella -infected broilers. (A) WB analysis: the protein bands of critical proteins. (B) WB analysis: the quantification of the protein levels by determining the band intensity and normalized to beta-actin band levels. (C) The mRNA expressions of tight junctions (ZO-1, claudin-1 and claudin-2). All experiments were repeated in triplicates. The data are expressed as the Mean +- SE (n = 15). ** P < 0.01, *** P < 0.001 vs. Control. # P < 0.05, ## P < 0.01, ### P < 0.001 vs. SL1344 infection. Figure 5
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
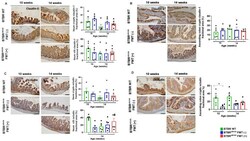
- Experimental details
- Immunohistochemical evaluation of claudin-1 and occludin proteins in ileum and ascending colon in BTBR ob/ob FMT (+) and BTBR ob/ob FMT (-) mice compared to BTBR WT mice. ( A ) Claudin-1 evaluation in the ileum crypts showed no significant difference according to age and treatment (all p > 0.99). Likewise, detection of claudin-1 in ileum villi was not statistically significant: 14-week-old BTBR WT versus 14-week-old BTBR ob/ob FMT (-) ( p > 0.99) and BTBR ob/ob FMT (+) ( p = 0.97) mice, and 14-week-old BTBR ob/ob FMT (-) versus FMT (+) ( p = 0.99) mice. ( B ) Claudin-1 expression in ascending colon crypts was not different among mice, regardless of age and treatment (all p > 0.99). ( C ) Occludin expression in ileum crypts was not significantly different between 14-week-old BTBR WT versus 14-week-old BTBR ob/ob FMT (-) ( p = 0.79) and FMT (+) ( p > 0.99) mice, and 14-week-old BTBR ob/ob FMT (-) versus FMT (+) ( p > 0.99) mice. In ileum villi, occludin expression was not different between 14-week-old BTBR WT versus 14-week-old BTBR ob/ob FMT(-) and FMT (+) ( p > 0.99 for both) mice, and 14-week-old BTBR ob/ob FMT(-) versus FMT (+) ( p > 0.99) mice. ( D ) Occludin expression in the ascending colon crypt was significantly different between 10-week-old BTBR ob/ob and age-matched BTBR WT mice (* p = 0.01), but not significantly different between 14-week-old BTBR WT versus 14-week-old BTBR ob/ob FMT (-) ( p = 0.06) and FMT (+) ( p = 0.27) mice, and 14-week-old BTBR ob/ob FMT (-) ve
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
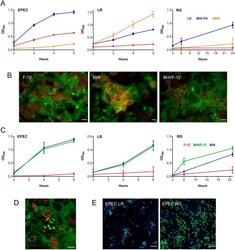
- Experimental details
- Fig. 2. Optimization of the apical VDC medium for co-incubation of EPEC, L. reuteri or R. gnavus and human T84 cell epithelia. (A) Growth of EPEC, L. reuteri (LR) and R. gnavus (RG) in LB, MRS and BHI-YH broth. Bacteria were incubated in the VDC without host cells and OD 600 was monitored over 5 h (EPEC and L. reuteri ) or 24 h ( R. gnavus ). Mean+-s.d., n =4. (B) Influence of BHI-YH medium on intestinal epithelial integrity and barrier function. Differentiated T84 cells were incubated in the VDC for 22 h with BHI-YH (BHI), DMEM/F-12 (F-12) or a 1:1 mixture of both media (BHI/F-12) on the apical side and DMEM/F-12 on the basal side. Epithelia were stained for F-actin (green) and occludin (red). Scale bars: 10 um. Images are representative of n =6. (C) Growth of EPEC, L. reuteri and R. gnavus in BHI-YH (BHI), DMEM/F-12 (F-12) and BHI-YH/F-12 medium (BHI/F-12). Bacteria were incubated in the VDC without host cells and OD 600 was monitored over 6 h (EPEC and L. reuteri ) or 23 h ( R. gnavus ). Mean+-s.d., n =4. (D) EPEC A/E lesion formation in T84 cells after 4 h incubation in apical BHI-YH/F-12 medium. Cells and EPEC were stained with F-actin (green) and DAPI (red), respectively. Scale bar: 5 um. Images are representative of n =6. (E) Optimization of bacterial inocula for co-incubations. EPEC was cultured in the VDC with different ratios of L. reuteri or R. gnavus for 4 h. Culture composition was evaluated by immunofluorescence staining for R. gnavus NanH or L. reuteri CmbA (gr
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
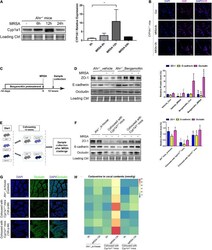
- FIGURE 7 Gut barrier function induced by commensal bacteria in an AHR-independent manner. (A) Immunoblot and densitometry plots of MRSA-induced CYP1A1 levels in the ileal epithelium from Ahr -/- mice ( n = 5-6). (B) Representative immunostaining of IEC AHR (purple) and DAPI (blue) from Cyp1a1 -/- mouse ileum before (Control; Con) or 6 h (MRSA-6h) and 12 h p-Mi (MRSA-12h). (C) Experimental design. Ahr -/- mice were fed bergamottin for 14 days before the MRSA challenge. Ileum mucosa collection at 12 h p-Mi for immunoblotting. (D) Immunoblot and densitometry plots of cell junction proteins in the ileal epithelium from Ahr -/- _Vehicle mice ( n = 7-8) or Ahr -/- _Bergamottin mice ( n = 7-8). (E) Experimental design. Ahr -/- mice were cohoused with Cyp1a1 +/+ mice and Cyp1a1 -/- mice before MRSA challenge for 10 weeks. Sample collection at 12 h p-Mi. (F) Immunoblot and densitometry plots of cell junction proteins in the ileal epithelium from Ahr -/- _si-house mice ( n = 7-8), Ahr -/- mice cohoused with Cyp1a1 +/+ mice ( n = 8-10) or Ahr -/- mice cohoused with Cyp1a1 -/- mice ( n = 8-10). (G) Immunofluorescence staining of ileum sections for occludin (green) and DAPI (blue) from each group. Scale bar: 40 mum. (H) Heatmap of the caecal contents from each group. Error bars, +-SEM. * P < 0.05. si-house, only Ahr -/- mice were housed in a cage; cohouse, Ahr -/- mice were housed in a cage with Cyp1a1 +/+ mice or Cyp1a1 -/- mice separately. Differences were analyzed using one-way ANOVA.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
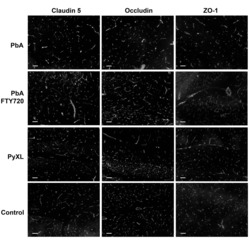
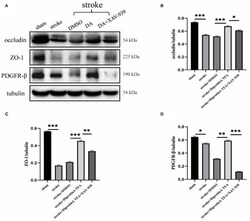
- Diprotin A TFA regulates the expression of tight junction protein at the BBB and pericyte. (A) Western blot images of BBB-associated tight junction protein (occludin, ZO-1) and pericyte (PDGFR-beta) in the penumbra tissue of five groups of mice with cerebral ischemic injury. (B-D) The results showed that the expression levels of occludin, ZO-1, and PDGFR-beta were upregulated in the group injected with DA compared with the vehicle control group, whereas the expression levels were downregulated in the group injected with DA and beta-catenin inhibitor. n = 3 per group. Data are represented as mean with SEM. * p < 0.05, ** p < 0.01, *** p < 0.001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
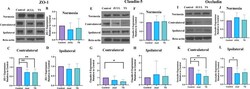
- Brain ZO-1, claudin-5, and occludin expression in mice after 14 days of JUUL/TS exposure with or without MCAO. A Western blot images of ZO-1 expression in normoxic brain and contralateral and ipsilateral brain hemispheres 24 h after MCAO. B - D Quantification of brain ZO-1 expression normalized to beta-actin and expressed as relative to control (1.0) in normoxic, contralateral, and ipsilateral brain regions. E Western blot images of claudin-5 expression in normoxic brain and contralateral and ipsilateral brain hemispheres 24 h after MCAO. F - H Quantification of brain claudin-5 expression normalized to beta-actin and expressed as relative to control (1.0) in normoxic, contralateral, and ipsilateral brain regions, respectively. I Western blot images of occludin expression in normoxic brain and contralateral & ipsilateral brain hemispheres 24 h after MCAO. J-L Quantification of brain occludin expression normalized to beta-actin and expressed as relative to control (1.0) in normoxic, contralateral, and ipsilateral brain regions. *P < 0.05, ***P < 0.001; n = 8 for each group ( B ), n = 6, 6, and 5 for control, JUUL, and TS respectively ( C ), n = 7, 7, and 6 for control, JUUL, and TS respectively ( D ), n = 9 for each group ( F ), n = 8, 7, and 6 for control, JUUL, and TS respectively ( G ), n = 8, 8, and 6 for control, JUUL, and TS respectively ( H ), n = 9 for each group ( J ), n = 7, 6, and 5 for control, JUUL, and TS respectively ( K ), n = 6 for each group ( L ). Cropped ima
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
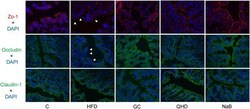
- Figure 5 Immunofluorescence staining of tight junction (ZO-1, Occludin and Claudin-1) in colon. White arrows point out the tight junction disruption (600 times of magnification). C, control, HFD, high-fat diet, GC, geniposide and chlorogenic acid combination, QHD, Qushi Huayu Decoction, NaB, sodium butyrate.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 2 Normal morphology of seminiferous tubules, interstitial area and cellular attachments (A), claudin11 (-, F) and occludin (-, K) expression. Irregular and atrophied (*, B) seminiferous tubules, immature germ cells in lumen (>, B), abnormal cellular attachments (insert, B) and loss of cellular tight junctions (G, L) in diabetic rats. Disorganised germinal epithelium, cytoplasmic vacuoles (v, C), immature germ cells in lumen (insert, C), slightly increased claudin11 (H) and occludin (M) expression in ATV treated diabetic rats. Round seminiferous tubules with minimal loss of cellular attachments and normal interstitial area (D), well organized tight junctions (I, N) comparable to control group in GLZ treated diabetic rats. Immature germ cells in lumen (>, F), amorphous material in interstitial area (*, F), depleted interstitial area (ti, insert, F), increased expression of claudin11 (J) and occluding (O) in ATV/GLZ combination group. Staining: H&E (first column); Immunohistochemistry: Claudin11 (second column) and occludin (third column); Scale bars: 50 um. STZ, diabetic rats; STZ + ATV, diabetic rats in Atorvastatin treatment; STZ + GLZ, diabetic rats in Gliclazide treatment; STZ + ATV/GLZ, diabetic rats in Atorvastatin/Gliclazide combination treatment.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
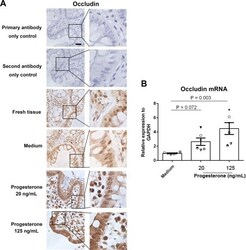
- Figure 3 Effect of progesterone on occludin expression in human primary gut tissues. Female primary gut tissues were treated with media alone or different concentrations of progesterone for 24 h. Occludin expression was then examined by immunohistochemistry assay ( A ). Data are representative of at least three independent experiments. Bar: 20 mum. Occludin expression was examined by qRT-PCR in female primary gut tissues treated by different conditions ( B ). One-way ANOVA.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
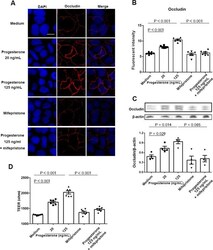
- Figure 4 Effect of progesterone on Caco-2 cells permeability. Caco-2 cells were treated with media alone or different concentrations of progesterone in the presence or absence of 1 muM mifepristone for 24 h. Occludin expression was then examined by immunofluorescence assay ( A ). Occludin and nuclei were detected by DyLight 594-labeled antibody and DAPI respectively. Data are representative of at least three independent experiments. Bar: 20 mum. Images were taken with a fluorescence microscope, and fluorescence intensity of occludin was analyzed using ImageJ software ( B ). Occludin expression in Caco-2 cells was examined by western blot ( C ). Relative expression of occludin was quantified to beta-actin by ImageJ software. Trans-epithelial electrical resistance (TEER) was measured in Caco-2 cells ( D ). Data are representative of at least three independent experiments. One-way ANOVA.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 2 Expression of TJ proteins in the intestine of rats from different experimental groups: ES--rats were orally gavaged with Saccharomyces cerevisiae fermentate and exposed to heat stress; EC--rats were orally gavaged with Saccharomyces cerevisiae fermentate and kept at room temperature; PS--rats were orally gavaged with PBS and exposed to heat stress; PC--rats were orally gavaged with PBS and kept at room temperature; * P < 0*05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
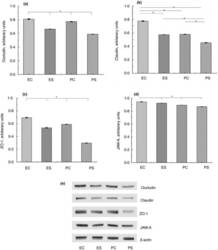
- Figure 3 Immunofluorescence staining of TJ proteins in the intestine of rats from different experimental groups. (a) Images of occludin immunostaining, Bar 50 um; (b) Analysis of occludin expression with immunostaining; (c) Images of ZO-1 immunostaining, bar 50 um; (d) Analysis of ZO-1 expression with immunostaining; (e) Images of claudin immunostaining, bar 10 um; (f) Analysis of claudin expression with immunostaining; (g) Images of JAM-A immunostaining, bar 10 um; (h) Analysis of JAM-A expression with immunostaining. A.U., arbitrary units. Rats were pretreated by oral gavage with B. subtilis BSB3 (BSB3) or with PBS and were either exposed forced running or remained sedentary ( n = 6 rats per condition). BEx, pretreated with BSB3, undergoing forced running; BCont, pretreated with BSB3 remaining sedentary; PEx, pretreated with PBS, undergoing forced running; PCont, pretreated with PBS remaining sedentary. Data with different superscript letters signify groups are significantly different ( P < 0*05). [Colour figure can be viewed at https://www.wileyonlinelibrary.com ]
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry